Etiological Association of Pruritus in End Stage Renal Disease Patients on Maintenance Hemodialysis
Article Information
Towfik Parvez1, Rana Mokarrom Hossain2, S M Remin Rafi3, S M Shamsuzzaman4, Md. Bedar Uddin5, Shoriful Islam6
1Medical officer, Upazila health complex, Sharsa, Jashore, Bangladesh
2Professor, Department of Nephrology, BSMMU, Dhaka, Bangladesh
3Medical officer, department of Nephrology, BSMMU, Dhaka, Bangladesh
4Assistant register, Department of nephrology, DMCH, Dhaka, Bangladesh
5Medical officer, 250 Bedded General Hospital, Jashore, Bangladesh
6Senior Consultant, Department of Medicine, 250 Bedded General Hospital, Jashore, Bangladesh
*Corresponding Author: Dr. Towfik Parvez, Medical officer, Upazila health complex, Sharsa, Jashore, Bangladesh.
Received: 16 December 2024; Accepted: 06 January 2025; Published: 24 January 2025
Citation:
Towfik Parvez, Rana Mokarrom Hossain, SM Remin Rafi, SM Shamsuzzaman, Md. Bedar Uddin, Shoriful Islam. Etiological Association of Pruritus in End Stage Renal Disease Patients on Maintenance Hemodialysis. Archives of Nephrology and Urology. 8 (2025): 09-17.
View / Download Pdf Share at FacebookAbstract
Background: Chronic kidney disease–associated pruritus (CKD-aP) is a common, troubling and, in some cases, debilitating problem for patients with CKD and end-stage renal disease (ESRD). Despite a prevalence rate of approximately 20% in CKD and 40% in ESRD and a clear association with poorer psychosocial and medical outcomes, this condition is often underreported by patients and overlooked by healthcare providers. This is likely due to uncertainty regarding its pathogenesis and treatment. Most commonly, CKD-aP is attributed to toxin build-up, peripheral neuropathy, immune system dysregulation, or opioid dysregulation. The exact pathogenesis remains largely elusive, which hampers the definite treatment protocol. Studies have shown changes in the immunochemical milieu of the skin in patients with CKD-aP, with several inciting stimuli identified. However, other unrecognized factors are likely to be involved. Aim of the study: To find out the etiological association of pruritus in ESRD patients on maintenance hemodialysis. Methods: This prospective observational study was carried out in the Department of Nephrology BSMMU and ShSMCH, Dhaka, Bangladesh. A total of 60 patients with CKD Stage 5 on MHD were included in this study. CKD stage 5 on MHD with pruritus and without pruritus were considered group I and group II. Patients aged above 18 years and undergoing hemodialysis for at least 3 months were enrolled in this study. Result: This study showed mean age was 47.24±15.63 years (range: 18-69) in Group I and 43.14±15.07 years (range: 18-73) in Group II, with the majority (76.5%) of the population being female in Group I. Glomerulonephritis (GN) was the predominant aetiology and hypertension (HTN) was a prominent comorbidity in both groups. Mild pruritus was most prevalent (47.1%) in a pruritic group of patients. Among pruritic patients, more than half (52.9%) patients had generalized pruritus. Almost three-fourths (70.6%) of patients rarely experienced sleep disturbance. The IL-31 exhibited a marked difference, with Group I showing a significantly higher mean of 128.23±77.34 xiii compared to Group II's mean of 60.52±36.25 (P0.05) with the severity of pruritus. Twice weekly treated patients (Mean±SD was 107.9±68.9) were more prone to develop pruritus than those getting thrice weekly with no significant difference in terms of IL-31, with the frequency of HD per week. Laboratory parameters had no significant differences between the two groups in terms of Hb, WBC, Circulatory eosinophil count, IgE, S. Ca, S. P04, Ca *P04 product and iPTH. Receiver-operator characteristic (ROC) was constructed by using IL-31, which cut value 87.7, with 70.6% sensitivity and 81.4% specificity. Conclusion: In this study, there was a significant difference in the IL-31 level between pruritic and nonpruritic patients. IL-31 levels didn't directly correlate with the severity of pruritus in ESRD patients on maintenance hemodialysis. Twice-weekly hemodialysis patients were more prone to develop pruritus than thrice-weekly treated patients. Besides, there was no significant difference in terms of IL-31 level with the frequency of HD.
