Epihtelioid Sclerosing Fibrosarcoma of the Chest Wall: A Case Report and Review of the Literature
Article Information
Derqaoui Sabrine1*, Laraqui Yasmine1, Raissouni Khouloud1, Jahid Ahmed1, Achir Abdellah2, Bernoussi Zakia1, Znati Kaoutar1
1Department of Pathology, Ibn Sina Teaching Hospital, Rabat, Morocco
2Thoracic Surgery Department, Ibn Sina Teaching Hospital, Rabat, Morocco
*Corresponding Author: Sabrine Derqaoui, Department of Pathology, Ibn Sina Teaching Hospital, Rabat, Morocco.
Received: 28 October 2019; Accepted: 27 November 2019 Published: 02 January 2020;
Citation: Derqaoui Sabrine, Laraqui Yasmine, Raissouni Khouloud, Jahid Ahmed, Aachir Abdellah, Bernoussi Zakia, Znati Kaoutar. Epihtelioid sclerosing fibrosarcoma of the chest wall: A case report and review of the literature. Journal of Cancer Science and Clinical Therapeutics 4 (2020): 042-048.
View / Download Pdf Share at FacebookAbstract
Background: SEF is a rare variant of fibrosarcoma, mostly occurring in extraosseous sites. It is a clinically challenging entity as no standardized treatment regimens are available. SEF’s literature is limited, due to its rarity and recent recognition. The current study reports the case of a patient with SEF arising from the anterior chest wall.
Case Presentation: A 26-year-old man presented with a 6 years history of a left superior chest mass. Imaging revealed an invasive tumor of the right pectoral region, without metastases. The patient undergone surgical resection of the tumor. Histology and immunohistochemistry confirmed the diagnosis and revealed a hypocellular tumor with large areas of hyalinized fibrous stroma. The patient was referred to the oncology department to undergo postoperative treatment (radiotherapy/chemotherapy).
Keywords
SEF; Fibrosarcoma; Extraosseous; Hypocellular tumor; Fibrous stroma
SEF articles, Fibrosarcoma articles, Extraosseous articles, Hypocellular tumor articles, Fibrous stroma articles
SEF articles SEF Research articles SEF review articles SEF PubMed articles SEF PubMed Central articles SEF 2023 articles SEF 2024 articles SEF Scopus articles SEF impact factor journals SEF Scopus journals SEF PubMed journals SEF medical journals SEF free journals SEF best journals SEF top journals SEF free medical journals SEF famous journals SEF Google Scholar indexed journals Fibrosarcoma articles Fibrosarcoma Research articles Fibrosarcoma review articles Fibrosarcoma PubMed articles Fibrosarcoma PubMed Central articles Fibrosarcoma 2023 articles Fibrosarcoma 2024 articles Fibrosarcoma Scopus articles Fibrosarcoma impact factor journals Fibrosarcoma Scopus journals Fibrosarcoma PubMed journals Fibrosarcoma medical journals Fibrosarcoma free journals Fibrosarcoma best journals Fibrosarcoma top journals Fibrosarcoma free medical journals Fibrosarcoma famous journals Fibrosarcoma Google Scholar indexed journals Extraosseous articles Extraosseous Research articles Extraosseous review articles Extraosseous PubMed articles Extraosseous PubMed Central articles Extraosseous 2023 articles Extraosseous 2024 articles Extraosseous Scopus articles Extraosseous impact factor journals Extraosseous Scopus journals Extraosseous PubMed journals Extraosseous medical journals Extraosseous free journals Extraosseous best journals Extraosseous top journals Extraosseous free medical journals Extraosseous famous journals Extraosseous Google Scholar indexed journals Hypocellular tumor articles Hypocellular tumor Research articles Hypocellular tumor review articles Hypocellular tumor PubMed articles Hypocellular tumor PubMed Central articles Hypocellular tumor 2023 articles Hypocellular tumor 2024 articles Hypocellular tumor Scopus articles Hypocellular tumor impact factor journals Hypocellular tumor Scopus journals Hypocellular tumor PubMed journals Hypocellular tumor medical journals Hypocellular tumor free journals Hypocellular tumor best journals Hypocellular tumor top journals Hypocellular tumor free medical journals Hypocellular tumor famous journals Hypocellular tumor Google Scholar indexed journals Fibrous stroma articles Fibrous stroma Research articles Fibrous stroma review articles Fibrous stroma PubMed articles Fibrous stroma PubMed Central articles Fibrous stroma 2023 articles Fibrous stroma 2024 articles Fibrous stroma Scopus articles Fibrous stroma impact factor journals Fibrous stroma Scopus journals Fibrous stroma PubMed journals Fibrous stroma medical journals Fibrous stroma free journals Fibrous stroma best journals Fibrous stroma top journals Fibrous stroma free medical journals Fibrous stroma famous journals Fibrous stroma Google Scholar indexed journals fibrotic articles fibrotic Research articles fibrotic review articles fibrotic PubMed articles fibrotic PubMed Central articles fibrotic 2023 articles fibrotic 2024 articles fibrotic Scopus articles fibrotic impact factor journals fibrotic Scopus journals fibrotic PubMed journals fibrotic medical journals fibrotic free journals fibrotic best journals fibrotic top journals fibrotic free medical journals fibrotic famous journals fibrotic Google Scholar indexed journals hyalinized stroma articles hyalinized stroma Research articles hyalinized stroma review articles hyalinized stroma PubMed articles hyalinized stroma PubMed Central articles hyalinized stroma 2023 articles hyalinized stroma 2024 articles hyalinized stroma Scopus articles hyalinized stroma impact factor journals hyalinized stroma Scopus journals hyalinized stroma PubMed journals hyalinized stroma medical journals hyalinized stroma free journals hyalinized stroma best journals hyalinized stroma top journals hyalinized stroma free medical journals hyalinized stroma famous journals hyalinized stroma Google Scholar indexed journals anterior chest wall articles anterior chest wall Research articles anterior chest wall review articles anterior chest wall PubMed articles anterior chest wall PubMed Central articles anterior chest wall 2023 articles anterior chest wall 2024 articles anterior chest wall Scopus articles anterior chest wall impact factor journals anterior chest wall Scopus journals anterior chest wall PubMed journals anterior chest wall medical journals anterior chest wall free journals anterior chest wall best journals anterior chest wall top journals anterior chest wall free medical journals anterior chest wall famous journals anterior chest wall Google Scholar indexed journals extraosseous sites articles extraosseous sites Research articles extraosseous sites review articles extraosseous sites PubMed articles extraosseous sites PubMed Central articles extraosseous sites 2023 articles extraosseous sites 2024 articles extraosseous sites Scopus articles extraosseous sites impact factor journals extraosseous sites Scopus journals extraosseous sites PubMed journals extraosseous sites medical journals extraosseous sites free journals extraosseous sites best journals extraosseous sites top journals extraosseous sites free medical journals extraosseous sites famous journals extraosseous sites Google Scholar indexed journals metastases articles metastases Research articles metastases review articles metastases PubMed articles metastases PubMed Central articles metastases 2023 articles metastases 2024 articles metastases Scopus articles metastases impact factor journals metastases Scopus journals metastases PubMed journals metastases medical journals metastases free journals metastases best journals metastases top journals metastases free medical journals metastases famous journals metastases Google Scholar indexed journals
Article Details
1. Introduction
Sclerosing epithelioid fibrosarcoma (SEF) has been originally described in 1995 by Meis–Kindblom [1] and lately been recognized as a distinct clinical entity. It is a rare variant of fibrosarcoma [2] showing predominantly epithelioid cells embedded in a fibrotic and hyalinized stroma [1]. Together with low-grade fibromyxoid sarcoma and hyalinizing spindle cell tumour with giant rosettes, SEF is classified as fibrosing fibrosarcomas [3]. SEFs mainly present as tumors of the lower extremities (39%) followed by the trunk (21%) and upper extremities [4] and sometimes involve the head and neck [1]. High frequencies of local tumor relapse and distant metastases have been reported (30%–40%) [4]. Due to its scarcity and relatively recent recognition as a distinct diagnostic entity, SEF has not been intensively investigated. Thus, SEF’s literature information is limited. The aim of the current study is to report on a patient with SEF arising from the anterior chest wall.
