Etiological, Clinical and Prognostic Characteristics of Comas in the Multipurpose Intensive Care Unit of The Zinder National Hospital
Article Information
MAGAGI Amadou1*, MAIKASSOUA Mamane2, HASSAN Maman L3 BOUKARI MB4, CHAIBOU MS5, DADDY H6, YAHAYA BN7
1,3 Resuscitation anesthesia department of the Zinder national hospital
2 Faculty of health sciences of the Dan Dicko Dankoulodo University of Maradi
4,5,6. Resuscitation Anesthesia Department of the National Hospital of Niamey
7Faculty of Health Sciences of the André Salifou University of Zinder
*Corresponding Author: MAGAGI Amadou, Faculty of Health Sciences of the André Salifou University of Zinder, department of anesthesia and intensive care at the Zinder national hospital.
Received: 05 December 2023; Accepted: 13 December 2023; Published: 19 January 2024
Citation: MAGAGI Amadou, MAIKASSOUA Mamane, HASSAN Maman L BOUKARI MB, CHAIBOU MS, DADDY H, YAHAYA BN. Etiological, Clinical and Prognostic Characteristics of Comas in the Multipurpose Intensive Care Unit of The Zinder National Hospital. Anesthesia and Critical Care 6 (2024): 01-08.
View / Download Pdf Share at FacebookAbstract
Coma is a frequent diagnostic and therapeutic emergency. The aim of the study was to enumerate the etiologies of comas and to determine the prognosis of patients. It was a descriptive, cross-sectional study with prospective data collection from 1er May to 31 December 2021, i.e. a period of 8 months in the polyvalent intensive care unit of Zinder National Hospital. The parameters studied were sociodemographic aspects, admission conditions, clinical status on admission, paraclinical data, etiologies of comas, complications, patient outcome. During the study period, 830 patients were admitted, including 112 cases of coma, representing a frequency of 13.5%. The average age was 41.64±22.54 years, with extremes of 3 and 90 years. Males predominated, with a sex ratio of 1.5. Housewives were the most affected (33.9%). High blood pressure was the most frequent antecedent condition. In 66.9% of cases, onset was abrupt, and 47.3% of patients were admitted within 24 hours of coma. The Glasgow score on admission was between 6 and 8 in 47.3% of cases. Traumatic comas accounted for 36.4% of cases, followed by metabolic comas (24.8%) and vascular comas (22.2%). The average length of stay was 5 days. Overall mortality was 48.2%. Poor prognostic factors were: age over 60 (P<0.01), history of hypertension (P=0.02), respiratory distress (P<0.01), tachycardia (P<0.01), coma depth (P<0.01) and hospital stay of less than 3 days (P=0.02). Patients' prognosis depends on early and appropriate management.
Keywords
Etiologies, Prognosis, Comas, HNZ Resuscitation, Introduction
Etiologies articles; Prognosis articles; Comas articles; HNZ Resuscitation articles; Introduction articles
Etiologies articles Etiologies Research articles Etiologies review articles Etiologies PubMed articles Etiologies PubMed Central articles Etiologies 2023 articles Etiologies 2024 articles Etiologies Scopus articles Etiologies impact factor journals Etiologies Scopus journals Etiologies PubMed journals Etiologies medical journals Etiologies free journals Etiologies best journals Etiologies top journals Etiologies free medical journals Etiologies famous journals Etiologies Google Scholar indexed journals Prognosis articles Prognosis Research articles Prognosis review articles Prognosis PubMed articles Prognosis PubMed Central articles Prognosis 2023 articles Prognosis 2024 articles Prognosis Scopus articles Prognosis impact factor journals Prognosis Scopus journals Prognosis PubMed journals Prognosis medical journals Prognosis free journals Prognosis best journals Prognosis top journals Prognosis free medical journals Prognosis famous journals Prognosis Google Scholar indexed journals Comas articles Comas Research articles Comas review articles Comas PubMed articles Comas PubMed Central articles Comas 2023 articles Comas 2024 articles Comas Scopus articles Comas impact factor journals Comas Scopus journals Comas PubMed journals Comas medical journals Comas free journals Comas best journals Comas top journals Comas free medical journals Comas famous journals Comas Google Scholar indexed journals HNZ Resuscitation articles HNZ Resuscitation Research articles HNZ Resuscitation review articles HNZ Resuscitation PubMed articles HNZ Resuscitation PubMed Central articles HNZ Resuscitation 2023 articles HNZ Resuscitation 2024 articles HNZ Resuscitation Scopus articles HNZ Resuscitation impact factor journals HNZ Resuscitation Scopus journals HNZ Resuscitation PubMed journals HNZ Resuscitation medical journals HNZ Resuscitation free journals HNZ Resuscitation best journals HNZ Resuscitation top journals HNZ Resuscitation free medical journals HNZ Resuscitation famous journals HNZ Resuscitation Google Scholar indexed journals Introduction articles Introduction Research articles Introduction review articles Introduction PubMed articles Introduction PubMed Central articles Introduction 2023 articles Introduction 2024 articles Introduction Scopus articles Introduction impact factor journals Introduction Scopus journals Introduction PubMed journals Introduction medical journals Introduction free journals Introduction best journals Introduction top journals Introduction free medical journals Introduction famous journals Introduction Google Scholar indexed journals
Article Details
1. Introduction
Coma is defined as a state of non-arousable unconsciousness due to dysfunction of the brain's ascending reticular activating system [1].It is a frequent diagnostic and therapeutic emergency requiring rapid and adequate treatment. In France in 2012, the admission rate to intensive care for coma was 48.83% [2]. In Africa, studies have reported an admission rate for comas to intensive care units ranging from 44.4 to 72% [5–8]. In Niamey, Niger in 2016, Yahaya's team recorded an admission rate for serious adult coma of 12.65%.[9]and in 2020, the Boukari MB team reported a rate of 24% among children[10]. The etiology partly determines the prognosis. At the Zinder National Hospital (HNZ), no work has been published on comas. What etiologies and prognosis for these patients? This is how we set ourselves the objective for this work, to list the etiologies and prognosis of comas at the level of our service.
2. Patient and Method
The study took place at the Zinder national hospital in its multipurpose intensive care unit. This was a descriptive, cross-sectional study with prospective data collection which was carried out at the HNZ multipurpose intensive care unit covering a period of 8 months from May 1 to December 31, 2021. All patients admitted to the department multipurpose resuscitation unit of the HNZ. All comatose patients (Glasgow score from 3/15 to 12/15) admitted to the multipurpose intensive care unit of the HNZ during our study period were included in the study. Not included were patients admitted to the intensive care unit with a Glasgow score greater than 12/15, patients operated on under sedation and those comatose after their admission (during hospitalization). The variables studied were age, gender, profession, origin, mode of transport, clinical condition at admission, etiologies, length of stay, outcome. The data collected were recorded and analyzed using Excel 2016 and Epi info version 7 software. Chi2 statistical tests were used to compare qualitative variables, and Kruskal-Wallis and Student to compare proportions and means. . The statistical significance threshold retained was less than 5%.
Informed consent was obtained from the family by explaining the purpose of our study.
The anonymity of the patients was respected: before data analysis, each patient's first and last name was replaced by a number.
3. Results
During the study period, 830 patients were admitted to the intensive care unit, including 112 cases of comatose patients, representing a frequency of 13.5%. The average age was 41.64 ±22.54 years with extremes of 3 and 90 years. The age group of 21 to 40 years was the most represented with 27.6% of cases (n=31).
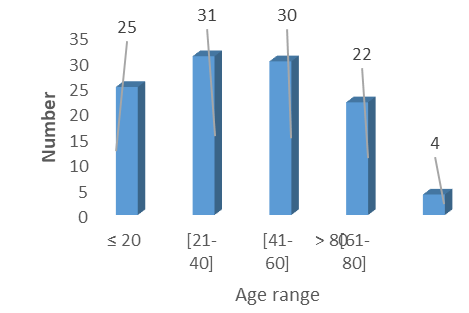
Figure 1: Distribution of patients by age group
The male gender represented 59.8% of cases (n=67), i.e. a sex ratio of 1.5.
Table 1: Distribution of patients according to profession
|
Occupation |
Workforce |
Percentage |
|
Housewives |
38 |
33.9 |
|
Farmer |
31 |
27.6 |
|
School |
18 |
16 |
|
Without fixed profession |
10 |
8.9 |
|
Official |
9 |
8 |
|
Trader |
6 |
5.3 |
|
Total |
112 |
100 |
Housewives were the most affected with a rate of 33.9% of cases (n=38).
Table 2: Distribution of patients according to origin
|
Origin |
Workforce |
Percentage |
|
Zinder region |
102 |
91.07 |
|
Maradi region |
4 |
8 |
|
Agadez region |
3 |
3.5 |
|
Diffa region |
1 |
2.6 |
|
Tahoua region |
1 |
0.8 |
|
Others |
1 |
0.8 |
|
Total |
112 |
100 |
The vast majority of patients came from the Zinder region with 91.07% of cases followed by the Maradi region with a rate of 8%.