Keywords
Etiological; Association; End Stage Renal Disease; Maintenance Hemodialysis
Etiological articles; Association articles; End Stage Renal Disease articles; Maintenance Hemodialysis articles
Article Details
1. Introduction
Chronic kidney disease-associated pruritus (CKD-aP), also known as uremic pruritus, is a prevalent and debilitating dermatological symptom among dialysis patients, significantly affecting their quality of life. It is particularly common in advanced chronic kidney disease (CKD) and is associated with increased morbidity and mortality [1]. Uremic pruritus has a broad prevalence, ranging from 22% to 90% in hemodialysis (HD) patients. A study from the Dialysis Outcomes and Practice Patterns Study (DOPPS) found that 37% of HD patients were moderately bothered by itching, with 18% experiencing severe pruritus [2]. The condition contributes to social stress, disturbed sleep, and psychological issues, further compromising patient well-being [3,4]. The pathophysiology of CKD-aP is multifactorial, involving uremic and non-uremic factors. Despite extensive research efforts and numerous suggested theories, CKD-aP remains a complex and poorly understood symptom associated with chronic kidney disease. The following factors can be listed as some of the most significant ones that contribute to the pathophysiology of CKD-aP: Immune dysregulation, xerosis of the skin, Hyperparathyroidism, uremic toxins accumulation, neural dysfunction, histamine mechanism, and opioid mechanism [5]. Hyperphosphatemia, hypocalcemia, and secondary hyperparathyroidism, common in CKD patients, contribute to pruritus by stimulating mast cells, which release histamine, and by promoting calcium salt deposition in the skin [6]. However, not all patients with severe hyperparathyroidism experience pruritus, indicating that pruritus results from a complex interplay of factors, not just elevated parathyroid hormone (PTH) levels [7]. Xerosis (dry skin) is another contributing factor in CKD-aP, particularly in patients undergoing hemodialysis. Xerosis results from dysfunctional sebaceous and apocrine sweat glands. It is exacerbated by dermal dehydration following dialysis, leading to a rough, cracked, and scaly skin surface that enhances the sensation of chronic pruritus [8,9]. Mast cell mediators, such as histamine and tryptase, play significant roles in pruritus, although antihistamines often fail to provide effective relief in CKD-aP [10]. Moreover, the opioid system, particularly overexpression of µ-opioid receptors and altered serum β-endorphin levels, is implicated in the modulation of itch sensation in CKD patients [11]. Uremic toxins, accumulating due to impaired renal clearance in CKD, have been linked to pruritus. Enhanced dialysis efficiency has been shown to alleviate pruritus by reducing the concentration of these toxins [12]. Inflammation, particularly systemic immune dysregulation, is also central to CKD-aP. Elevated levels of inflammatory markers, such as C-reactive protein (CRP) and interleukins (IL)-2 and IL-6, are associated with the condition [13], as well as elevated white blood cell count, ferritin, and a decrease in albumin [14]. Interleukin-31 (IL-31), a T-cell-derived cytokine, has recently been identified as a potential key player in CKD-aP. Elevated IL-31 levels in hemodialysis patients correlate with pruritus, and IL-31’s role in immune modulation and pruritus persistence suggests that it could be a therapeutic target [1]. Additionally, IL-31 has been implicated in pruritic diseases such as atopic dermatitis and psoriasis [15], further supporting its potential role in CKD-aP. Despite these findings, the exact correlation between IL-31 and pruritus intensity remains unclear [1]. Agarwal et al. (2021) describe pruritus in chronic kidney disease (CKD) patients as persistent, recurrent, and typically bilateral, often worsening at night [5]. It commonly affects the trunk and limbs, particularly the back, with exacerbating factors such as heat, dryness, and stress. CKD-associated pruritus (CKD-aP) usually occurs without primary skin lesions, though secondary lesions from scratching, including excoriations, ulcerations, and prurigo nodularis, may be present. The condition tends to worsen with showers, dialysis, heat, cold, and physical exertion. It is noteworthy that CKD-aP is often recurrent and does not respond to available therapeutic methods, necessitating further investigation into its pathophysiology for improved therapeutic strategies. This study aims to explore the association of various etiological factors contributing to pruritus in maintenance hemodialysis patients.
2. Methodology & Materials
This cross-sectional observational study was conducted in the Department of Nephrology at Bangabandhu Sheikh Mujib Medical University (BSMMU) and Shaheed Suhrawardy Medical College Hospital in Dhaka, Bangladesh, from November 2022 to August 2023. A total of 60 patients diagnosed with chronic kidney disease (CKD) stage 5 (ESRD) undergoing maintenance hemodialysis for at least 3 months were included in the study. After inclusion, the patients were divided into two groups, Group A and Group B. The Group included patients on maintenance hemodialysis with pruritus. Group B has included patients on maintenance hemodialysis without pruritus.
- • Group A: CKD stage 5D patients on maintenance hemodialysis with pruritus.
- • Group B: CKD stage 5D patients on maintenance hemodialysis without pruritus.
Inclusion Criteria
- • Patients aged ≥18 years.
- • Patients on maintenance hemodialysis for ≥3 months with or without pruritus.
Exclusion Criteria
- • Active malignancy.
- • Active infection.
- • Immunosuppressant therapy.
- • Primary dermatologic conditions (e.g., dermatitis, psoriasis).
- • Psychotic illness or non-cooperative behavior.
- • Active hepatitis or cholestatic liver disease.
The study was approved by the Ethical Review Committee of BSMMU, Dhaka, ensuring that the rights of participants were protected. All participants were informed about the study, including its risks and benefits, and written informed consent was obtained. After getting consent, meticulous history was taken, including pruritus duration, distribution, frequency and severity measurement by Pruritus Visual analog scale (PVAS) and pruritus Grading System Score (PGSS) & relevant clinical examinations were performed and recorded in predesigned structured proforma. The medical records were collected to extract demographic information (age and sex). During enrollment in the study, different hematological, biochemical and hormonal (CBC et al. calcium, Serum inorganic phosphate, Serum Albumin, Serum Ferritin, S. iPTH) tests were done and recorded from the study population, which the Department of Laboratory Medicine, Kidney research laboratory and department of microbiology and immunology of BSMMU did. Measurement of IL-31 serum levels was done by enzyme-linked immune sorbent assay (ELISA) technique from the Department of Microbiology and Immunology, BSMMU.