2. Case Presentation
A 26-year-old man presented with a 6 years history of a left superior chest mass. Except his smoking history, he had no major medical troubles. According to the patient, the mass had progressively increased in size during this time, and dramatically especially during the last 6 months. On examination, he was a healthy male with stable vital signs. The chest examination showed a firm, non-tender, and adherent to deeper structures mass; measuring 12 cm × 10 cm with a normal overlying skin. Rest of the examination was unremarkable. The patient’s blood cell counts, serum electrolyte levels, renal and liver functions were within normal range. Conventional thoracic radiographs showed a soft tissue mass on the left side of the chest with normal lung fields.
Chest magnetic resonance imaging (MRI) revealed an invasive tumor of the right pectoral region measuring 12.25 × 9.5 × 9 cm, which was hypointense on T1-weighted images and heterogeneously hyperintense on T2-weighted images (Figure 1). There were no intra pulmonary’s invasion signs. The primary site of involvement could not be identified; the tumor might have arised in the soft parts or the superior ribs. The radiological findings were suggestive for an osteosarcoma. The routine staging was negative for metastases. The patient undergone surgical resection of the tumor. The diagnosis was confirmed by the histological study of the resected tumor. On gross, the mass measured 12 × 9.5 × 9 cm, it was well circumscribed, lobulated, firm and gray-white. It shows a myxoid pattern and calcifications. Hematoxylin-eosin stained sections revealed a hypocellular tumor with large areas of hyalinized fibrous stroma. It consists of nests and cords of small to medium sized, round, relatively uniform epithelioid cells (Figure 2). Nuclei were vesicular with finely stipple chromatin and small nucleoli. Mitoses were inconspicuous (3 MF / 10 HPF). The resection’s margins were negative (RO resection). On immunohistochemistry, the neoplastic cells were nagative for MUC4, cytokeratin (AE1/AE3), EMA, CD34, SMA, H caldesmon, desmin and beta catenin (Figure 3). The detection of translocation t(X;18)(p11.2;q11.2) by fluorescence in situ hybridization was negative. The patient was referred to oncology department to undergo postoperative treatment (radiotherapy/chemotherapy).
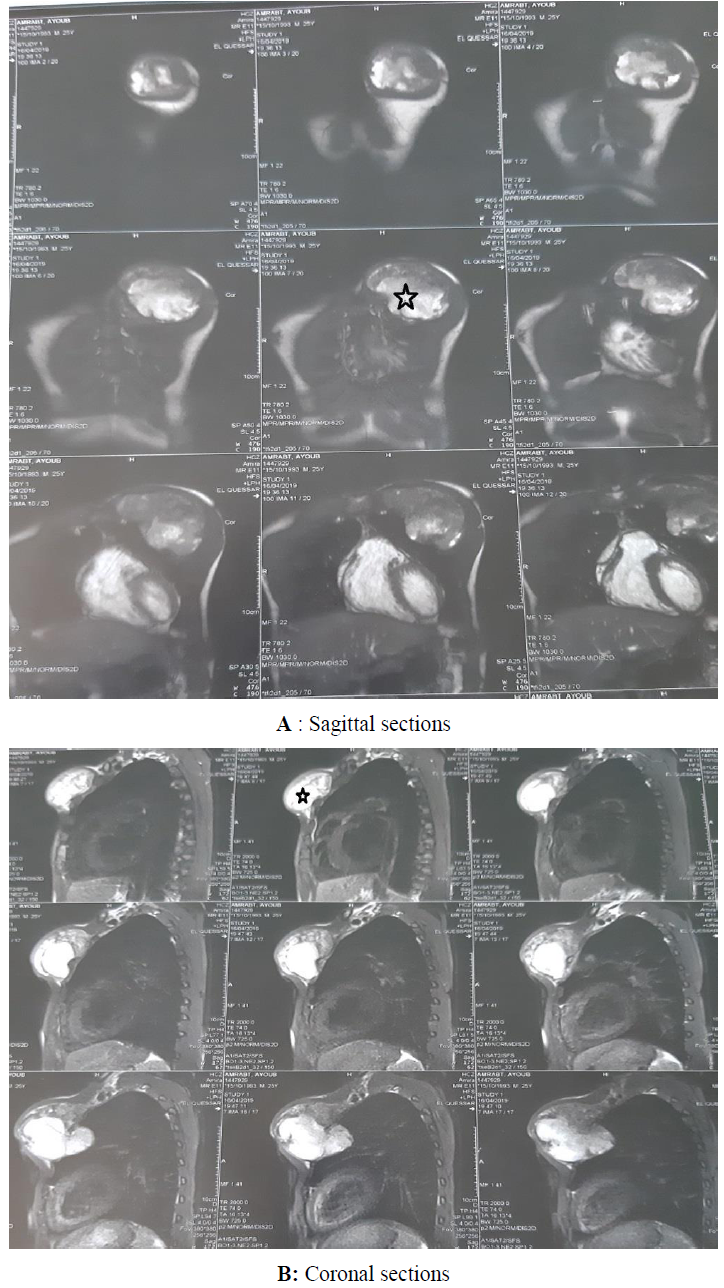
Figure 1: Chest magnetic resonance imaging (MRI) showing an invasive tumor of the right pectoral region measuring (black asterisk) 12.25 × 9.5 × 9 cm, which is heterogeneously hyperintense on T2-weighted images
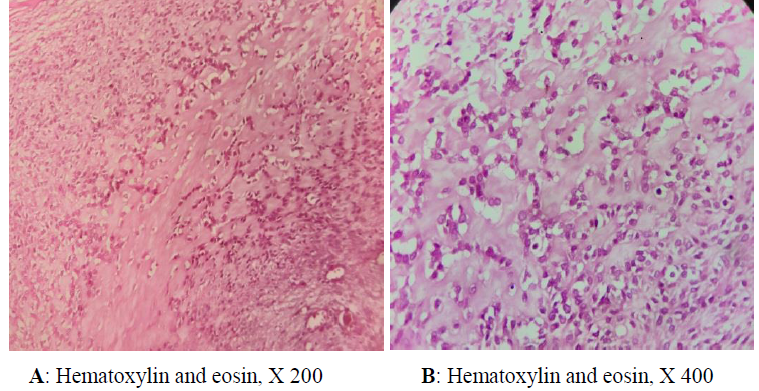
Figure 2: Sclerosing epithelioid fibrosarcoma, showing epithelioid cells, arranged in cords within a sclerotic matrix.
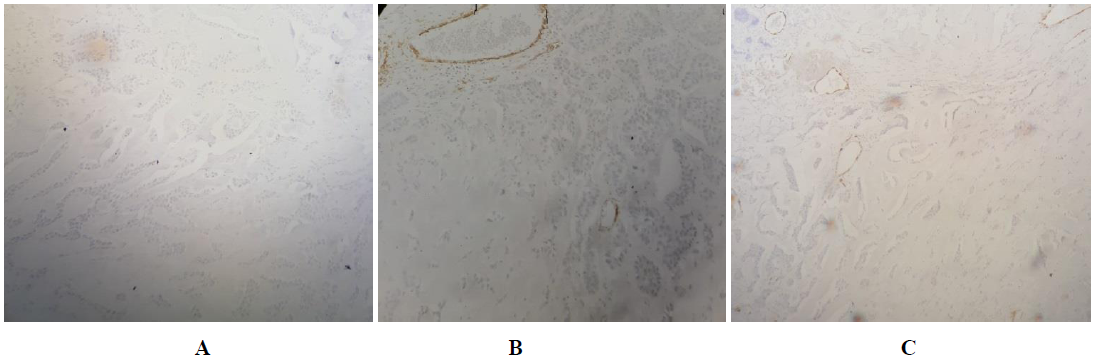
Figure 3: The neoplastic cells are MUC4 negative (A), SMA (B) negative and CD34 negative (C). (Immunohistochemical stain, X 200).