Table 3: Distribution of patients according to history
|
Background |
Workforce |
Percentage |
|
HT |
12 |
10 |
|
Diabetes |
4 |
3 |
|
Diabetes, hypertension |
4 |
3 |
|
Coma, IR |
1 |
0.8 |
|
Orchiectomy, herniorrhaphy |
1 |
0.8 |
|
hypertension, DALY |
1 |
0.8 |
|
Hypertension, heart disease |
1 |
0.8 |
|
None |
88 |
78 |
|
Total |
112 |
100 |
The history consisted mainly of arterial hypertension in 12 patients. More than half (78%) of the patients had no history (n=88). The onset of symptoms was sudden in 66.9% of patients (n=75).More than half (49.1%) of the patients (n=55) were admitted 24 hours after the onset of symptoms. The average delay was 25.5 hours. Patients benefited from medical transport in 59.8% of cases.
Table 4: Distribution of patients according to admission constants
|
Constants Workforce Percentage |
|
Temperature ?Normal 41 56.9 ?Hyperthermia 31 43.1 FC ?Normal 31 42.5 ?Tachycardia 42 57.5 P.A. ?Normal 46 54.1 ?HT 39 45.9 SpO2 ?≥ 95% 48 53.9 ?<95% 41 46.1 |
Hyperthermia, tachycardia and hypertension were observed in 43.1% respectively; 57.5%; 45.9%. SpO2 was below 95% in 46.1% (n=41) of patients.
In 47.3% (n=53) of patients, the Glasgow score at admission was between 6 and 8, 38.3% between 9 and 12 and 14.2% had a score between 3 and 5.
Hemiplegia was the main focal sign observed in 24.2% of cases (n=25).
Table 5: Distribution of patients according to additional examinations
|
Additional tests |
Workforce |
Percentage |
|
Blood sugar |
80 |
71.4 |
|
NFS |
64 |
57.1 |
|
Creatininemia |
60 |
53.5 |
|
Azotemia |
59 |
52.6 |
|
Serum electrolytes |
52 |
46.4 |
|
GE |
36 |
32.1 |
|
Brain scan |
40 |
35.7 |
|
TP |
2 |
1.7 |
|
ASAT |
2 |
1.7 |
|
ALAT |
2 |
1.7 |
The main tests carried out were blood sugar, CBC, serum creatinine in 71.4% respectively; 57.1%; 53.5%. Brain scanning was only performed in 35.7% of patients.
Table 6: Distribution of patients according to etiologies
|
Etiologies Numbers Percentage |
|
Cranio-encephalic trauma 41 36.60% ?Brain contusion 11 09.8 ?Extradural hematoma 02 01.7 ?Subdural hematoma 03 02.6 ?ND 25 22.3 Metabolic disorders 28 25% ?Renal failure 16 14.2 ?Diabetes 11 09.8 ?Hepatic encephalopathy 01 00.8 Infections 15 13.39% ?Severe malaria 09 08.0 ?Meningoencephalitis 03 02.6 ?Brain abscess 01 00.8 ?Others 02 01.7 Stroke 25 22.32% ?DALY 14 12.5 ?AVCH 08 07.1 ?ND 03 02.6 Others 03 02.6% Total 112,100 |
Others: intoxication, obstetric, not determined
Cranioencephalic trauma was the most common etiology with a rate of 36.4% (n=41) followed by metabolic coma (25%).
Table 7: Distribution of patients according to decompensations
|
Decompensations |
Workforce |
Percentage |
|
Respiratory distress |
26 |
23.6 |
|
Convulsive seizures |
7 |
6.3 |
|
Septic shock |
4 |
3.6 |
|
Respiratory and hyperthermic distress |
4 |
3.6 |
|
Septic shockand metabolic disorder |
1 |
0.9 |
|
Cardiovascular disorder |
3 |
2.7 |
|
Hypoglycemia |
1 |
0.9 |
|
Hyperthermic |
3 |
2.7 |
|
Respiratory and cardiovascular distress |
2 |
1.8 |
|
Hyperthermia and cardiovascular |
1 |
0.9 |
|
Seizures and hyperthermia |
2 |
1.8 |
|
Seizures and cardiovascular disorder |
1 |
0.9 |
|
Bed sore |
1 |
XX0.9 |
|
None |
54 |
49 |
|
Unspecified |
2 |
1.8 |
|
Total |
112 |
100 |
Respiratory distress was the main decompensation recorded with a rate of 23.6% (n=26).