Statistical Analysis:
Computer-based statistical analysis was carried out with appropriate techniques and systems. All data were recorded systematically in the preformed data collection form. Quantitative data were expressed as mean, and standard deviation, and qualitative data were expressed as frequency distribution and percentage. Statistical analyses were performed by using windows-based computer software with Statistical Packages for Social Sciences (SPSS-27) (SPSS Inc, Chicago, IL, USA). Mann–Whitney U test for abnormally distributed quantitative variables was used to compare between two studied groups. The chi-square test for categorical variables was used to compare different groups. Student’s t test for normally distributed quantitative variables was used to compare between two studied groups. Kruskal–Walli’s test was used to assess the statistical significance of the difference between more than two study group ordinal variables. Spearman’s correlation coefficient (r) was used to measure the association between two quantitative variables not normally distributed or one quantitative and other qualitative variables; p p-value of <0.05 was considered statistically significant.
3. Results
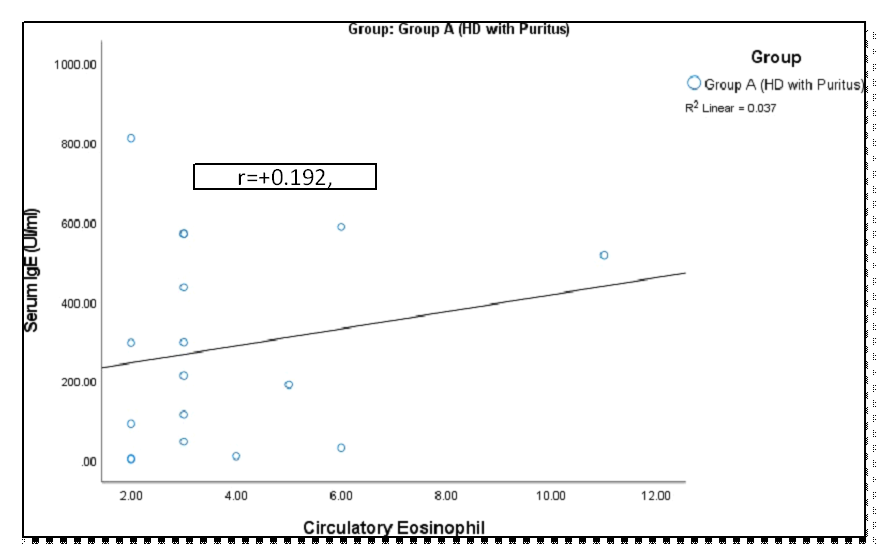
The study included 60 patients diagnosed with stage 5 chronic kidney disease (end-stage renal disease, ESRD), all of whom had been undergoing maintenance hemodialysis for a minimum of 3 months. The distribution of patients based on pruritus status reveals that 28.3% of hemodialysis (HD) patients experienced pruritus, while 71.7% did not (Figure 1). A comparative demographic analysis between patients with pruritus (Group I) and those without (Group II) shows that the majority of patients in Group II (69.8%) were male, whereas Group I had a significantly higher proportion of female patients (76.5%), with a statistically significant gender difference (p = 0.001) (Table 1). Additionally, the study population displayed a wide age range, with Group I having a mean age of 47.24 ± 15.63 years, while Group II had a mean age of 43.14 ± 15.07 years. No statistically significant difference was observed between the two groups (P = 0.352). However, the most common age range in both groups was 41–50 years. Regarding the etiology of end-stage renal disease (ESRD), both groups had similar proportions of diagnoses, with hypertension being the most common cause in both groups (100% in Group I and 83.7% in Group II). However, the differences between the groups in terms of diagnosis did not reach statistical significance (P > 0.05) (Table 2). Additionally, no significant association was observed between pruritus status and specific underlying causes such as glomerulonephritis (GN), diabetes mellitus (DM), or obstructive nephropathy. The duration of hemodialysis was significantly different between the groups (P = 0.007). Group I had a higher percentage of patients with intermediate-term HD (58.8%) compared to Group II (41.9%). In contrast, Group II had a greater proportion of patients on short-term HD (55.8%), whereas Group I had only 23.5% on short-term HD (Table 3). Analysis of IL-31 levels showed higher levels in patients who underwent three sessions of dialysis per week compared to those on two sessions. However, the difference was not statistically significant (p = 0.18) (Table 4). Figure 2 illustrates the distribution of pruritus status and grading among 17 patients in Group I who are undergoing hemodialysis (HD) and experiencing pruritus. The grading of pruritus in these patients is categorized as mild (47.1%), moderate (35.3%), and severe (17.6%). Table 5 shows the distribution of the clinical characteristics of pruritus based on the pruritus grading system score. It was observed that more than half (52.9%) of the patients had generalized pruritus, and 47.1% had pruritus at multiple sites. Almost two-thirds (64.7%) of the patients experienced episodic pruritus, followed by 29.4% with frequent pruritus and 5.9% with continuous pruritus. More than half (52.9%) of the patients had scratching, followed by 23.5% with generalized excoriation, 11.8% with rubbing, and