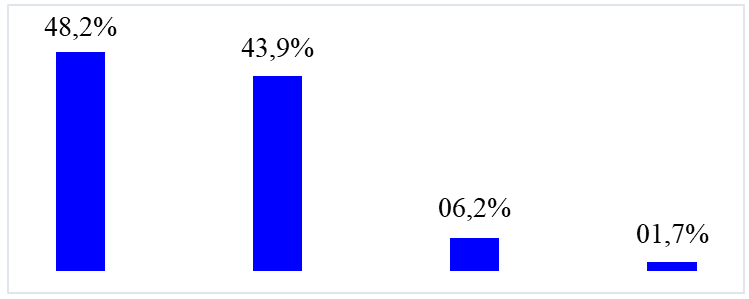
Figure 2: Distribution of patients according to prognosis
The death rate was 48.2% (n=54).
Table 8: Influence of age on prognosis
|
Age |
Alive (%) Dead (%) Total (%) |
|
> 60 ≤ 60 |
07(28) 18(72) 25(100) 51(58.6) 36(41.3) 87(100) |
Chi2=7.29 p-value<0.01
Age over 60 years was significantly linked to the occurrence of patient death.
Table 9: Influence of history on prognosis
|
History Living(%) Deceased(%) Total(%) |
|
Yes 08(32) 17(68) 25(100) No 50(57.4) 37(42.5) 87(100) |
Chi2=5.04 p-value=0.02
In 17 cases or 68%, the presence of a history was a factor favoring death.
Table 10: Influence of time to admission to intensive care on prognosis
|
Admission deadline |
Alive(%) Dead(%) Total(%) |
|
≤ 6 hours > 6 hours Unspecified |
06(66.6) 03(33.3) 09(100) 51(51.5) 48(48.4) 99(100) 01(25) 03(75) 04(100) |
Fisher exact= 0.30 p-value= 0.49
Deceased patients were admitted beyond 6 hours in 48.4% of cases.
Table 11: Influence of mode of transport on prognosis
|
Transport Living(%) Dead(%) Total(%) |
|
Medicalized 29(64.4) 16(35.5) 45(100) Non-medicalized 30(44.7) 37(55.2) 67(100) |
Chi2=0.78 p-value=0.37
Non-medical transport had a mortality rate of 55.2%.
Table12: Influence of altered state of consciousness on prognosis
|
Glasgow score |
Alive(%) Deceased(%) |
Total(%) |
||
|
3 and 5 6 and 12 |
3(18.7) 13(81.2) 55(57.2) 41(42.7) |
16(100) 96(100) |
||
Fisher exact=0.004 p-value<0.01
The deterioration of consciousness significantly influenced the occurrence of death.
4. Discussion
During the study period, 830 patients were recorded, including 112 cases of comas, representing a frequency of 13.5%. Mapoure in Senegal in 2009[5]and Kouamé in Ivory Coast in 2021 [6] had recorded respectively with a rate of 48.99% and 72.11%. The result of this study could be explained by the death of certain patients before admission to the intensive care unit. The average age was 41.64 years with extremes of 3 and 90 years. The age group of 21 to 40 was the most affected with a rate of 27.6%. The youth of the patients was often reported by African authors. Indeed Otiobanda and Mohamed had found a respective average age of 40.5 and 43.9 years [36.37]. However, Adukauskienè in Lithuania in 2020 reported a mean age of 67.6 ± 15.2 years [38]. In Latin America, 69% of patients admitted to the intensive care unit for traumatic coma were aged under 40 [3].The young age found in this study could be explained by the youth of the Nigerien population [39] and the exposure of these young people to public road accidents. We recorded a male predominance with a rate of 59.8%. Which is similar to literature data [40–44]. The explanation lies in the fact that men are more exposed to potential risk factors such as road accidents. But for the Vallin team, to this is added caution, which was much higher among women than men [45]. Housewives were the most affected with a rate of 33.9% in this study. This observation was made by Maimouna Saidou in Mali with 34.2% of housewives [46]. On the other hand, in Benin, Tchaou and Adelin had observed a predominance of independent workers [47.48].This proves that the occurrence of coma is not correlated with occupation. Most patients came from the city of Zinder followed by the department of Magaria and the region of Maradi. This result could be explained for the city of Zinder by the proximity of the intensive care unit; for Magaria it was the absence of an intensive care unit and for Maradi it was the non-functioning of certain services. Arterial hypertension was the main antecedent recorded in 10% of cases. In India in 2020, stroke was the most common cause of non-traumatic coma with a rate of 48.99% [4]. The result of this series was similar to those of Sène Diouf in Senegal in 2009 [49] and Mahamadou Coulibaly in Mali in 2019 [50]. The growing number of hypertensive patients in Niger could be the cause[51–53]. In this series, the beginning was brutal in66.9% of cases.
HoweverBoukari in Niger in 2020 [10] found 71.43% of cases of progressive onset of non-traumatic comas in children aged 7 days to 15 years. In addition, in children, the majority of cases of coma occurred gradually. Infection was the etiology the main etiology of coma in pediatric settings [54–57].Patients were admitted to intensive care 24 hours after the start of symptoms in 49.1% of cases. This result waslower than that ofIbekwe who reported 80% of admission cases after the onset of signs [58]. For Lukman in Nigeria in 2012 59.8% of patients were admitted within 24 hours[59].The result of this study could be explainedby the lack of medical transport and the delay in carrying out the brain scan.Nearly half of the patients had hyperthermia (43.1%), tachycardia (57.5%), hypertension (45.9%) and low SPO2 (46.1%) in this series. In this series, patients with a Glasgow score between 6 and 8 represented 47.3% of cases. Masson had recorded a score below 8 in 69% of patients [61]. The result of this study could be explained by the severity of comas due to the delay in early consultation.
In the majority of patients essentially benefited from: blood sugar, blood count, and serum creatinine. CT was performed in only 35.7% of patients due to the fact that the HNZ did not have a scanner during the study period.The dominant etiologies were cranioencephalic trauma followed by metabolic coma with respectively 36.4% and 24.8% of cases. This same observation was made by Adelin in Benin in 2015. Which also justified the compulsory wearing of helmets in Benin [47]. However, for William's team in the United States in 2002, cranio-encephalic trauma was the third cause of coma with 14% of cases [62]. The result of this study could be explained by the non-compliance with road safety rules favoring public road accidents (AVP), mainly source of the traumatic comas observed in our regions.[63,64]. Respiratory distress was the maindecompensationin this series with a rate of 23.6%. Rate lower than that of Djibril in Togo which reported 51% of cases of respiratory distress[65]. This disorder is due to lesional or metabolic damage to the central structures controlling breathing.[66]. In this series, 48.2% of patients died. However, this result was close to the data in the European literature varying between 43 to 46% [67–71].However, higher mortality was reported by Obiako in Nigeria with a rate of 76% of cases.[72].
The delay in consulting, the delay in treatment and the insufficiency of therapeutic means could explain the result of this study. In this study, older age (over 60 years) was statistically correlated with death (P<0.01). The same observation was reported byOssou-Nguiet PM et al in Brazzaville in 2013[73]and Firsching in Germany in 2017 [74].In fact, elderly people present comorbidities with an increased risk of decompensations further worsening the management [75].Previous history was associated with a significant increase in mortality in this series (P=0.02).Raveloson made the same observation[76]. Delay in seeking consultation was correlated with death (P=0.49). Indeed, the short time to consult would improve the vital prognosis of patients. For some authors this time should not exceed 1 to 3 hours to obtain a good prognosis [77.78].In this series, medical transport improved the vital prognosis of patients. Mendy J in a study carried out in Senegal in 2014 reported a medical transport rate of 5.4% with an overall mortality rate of 34.8% [79]. The result of this study was explained by the absence of an emergency medical aid service (SAMU) in the city and region of Zinder.The most lethal etiologies were stroke, comas of metabolic and infectious origin (P respectively 0.18, 0.51 and 0.49). The study carried out byAdukauskiene in Lithuania in 2020 showed that 74% of deaths were from metabolic coma (P=0.004)[38]. In Niger in 2019, Mansour reported that malaria comas significantly increased the case fatality rate (P=0.0001)[30].
References
- Young GB, Coma, Ann NY. Acad Sci. 1157 (2009): 32-47.
- Weiss N, Regard L, Vidal C, et al. Causes of coma and their evolution in the medical intensive care unit. J Neurol 259 (2012): 1474-1477.
- Bonow RH, Barber J, Temkin NR, et al. The outcome of severe traumatic brain injury in Latin America. World Neurosurg 111 (2018): 82-90.
- Pijush KB, Arijit S. Study on etiology and immediate outcome of adult comatose patients in medical ward. Int J Cur Res Rev 12 (2020):19-22.
- Mapoure NY, Diouf FS, Ndiaye M, et al. Study prospective longitudinal study of comas in an African neurological environment: experience of Dakar, Senegal. Rev Med Brux 30 (2009): 163-169.
- Kouamé KI, Boo KJ, Mobio MP, et al. Factors prognosis of comas in intensive care at Yopougon University Hospital. RAMUR 25 (2020): 39-45.
- Sanou J, Bonkoungou PZ,Kinda B, et al. Trauma serious cranial injuries at the Yalgado Ouédraogo university hospital center: Aspects epidemiological, clinical and factors limiting the performance of brain scanning. RAMUR. 17 (2012): 01
- Ouédraogo PV, Savadogo AA, Samadoulougou S, et al. Mortality of strokes in the acute phase at the Souro Sanou University Hospital Center Bobo-Dioulasso; Burkina Faso. African Journal of Neurological Sciences 38 (2019): 22.