localized excoriation, respectively. Almost three-fourths (70.6%) of the patients experienced rare sleep disturbances, followed by 17.6% with occasional and 11.8% with frequent disturbances. The comparison of IL-31 levels across pruritus severity groups was analyzed. The mean IL-31 levels for mild, moderate, and severe pruritus were 136.5 ± 88.7, 143.3 ± 62.1, and 76.2 ± 75.3, respectively. However, the p-value of 0.304 indicated no statistically significant difference in IL-31 levels among the severity groups (Table 6). When comparing laboratory parameters between Group I (HD with pruritus) and Group II (HD without pruritus), as shown in Table 7, significant differences were observed in IL-31 levels (p < 0.001), with Group I having higher mean IL-31 levels (128.23 ± 77.34) compared to Group II (60.52 ± 36.25). However, no significant differences were observed for serum creatinine, albumin, ferritin, hemoglobin, or CRP levels between the two groups, as their respective p-values were above the conventional threshold of 0.05. Further analysis of IgE levels and circulatory eosinophil counts between the two groups, presented in Table 8, revealed no significant differences. The mean IgE levels in Group I were 281.49 ± 255.13, and in Group II, they were 329.81 ± 448.19 (p = 0.876). Similarly, the mean circulatory eosinophil count was 256.99 ± 113.72 in Group I and 260.57 ± 228.67 in Group II, with a p-value of 0.218, indicating no statistical significance. In Table 9, the comparison of intact parathyroid hormone (PTH), calcium (Ca), and phosphate (PO4) levels between Group I and Group II also showed no significant differences. The mean intact PTH was higher in Group I (257.33 ± 195.50) compared to Group II (180.43 ± 104.55), but this difference was not statistically significant (p = 0.309). Likewise, the levels of calcium (9.49 ± 0.90 in Group I vs. 9.17 ± 0.85 in Group II) and phosphate (47.13 ± 19.27 in Group I vs. 38.63 ± 14.53 in Group II) did not differ significantly between the groups, with p-values of 0.203 and 0.241, respectively. Figure 3 illustrates the receiver operating characteristic (ROC) curve analysis used to determine the optimal cutoff value of IL-31 for detecting pruritus. The curve plots sensitivity versus 1-specificity at various IL-31 threshold levels, providing insights into its diagnostic accuracy. Table 10 presents the diagnostic validity test for interleukin-31 (IL-31) in predicting pruritus in patients with end-stage renal disease undergoing maintenance hemodialysis. The results show that a cutoff value of 87.7 pg/ml for IL-31 yields a sensitivity of 70.59%, specificity of 81.4%, positive predictive value (PPV) of 60%, negative predictive value (NPV) of 87.5%, and an overall accuracy of 78.33%. Figures 4, 5, and 6 demonstrate that there was no significant correlation between serum IgE levels and circulating eosinophil counts in patients with pruritus (r = 0.192; p > 0.05). Similarly, in patients without pruritus, a non-significant negative correlation was observed (r = -0.20; p > 0.05).
Table 1: Comparison of Demographic Characteristics Between Group I and Group II (N=60).
|
Demographic characteristics |
Group I (HD with Pruritus) (N=17) |
Group II (HD without Pruritus) (N=43) |
P value |
||
|
n |
% |
n |
% |
||
|
Age (years) |
|||||
|
≥20 |
1 |
5.9 |
2 |
4.7 |
|
|
21-30 |
2 |
11.8 |
8 |
18.6 |
|
|
31-40 |
2 |
11.8 |
11 |
25.6 |
|
|
41-50 |
5 |
29.4 |
9 |
20.9 |
0.352 |
|
51-60 |
3 |
17.6 |
6 |
14 |
|
|
>60 |
4 |
23.5 |
7 |
16.3 |
|
|
Mean±SD |
47.24±15.63 |
43.14±15.07 |
|||
|
Gender |
|||||
|
Male |
4 |
23.5 |
30 |
69.8 |
0.001 |
|
Female |
13 |
76.5 |
13 |
30.2 |
|
Table 2: Comparison of Diagnosis of End-Stage Renal Disease (ESRD) Between Group I and Group II.
|
Group I (HD with Pruritus) (N=17) |
Group II (HD without Pruritus) (N=43) |
||||
|
Variable |
n |
% |
n |
% |
P value |
|
GN |
6 |
35.3 |
20 |
46.5 |
0.429 |
|
HTN |
17 |
100 |
36 |
83.7 |
0.077 |
|
DM |
7 |
41.2 |
15 |
34.9 |
0.649 |
|
Obstructive nephropathy |
0 |
0 |
1 |
2.3 |
0.526 |
|
SLE, LN |
0 |
0 |
1 |
2.3 |
0.526 |
Table 3: Comparison of Hemodialysis (HD) Duration Between Group I and Group II.