- Yahaya A.O. Severe comas in adults admitted to intensive care: prospective study about 30 cases collected at the Niamey national hospital Niamey: Abdou Moumouni University; (2016): 93p.
- Boukari BM, Hounkpè PC, Magagi A, et al. Epidemiology of non-traumatic coma in children aged 7 days to 15 years. Journal of the Society of Clinical Biology of Benin, 2020; 33 (2020): 11-17.
- Bauer ZA, De Jesus O, Bunin JL. StatPearls. Treasure Island (FL): Unconscious Patient (2021).
- Karpenko A, Keegan J. Diagnosis of coma. Emerg Med Clin North Am 39 (2021): 155-172.
- Huff JS, Tadi P. StatPearls. Treasure Island (FL): Coma. (2021).
- Rohaut B, Kandelman S, Sharshar T. Book of intensive care interns. France: Medicine Sciences publications Lavoisier; 2014. Disorders of consciousness, coma 427-440.
- Elaine NM. Anatomy physiology. 4th edition. Paris. De Boeck. System central nervous 12 (1999): 405-450.
- Kamina P. Clinical anatomy, T5 Neuroanatomy. 2nd edition. Paris. Maloine. Brain stem; (2013): 241-261.
- Itani A, Khayat E. KB Neurology. 5th edition. Paris. Vernazobres-Grego. 2011. Non-traumatic coma (2011): 369-381.
- Osman D, Bonnet MP, Bouferrache K, et al. Emergency resuscitation anesthesia. 2nd edition. Paris. Masson. Nontraumatic Coma (2010): 133-144.
- Dhafer BL. Coma (2019)
- Benaïm C, Benatru I. The head trauma patient: prognosis. The Neurologist’s Letter. 2009; 13(11): 359-65.
- Fatigba OH, Padonou J. Epidemiology of cranioencephalic trauma in Parakou (benign). African Journal of Neurological Sciences 29 (2010): 25-33.
- Enicker B, Louw H, Madiba T. Acute extradural haematomas in children: A 12-year experience from a single neurosurgery unit. SAJS 54 (2016): 28-33.
- Lucien MO, Fifi Claire AL, Paul Marie L. Treatment of subdural hematomas chronicles in Libreville (Gabon): review of 102 cases. African Journal of Neurological Sciences 30 (2011): 28-38.
- Thierry AA, Donald A, Mendinatou A, Kofi-Mensa S, Gottfried A, Oyéné K. Encephalitis presentation revealing an aneurysm of the posterior communicating artery in Parakou (benign). African Journal of Neurological Sciences 38 (2019): 67-72.
- Tchala AB, Djagadou KA, Tchamdja T, et al. Cerebrovascular accidents in elderly people at the Sylvanus University Hospital Center Olympia de Lome. Journal of Scientific Research of the University of Lomé. 21 (2019): 313-319.
- Dawodu CO, Danesi MA. Factors Influencing Mortality in Hemorrhagic Stroke. Nigerian Journal of Clinical Medicine 1 (2008):1
- Tekpa G, Gbangba NE, Yangatimbi E, et al. Clinical and bacteriological aspects of purulent meningitis in rural Central African areas. Rev Mali Infect Microbio 15 (2020): 44-53.
- Kané B, Abdou M, Koné O, et al. Causes of Bacterial meningitis in children aged 1 month to 15 years in the pediatric department of the Mali hospital from 2012 to 2018. Rev Mali Infect Microbiol 15 (2020): 72-76.
- Sofiene B, Imed BS, Asma B, et al. Amoebic cerebral abscess: report of three cases with review of the literature. African Journal of Neurological Sciences 33 (2014): 3-9.
- Mansour MA, Samaila B, Mahamane ML, et al. Factors associated with severe malaria in children and its prognosis at the National Hospital of Niamey, Niger. Black African medicine. 66 (2019): 465-476.
- Mégarbane B, Baud F. Main acute poisonings. THE PRACTITIONER’S REVIEW. 56 (2006):1603-1613.
- Sanoussi S, Assoumane I, Kelani A, et al. Surgical treatment of posterior fossa tumors: prospective study of 36 cases. African Journal of Surgery and Specialties 6 (2012): 13-18.
- Jacquens A, Degos V. Coma following anesthesia. The Anesthesia Resuscitation Practitioner 22 (2018): 149-156.
- Vincent Castelain. Attitude towards a coma. France: Hautepierre Hospital (2011).
- Cassol H, Aubinet C, Thibaut A, e al. Diagnosis, prognosis and treatments of disorders of consciousness. NPG 18 (2011): 47-59.
- Mohamed MA, Bekele N, Diro E, et al. No- traumatic coma: causes and outcome of adult patients at university of Gondar hospital, northwest Ethiopia. Clinical Medicine Research 4 (2015): 198-203.
- Otiobanda GF, Elombila M, Mpoy Emy Monkessa CM,Leyono-Mawandza Peggy DG,Niengo OG.Profile of patients admitted to the Multipurpose Intensive Care Unit of the Brazzaville Hospital Center. RAMUR 22 (2017): 65-69.
- Adukauskiene D, Jašinskas V, eds. The link between risk factors and mortality rate in coma patients. 33rd Annual Congress; December 06-09, 2020; Brussels, Belgium. Brussels: Digital ESICM (2020).
- Ministry of Economy and Finance. Decree No. 2011-059/PCSRD/ME/F 2011
- Kamat G. Clinical profile, etiology and outcome of non-traumatic coma in a tertiary care center. IJCMR 6 (2019): 33-36.
- Balaka B, Douti L, Azoumah D, et al. Etiologies and prognosis of non-traumatic comas in children at the Lomé university hospital. Diary of Scientific Research at the University of Lomé 14 (2012): 33-40.
- Samaké BM, Mangané SM, Togola M, et al. Admission of elderly people to intensive care. MALI MEDICAL 1 (2015): 25-27.
- Obiako OR, Oparah SK, Ogunniyi A. Prognosis and outcome of acute stroke in the University College Hospital Ibadan, Nigeria. Nigerian Journal of Clinical Practice 14 (2011): 359-362.
- Tomta K, Djibril MA, Mouzou T, et al. Trauma cranioencephalic diseases (TCE) at the Tokoin University Hospital Center in Lome (Togo). Newspaper of Scientific Research at the University of Lomé 13 (2011): 47-57.
- Vallin M, Chains. Road legislation criminal procedure code. INED 1267 (1967): 55-66.
- Dembele Maimouna SS. Epidemiological and clinical aspects of comas in the anesthesia and intensive care unit at the Gabriel Touré university hospital center [Thesis]. Mali: University of Bamako (2007): 92.
- Adelin TB, Armel AT, Eugene Z, et al. Prognostic Factors of Comas in the Intensive Care Unit of the University Teaching Hospital of Parakou (Benin). JBBS 5 (2015): 503-512.
- Tchaou BA, Tchégnonsi NCF, Houndjè CYP, et al Management of non-traumatic comas in intensive care at the Parakou University Hospital in Benin. RAMUR 23 (2018):10-16.
- Sène Diouf F, Mapoure NY, Ndiaye M, et al.Prognosis of intracerebral hemorrhages with coma in a neuroresuscitation unit tropical. Tropical Medicine 68 (2008): 606-610.
- Mangane SA, Diallo D, Meimoune AHA, et al. THE Cerebral Vascular Accidents at “Le Luxembourg” Bamako University Hospital: Clinical Aspects, Therapeutics and Prognosis. Diabetes. Health Sci. Say 20 (2019): 73-77.
- Tahirou I, Moussa IDJ, Ada A, et al. Analysis of preliminary results of myocardial scintigraphy carried out at the Institute of Radio- isotopes (IRI) of Niger. About 37 cases. Nuclear medicine 36 (2012): 591-599.
- Sani MM, Ada A, Tchatath NNV, et al. Particularities of diabetes in subjects aged over 60 in Niger. Health Sci Dis 19 (2018), 10-14.
- Salymatou KM. Epidemiological and etiological aspects of non-traumatic comas in adults in the intensive care unit at the Niamey national hospital [Thesis]. M li: University of Bamako (2007): 130.
- Ahmed S, Ejaz, Shamim MS, et al. Non-traumatic coma in pediatric patients: etiology and predictors of outcome.J Pak Med Assoc 61 (2011): 671-675.
- Brisset J, Kinkpé E, Bailly J, et al. Coma no trauma in young children in Benin: does everything revolve around the management of malaria ? Medicine and Infectious Diseases 5 0(2020): S174.
- Duyu M, Altun ZK, Yildiz S. Nontraumatic coma in the pediatric intensive care unit: etiology, clinical characteristics and outcome. Turk J Med Sci 51 (2021): 214-223.
- Fariba K, Najmeh GN. Etiology and outcome of nontraumatic coma in children admitted to pediatric intensive care unit.Iranian Journal of Pediatrics 19 (2009): 3938.