|
HD duration |
Group I (HD with Pruritus) (N=17) |
Group II (HD without Pruritus) (N=43) |
P value |
||
|
n |
% |
n |
% |
||
|
Short-term HD (< 1 year) |
4 |
23.5 |
24 |
55.8 |
|
|
Intermediate-term HD (1-5 years) |
10 |
58.8 |
18 |
41.9 |
0.007 |
|
Long-term HD (> 5 years) |
3 |
17.6 |
1 |
2.3 |
|
Table 4: Comparison of IL-31 with frequency of dialysis per week (N=17)
|
IL-31 (pg/ml) |
Frequency of dialysis per week |
p-value |
|
|
Two (N=11) |
Three (N=6) |
||
|
Mean±SD |
107.9±68.9 |
165.4±84.2 |
0.18 |
|
Median |
93.8 |
176.4 |
|
|
Range (min-max) |
18.9-222.7 |
50.2-285.0 |
|
Table 5: Pruritus Status and Pruritus Grading in Group I (HD with Pruritus) (N=17)
|
Variable |
Number of patients (n) |
Percentage (%) |
|
Distribution |
||
|
Multiple sites |
8 |
47.1 |
|
Generalized |
9 |
52.9 |
|
Frequency |
||
|
Episodic |
11 |
64.7 |
|
Frequent |
5 |
29.4 |
|
Continuous |
1 |
5.9 |
|
Severity |
||
|
Rubbing |
2 |
11.8 |
|
Scratching |
9 |
52.9 |
|
Localized excoriation |
2 |
11.8 |
|
Generalized excoriation |
4 |
23.5 |
|
Sleep disturbance |
||
|
Rare |
12 |
70.6 |
|
Occasional |
3 |
17.6 |
|
Frequent |
2 |
11.8 |
Table 6: Comparison of IL-31 among the severity of pruritus (N=17)
|
IL-31 |
Severity of pruritus |
p-value |
||
|
Mild (N=8) |
Moderate (N=6) |
Severe (N=3) |
||
|
Mean±SD |
136.5±88.7 |
143.3±62.1 |
76.2±75.3 |
0.304 |
|
Median |
115.9 |
155 |
48.2 |
|
|
Range |
26-285 |
50.2-212 |
18.9-161.5 |
|
Table 7: Comparison of Laboratory Parameters Between Group I and Group II
|
Group I (HD with Pruritus) (N=17) |
Group II (HD without Pruritus) (N=43) |
P value |
|
|
Variable |
Mean±SD, |
Mean±SD, |
|
|
S. creatinine |
8.00±1.42 |
7.42±1.87 |
0.253a |
|
IL-31 |
128.23±77.34 |
60.52±36.25 |
<0.001b |
|
S. albumin |
53.51±87.21 |
33.19±11.06 |
0.850b |
|
S. ferritin |
1273.46±703.56 |
1254.60±1310.14 |
0.163b |
|
Hb |
12.90±15.24 |
9.53±1.18 |
0.421b |
|
CRP |
24.4±11.54 |
21.8±9.31 |
0.365a |
Table 9: Comparison of Intact PTH, Ca and PO4 Between Group I) and Group II
|
Laboratory parameters |
Group I (HD with Pruritus) (N=17) |
Group II (HD without Pruritus) (N=43) |
P value |
|
Mean±SD, |
Mean±SD, |
||
|
IgE |
281.49±255.13 |
329.81±448.19 |
0.876 |
|
Circ. Eosinophils |
256.99±113.72 |
260.57±228.67 |
0.218 |
Table 10: Diagnostic validity test to measure performance of IL-31 to predict the pruritus.
|
IL-31 |
Clinical diagnosis |
|
|
Pruritus (N=17) |
No-Pruritus (N=43) |
|
|
>87.7 pg/ml |
12 |
8 |
|
(TP) |
(FP) |
|
|
<87.7 pg/ml |
5 |
35 |
|
(FN) |
(TN) |
|
|
Diagnostic performance value |
Values (%) |
95%CI (%) |
|
Sensitivity |
70.59 |
44.04 to 89.69 |
|
Specificity |
81.4 |
66.60 to 91.61 |
|
Positive Predictive Value |
60 |
42.78 to 75.06 |
|
Negative Predictive Value |
87.5 |
76.78 to 93.68 |
|
Accuracy |
78.33 |
65.80 to 87.93 |
4. Discussion
This prospective observational study was carried out with an aim to compare maintenance hemodialysis patients with and without pruritus in relation to metabolic and inflammatory factors and to see the association of immunological factor (IL-31) with or without pruritus on maintenance hemodialysis patients as well as to find out the severity of pruritus on maintenance hemodialysis patients. A total of 60 patients with CKD (Stage 5D) who attended the Nephrology department of Bangabandhu Sheikh Mujib Medical University, Dhaka & Shaheed Suhrawardy Medical College Hospital from November 2022 to August 2023 were included in this study. CKD stage 5 on maintenance hemodialysis with pruritus and without pruritus were considered as group I and group II. Age above 18 years and patients undergoing hemodialysis for at least 3 months with or without pruritus were enrolled in this study. Our study found that 28.3% of patients with end-stage renal disease (ESRD) undergoing maintenance hemodialysis experienced pruritus, while 71.7% did not report any symptoms of pruritus (Figure 1). This finding provides valuable insights into the understanding and management of pruritus in ESRD patients. Previous studies have reported a wide variation in the prevalence of uremic pruritus among hemodialysis patients, with estimates ranging from 22.0% to as high as 90.0% [7,16]. Regarding demographic characteristics, we observed a higher proportion of female patients in Group I (76.5%), which contrasts with the more balanced gender distribution in Group II (30.2% female) (Table 1). Gender bias was not evident in the emergence of pruritus among individuals with CKD across various studies [17,18]. The study population exhibited a broad age range, reflecting the demographic diversity typically seen in patients with end-stage renal disease (ESRD) undergoing hemodialysis. Group I, consisting of patients with pruritus, had a marginally higher mean age compared to Group II, the control group. However, this difference in age was not statistically significant. This observation aligns with previous studies, which have identified pruritus as a prevalent symptom among ESRD patients across a wide spectrum of age groups [3,18,19]. The findings of this study indicated that glomerulonephritis (GN) was the predominant etiology in both Group I and Group II, accounting for 35.3% and 46.5% of the patient populations, respectively. Notably, statistical analysis revealed no significant difference (p>0.05) between the two groups regarding GN as the underlying cause. Hypertension (HTN) was a common comorbidity in both groups, affecting all patients in Group I and 83.7% of those in Group II. In contrast, diabetes mellitus (DM) was present in 41.2% of Group I and 34.9% of Group II, with no significant difference observed (p>0.05). This suggests that DM may not be a statistically significant factor contributing to pruritus in end-stage renal disease (ESRD) patients undergoing maintenance hemodialysis. Additionally, obstructive