- Ibekwe RC, Ibekwe MU, Onwe OE, et al. Non-traumatic childhood coma in Ebonyi State University Teaching Hospital, Abakaliki, south eastern Nigeria. Nigerian Journal of Clinical Practice 14 (2011): 43-46.
- Owolabi FL, Mohammed AD, Okatubo G, et al. Etiology and outcome of medical coma in a tertiary hospital in Northwestern Nigeria. Annals of Nigerian Medicine 6 (2012): 92-97.
- Stevens RD, Bhardwaj A. Approach to the comatose patient. Critical care medicine 34 (2006): 31-41.
- Masson F, Thicoipe M, Mokni T, et al. Epidemiology of traumatic comas: a prospective population-based study. Brain Injury 17 (2003): 279-293.
- William K ,William JB, HuffJS, et al. Altered mental status: assessment and etiology in the ED.Am J Emerg Med 20 (2002): 613-617.
- Daddy H,Chaibou MS, James Didier L, et al. Taken in burden of road accidents among adults admitted to hospital emergency departments national office of Niamey–Niger. RAMUR 22 (2017):17-21.
- Holden FO, Jidjoho P. Clinical studies / clinical studies epidemiology of trauma cranioencephalic diseases in Parakou (Benin). African Journal of Neurological Sciences 29 (2010): 25-33.
- Djibril MA, Balaka A, Nemi KD, et al. Diabetic emergencies a lome: epidemiological and prognostic aspects. Journal of Scientific Research the University of Lomé 15 (2013): 353-358.
- Gagnadoux F, Gonzalez-Bermejo J, Duguet A, et al. Pathophysiological mechanisms of respiratory damage. Journal of Respiratory Diseases News 4 (2012): 123-126.
- Forsberg S, Höjer J, Ludwigs U. Prognosis in patients presenting with nontraumatic coma. Crit Care 14 (2010): S333.
- Chesneau AS, Bretonniere C, Nicolet I, et al. Elderly people and coma on admission to intensive care: prognosis and impact on PMO activity. Resuscitation. 2012; 21 (2012): 21-24.
- Chrysanthopoulou E, Karampela I, Patsilinakou S, et al. Admission to Intensive Care Unit due to non-traumatic coma: etiology and outcome. European Respiratory Journal 54(2019): S2282.
- Simpson HK, Clancy M, Goldfrad C, et al. Admissions to intensive care units from emergency departments: a descriptive study. Emerg Med J. 2005; 22(6): 423-428.
- Wong CP, Forsyth RJ, Kelly TP, et al. Incidence, aetiology, and outcome of non-traumatic coma: a population based study. Archives of Disease in Childhood 84 (2001): 193-199.
- Obiako OR, Ogunniyi A, Anyebe E. Prognosis of non-traumatic coma: The role of some socio-economic factors on its outcome in Ibadan, Nigeria. Annals of African Medicine 8 (2009): 115-121
- Ossou-Nguiet PM,Gombet TR,Ossil-Ampion M, et al. Mortality factors of stroke in Brazzaville University Hospital. RAMUR 18 (2013).
- A. Coma after acute head injury. Dtsch Arztebl Int 114 (2017): 313-320.
- Chassagne P. Frailty-comorbidity: a determining association of care of a pathology in the elderly. cah year gerontol 4 (2012): 1.
- Chrakotoarivony ST, Rajaonera AT, Ramorasata JA, et al. Poor prognostic factors for non-traumatic comas (About 449 cases observed in the medical intensive care unit of the University Hospital. A/JRB. Revue d 'Anesthesia-Resuscitation and Emergency Medicine 1 (2010): 23-27.
- Elmehdawi RR, Elmagerhei HM. Profile of diabetic ketoacidosis at a teaching hospital in Benghazi, Libyan Arab Jamahiriya. EMHJ 16 (2010): 292-299.
- Ahmed YK, Kheireddine AB. Post-traumatic brain death.pdf. Pan African Medical Journal 31 (2018): 1-4.
- Mendy J, Kpelao E, Sakho Y, et al. Severe head injuries in children: management and short-term prognosis in Dakar (Senegal). RAMUR. 19 (2014)
- Asse KV, Plo KJ, Yenan JP ,et al. Non-traumatic comas of children in Abidjan. RAMUR. 17 (2012).
- Thula N, Patel J. A study of clinical profile of patients presented with non-traumatic coma. IAIM ; 7 (2020): 20-27.
- Kafle DR, Sah RP, Karki DR. Non Traumatic Coma in the Intensive Care Unit: Etiology and Prognosis. Kathmandu University Medical Journal. 19 (2021): 371-374.
- Sinclair JR, Watters DAK, Bagshaw A. Non-Traumatic Coma in Zambia. Tropical doctorate. 19 (2016).