nephropathy and systemic lupus erythematosus (SLE) with lupus nephritis (LN) were identified as fewer common etiologies for pruritus in ESRD patients, with no statistically significant differences between the two groups (p>0.05). These findings align with the results of Adejumo et al. (2016), who also reported similar prevalence rates of pruritus in ESRD patients with these conditions, suggesting that these factors may not be primary contributors to pruritus in this patient population [20]. Several studies have reported no statistically significant difference in the etiology of uremia with respect to its association with pruritus in patients with end-stage renal disease [3,19]. Our study identified a significant variation in hemodialysis (HD) duration between the two groups, highlighting a potential link between HD exposure length and pruritus prevalence in end-stage renal disease (ESRD) patients. In Group I, a notable portion of patients (23.5%) had undergone HD for less than a year, while the majority (58.8%) had been on HD for 1–5 years and 17.6% for over five years. In contrast, most of Group II (55.8%) had a short-term HD history, with 41.9% in the 1–5-year range and only 2.3% undergoing HD for more than five years (Table 3). These results suggest a potential cumulative effect of HD duration on pruritus, as the longer-term HD patients in Group I experienced a higher prevalence of pruritus. In contrast, Group II, primarily short-term HD patients, had fewer cases. This finding is in contrast with studies by Narita et al. (2006), Akhyani et al. (2005), and Cho et al. (1997), which did not establish a significant relationship between HD duration and pruritus in ESRD [3,19]. However, Rehman et al. (2018) reported only a minimal impact of prolonged HD on pruritus, underscoring the complexity of pruritus in ESRD patients [21]. Table 4 presents a comparison of IL-31 levels in relation to dialysis frequency among Group I patients (those receiving hemodialysis with pruritus) in our study. Patients undergoing twice-weekly hemodialysis showed elevated IL-31 levels (107.9±68.9 pg/ml) compared to those receiving thrice-weekly treatments (65.4±85.2 pg/ml), suggesting a potential association with increased pruritus risk. Although previous studies have observed a reduction in uremic pruritus with advancements in hemodialysis techniques, our findings did not reveal a statistically significant difference in IL-31 levels between patients undergoing twice-weekly versus thrice-weekly dialysis sessions [4,22]. Pruritus, a frequent and distressing symptom in ESRD patients, affected 47.1% mildly, 35.3% moderately, and 17.6% severely, as shown in Figure 2. This distribution aligns with findings by Oweis et al. (2021), Rehman et al. (2018), and Ozen et al. (2018) [1,21,23]. Similar findings were reported by Yousef et al. (2020), who observed that 31.8% of patients experienced mild pruritus, 27.3% had moderate pruritus, and 40.9% suffered from severe pruritus, further emphasizing the high prevalence of pruritus in individuals with end-stage renal disease (ESRD) [24]. Pruritus was prevalent in ESRD patients on maintenance hemodialysis, with 52.9% experiencing generalized pruritus and 47.1% reporting pruritus at multiple sites. Most patients (64.7%) had episodic itching, followed by 29.4% with frequent episodes and 5.9% with continuous pruritus. Scratching was the most common manifestation (52.9%), followed by generalized excoriation (23.5%) and localized excoriation (11.8%). These findings are consistent with previous studies on pruritus in ESRD patients [24]. This study found that the majority of patients (70.6%) experienced rare sleep disturbances, with 17.6% reporting occasional disruptions and 11.8% suffering from frequent sleep disturbances attributed to pruritus. In line with findings from Ozen et al. (2018), 50.4% of patients reported moderate pruritus, with 33.8% of them indicating that pruritus contributed to sleep disturbances [1]. Additionally, the study by Rehman et al. (2018) revealed that 53.4% of patients experienced moderate sleep disturbances, while 8.4% reported severe disturbances [21]. Table 6 shows the potential etiological link between pruritus and End Stage Renal Disease (ESRD) in patients undergoing maintenance hemodialysis, focusing on interleukin-31 (IL-31) levels. The study found no significant difference in IL-31 levels among patients with mild (136.5±88.7), moderate (143.3±62.1), and severe (76.2±75.3) pruritus (p>0.05), suggesting that IL-31 may not correlate with pruritus severity in this population. Oweis et al. (2021) observed elevated IL-31 levels in uremic pruritus (UP) patients but found no direct correlation with itch severity. Additionally, IL-13 levels, rather than IL-31, were associated with itch severity, consistent with findings by Gibbs et al. (2019) linking elevated IL-31 to other pruritic skin conditions [25]. In this study, the mean IL-31 levels were significantly higher in Group I (HD with pruritus) at 128.23±77.34, compared to Group II (HD without pruritus) at 60.52±36.25 (Table 7). These results suggest a strong association between elevated IL-31 levels and pruritus in patients with end-stage renal disease (ESRD) on maintenance hemodialysis. Swierczynska et al. (2022) also observed elevated IL-31 levels in hemodialysis patients with chronic kidney disease-associated pruritus (CKD-aP), with a mean of 679.9±1112.3 pg/mL in those with pruritus, compared to 176.1±290.7 pg/mL in those without [26]. These findings also align with Ko et al. (2014), who demonstrated higher IL-31 levels in pruritus patients, and Oweis et al. (2021), whose cross-sectional study reported similar results, reinforcing the role of IL-31 in pruritic conditions in hemodialysis patients [27]. Table 7 also demonstrated no significant differences in serum creatinine, albumin, ferritin, hemoglobin, WBC, eosinophils, and CRP levels between Group I (HD with pruritus) and Group II (HD without pruritus). This suggests that pruritus in ESRD patients on hemodialysis correlates with elevated IL-31 levels but not with other laboratory parameters. Contrary to our findings, Sarhan et al. (2020) reported a significant positive correlation between CRP levels and uremic pruritus (p<0.001) [28]. In this study, no significant differences were observed between Group I and Group II in terms of immunoglobulin E (IgE) levels (Group I: 281.49±255.13, Group II: 329.81±448.19; P=0.876) or circulating eosinophil levels (Table 8). Despite some studies suggesting a link between elevated IgE and eosinophils with pruritus in hemodialysis patients, results remain inconclusive or contradictory. Similarly, no significant differences were found between the groups regarding intact parathyroid hormone (PTH) levels (Group I: 257.33±195.50, Group II: 180.43±104.55; P>0.05), serum calcium levels (Group I: 9.49±0.90, Group II: 9.17±0.85; P>0.05), phosphate levels (Group I: 47.13±19.27, Group II: 38.63±14.53; P>0.05), or the calcium-phosphate product (Group I: 47.13±19.28, Group II: 38.63±14.53; P>0.05) (Table 9). These findings align with previous studies, such as those by Narita et al. (2006), Akhyani et al. (2005), and Cho et al. (1997), which also found no significant association between pruritus and serum PTH, calcium, or phosphate levels in end-stage renal disease patients [3,19,29]. Similarly, Hasan et al. (2019) reported no significant association between pruritus and serum PTH levels [30]. Table 10 presents the findings of the current study, which indicate that IL-31 exhibited moderate diagnostic accuracy in detecting pruritus in these patients. The sensitivity of IL-31 was calculated at 70.59%, implying that it correctly identified pruritus in approximately 71% of cases. The specificity, which indicates the test's ability to identify non-pruritic cases correctly, was found to be 81.40%. This demonstrates that IL-31 is fairly good at excluding individuals who do not experience pruritus. The overall accuracy of the test was 78.33%, indicating that IL-31's performance is reasonably reliable in the context of pruritus diagnosis. In this study, the PPV for IL-31 was 60.0%, indicating that when IL-31 identified pruritus, there was a 60% chance that it was a true positive. The NPV was notably higher at 87.50%, suggesting that when IL-31 did not identify pruritus, there was an 87.5% chance that it was a true negative. While this study's finding provides valuable insights into the diagnostic performance of IL-31 in identifying pruritus in ESRD patients undergoing hemodialysis, it is essential to contextualize these results within the broader landscape of existing research. This study examined the relationship between serum IgE, eosinophil levels, IL-31, and pruritus severity in maintenance hemodialysis (MHD) patients. Figures 3, 4, and 5 show no significant correlation between serum IgE and eosinophil levels in patients with pruritus (r=0.192; p>0.05) or without pruritus (r=-0.20; p>0.05). Additionally, a non-significant positive correlation was observed between IL-31 and the Pruritus Grading Severity Scale (PGSS) in MHD patients with pruritus (r=0.327; p=0.200). These findings suggest that serum IgE levels do not significantly contribute to pruritus in this population. Similarly, the non-significant correlation between IL-31 and PGSS points to the potential, though unproven, role of IL-31 as a biomarker for pruritus. These results contrast with previous studies, such as Rayner et al. (2019), which reported a significant positive correlation between IL-31 and pruritus severity [31]. Differences in sample sizes, patient demographics, and assay techniques may explain these discrepancies. Moreover, Oweis et al. (2021) found no significant correlation between IL-31 and itch score (r=-0.094; p>0.05), reinforcing the complexity of pruritus in end-stage renal disease [1]. Although this study did not identify a significant correlation between serum IgE and eosinophils, the positive yet non-significant IL-31-PGSS relationship warrants further investigation. This research highlights the need for continued exploration of potential biomarkers and better management strategies for pruritus in MHD patients.
Limitations of the study
- This is a cross-sectional study, which cannot establish the causality and temporality between serum levels of IL-31 and uremic pruritus.
- This study did not adjust for other various inflammatory cytokines because of financial constrain, limited time.
- The present study was conducted at a very short period of time.
5. Conclusion and Recommendations
In this study there was significant difference of IL -31 level in between pruritic and non-pruritic patients. IL-31 levels didn’t directly correlate with the severity of pruritus in ESRD patients on maintenance hemodialysis. Twice weekly hemodialysis patients were more prone to develop pruritus than thrice weekly treated patients. Besides, there was no significant difference in terms of IL-31 level with the frequency of HD.
Declarations
Funding:
No funding sources
Ethical approval
The study was approved by the Institutional Ethics Committee.
Conflict of interest
None declared
References
- Oweis AO, Firas AQ, Bodoor K, et al., Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Cytokine 138 (2021): 155369.
- Rayner H, Baharani J, Smith S, et al., Uraemic pruritus: relief of itching by gabapentin and pregabalin. Nephron Clinical Practice 122 (2013): 75-79.
- Narita I, Alchi B, Omori K, et al., Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney international 69 (2006): 1626-1632.
- Weng CH, Hu CC, Yen TH, et al., Uremic pruritus is associated with two-year cardiovascular mortality in long term hemodialysis patients. Kidney and Blood Pressure Research 43 (2018):1000-1009.
- Agarwal P, Garg V, Karagaiah P, et al., Chronic kidney disease-associated pruritus. Toxins 13 (2021): 527.
- Kimmel M, Alscher DM, Dunst R, et al., The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrology Dialysis Transplantation 21 (2006): 749-755.
- Hu T, Wang B, Liao X, et al., Clinical features and risk factors of pruritus in patients with chronic renal failure. Experimental and therapeutic medicine 18 (2019): 964-971.
- Szepietowski JC, Salomon J. Uremic pruritus: still an important clinical problem. Journal of the American Academy of Dermatology 51 (2004): 842-843.
- Molina P, Ojeda R, Blanco A, et al., Etiopathogenesis of chronic kidney disease-associated pruritus: putting the pieces of the puzzle together. Nefrología (English Edition). 43 (2023): 48-62.
- Dugas-Breit S, Schöpf P, Dugas M, et al., Baseline serum levels of mast cell tryptase are raised in hemodialysis patients and associated with severity of pruritus: Basale Mastzelltryptase-Serumspiegel sind bei Haemodialyse-Patienten erhöht und mit der Pruritus-Intensität assoziiert. JDDG: Journal der Deutschen Dermatologischen Gesellschaft 3 (2005): 343-347.
- Kfoury LW, Jurdi MA. Uremic pruritus. J Nephrol 25 (2012): 644-652.
- Cheng AY and Wong LS. Uremic pruritus: from diagnosis to treatment. Diagnostics, 12 (2022): 1108.
- Fallahzadeh MK, Roozbeh J, Geramizadeh B, et al., Interleukin-2 serum levels are elevated in patients with uremic pruritus: a novel finding with practical implications. Nephrology Dialysis Transplantation 26 (2011): 3338-3344.
- Pisoni RL, Wikström B, Elder SJ, et al., Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrology Dialysis Transplantation 21 (2006): 3495-3505.
- Kabashima K, Matsumura T, Komazaki H, et al., Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. New England Journal of Medicine 383 (2020):141-150.
- Khan TM, Wu DB, Goh BH, et al., An observational longitudinal study investigating the effectiveness of 75 mg pregabalin post-hemodialysis among uremic pruritus patients. Scientific reports 6 (2016): 36555.
- Tajbakhsh R, Joshaghani HR, Bayzayi F, et al., Association between pruritus and serum concentrations of parathormone, calcium and phosphorus in hemodialysis patients. Saudi Journal of Kidney Diseases and Transplantation. 24 (2013):702-706.
- Tinôco JD, Paiva MD, Macedo BM, et al., Prurido no paciente em hemodiálise: associação com ingestão de fósforo e nível sérico de cálcio. Revista Gaúcha de Enfermagem 39 (2018): e2017-0081.
- Akhyani M, Ganji MR, Samadi N, et al., Pruritus in hemodialysis patients. BMC dermatology 5 (2005): 1-6.
- Adejumo O, Akinbodewa A, Alli O, et al., Prevalence, pattern and association of pruritus with quality of life in chronic kidney disease patients attending kidney care centre, Ondo City, Southwest Nigeria. Ethiopian journal of health sciences 26 (2016): 549.
- Rehman IU, Munib S, Ramadas A, et al., Prevalence of chronic kidney disease-associated pruritus, and association with sleep quality among hemodialysis patients in Pakistan. PloS one. 13 (2018): e0207758.
- Mettang T, Kremer AE. Uremic pruritus. Kidney international. 87 (2015): 685-691.
- Ozen N, Cinar FI, Askin D, et al., Uremic pruritus and associated factors in hemodialysis patients: A multi-center study. Kidney research and clinical practice 37 (2018): 138.
- Yousef AM, Yasien HA, Haggag MM, et al., Study of serum level of interleukin-31 in patients with uremic pruritus. Menoufia Medical Journal 33 (2020): 257-261.
- Gibbs BF, Patsinakidis N, Raap U. Role of the pruritic cytokine IL-31 in autoimmune skin diseases. Frontiers in immunology 10 (2019): 1383.
- Świerczyńska K, Krajewski PK, Nowicka-Suszko D, et al., The serum level of IL-31 in patients with chronic kidney disease-associated pruritus: what can we expect?. Toxins 14 (2022): 197.
- Ko MJ, Peng YS, Chen HY, et al., Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. Journal of the American Academy of Dermatology 71 (2014): 1151-1159.
- Sarhan II, Ibrahim MA, Kamel NM, et al., Association of high sensitive C reactive protein and dialysis adequacy with uremic pruritus in hemodialysis patients. Alexandria Journal of Medicine 56 (2020): 111-117.
- Cho YL, Liu HN, Huang TP, et al., Uremic pruritus: roles of parathyroid hormone and substance P. Journal of the American Academy of Dermatology 36 (1997): 538-543.
- Hasan R, Chowdhury MN, Islam MN, et al., Distribution of Pruritus and Its Association with Serum Parathormone Level in Chronic Kidney Disease (Stage-5) Patients On Maintenance Hemodialysis. Journal of Dhaka Medical College 28 (2019): 54-59.
- Rayner HC, Larkina M, Wang M, et al., International comparisons of prevalence, awareness, and treatment of pruritus in people on hemodialysis. Clinical journal of the American Society of Nephrology 12 (2017): 2000-2007.