3. Discussion
SEF is a very rare distinctive variant of fibrosarcoma with a metastatic potential. It is characterized by the World Health Organization (WHO) as a malignancy of deep soft tissue [5]. Although the majority of the reported cases arise in the extremities, limb girdle, and trunk, SEF can also affect unusual sites including the kidney; the ovary, the ceacum, the liver, the lung and the pancreas [6]. It mainly affects patients of middle age (our case: 26 years old) with equal gender predisposition. SEF presents as a very slow growing
mass, in most cases, taking around 33 months from the onset of symptoms to the diagnosis. It had an extremely high potential to metastasize and a high rate of local recurrence because of its locally and systemically infiltrative phenotype [7]. Due to its rare occurrence and confusing imaging characteristics, SEF preoperative imaging diagnosis remains challenging. In fact, it can mimic other common soft tissue tumor [8].
To the best of our knowledge, radiological aspects of SEF were not described in the three largest series of SEF in the litterature (Antonescu CR et al. [9], Meis-Kindblom JM et al. [10] and Chew W et al. [11]. In fact, radiological findings were distinctive in only few case reports such as the study of Xu, Jingjing et al; where MRI revealed a focal mass, with hypo- and iso-signal intensity on T1-weighted imaging and mixed-signal intensity on T2-weighted imaging. In our case, the mass showed the same radiological features on MRI, but no evidence of primary tumor’s site could be found (rib or soft part). The fluorodeoxyglucose positron emission tomography/computed tomography (FDG PET/CT) findings have been reported in 4 studies [8], revealing that the FDG uptake of SEF is closely associated with its histopathologic features regarding differentiation and aggressiveness. In fact the high-grade SEF is probably FDG-avid, and the low-grade one is likely to show less FDG uptake [8]. Histologically, the tumor’s classical morphology is nests and cords of mildly atypical cells within a dense collagenous matrix [12]. Malignant epithelioid cells are round to ovoid with sparse and sometimes clear cytoplasm, embedded in hyaline sclerosis. The tumor may exhibit other changes such as areas of conventional fibrosarcoma, myxoid zones, and sometimes foci of hyaline cartilage, calcification, or metaplastic bone formation. In addition, SEF sometimes shares features with low-grade fibromyxoid sarcoma (LGFMS) and hyalinizing spindle cell tumor with giant rosettes [13]. In some cases, more or less extensive areas reminiscent LGFMS can be seen, either in synchronous or metachronous combination with SEF areas; such tumors are known as hybrid SEF/LGFMS [14]. The SEF may not show the typical morphologic features of sarcomas including infiltrative growth pattern, pronounced pleomorphy, mitotic activity and necrosis [2].
Immunohistochemistry and molecular studies may be of substantial utility in supporting a diagnosis of SEF. The immunohistochemistry profile includes positive MUC4 (sensitive and specific in up to 70% of cases), focal or weak positivity of EMA (epithelial membrane antigen ), S100 and cytokeratins and negativity for CD34, leukocyte markers, HMB45, CD68, desmin, H-caldesmon and SMA (smooth muscular actin). Transducin-like enhancer of split 1 (TLE1) positive staining have been reported in one case [15]. In our case, the neoplastic cells were negative for MUC4, cytokeratin (AE1/AE3), EMA, CD34, H caldesmon, desmin and beta catenin. SEF’s genomic alterations are complex [14]. The majority of the cases exhibited a reciprocal chromosomal translocation t(11;22)(p11;q12) resulting in generation of EWSR1-CREB3L1 fusion gene [6] which leads to higher expression of the CD24 gene [14]. Genomic profiling by clinical-grade next generation sequencing (NGS) revealed a fusion gene between intron11 of EWSR1 (22q12.2) and intron5 of CREB3L1 (11p11.2) [16]. Other complex genomic rearrangements have been reported, including recurrent intragenic deletions of the DMD gene encoding dystrophin. Thus DMD and CD24 represent promising treatment targets [14]. The shared and distinct genetic features could perhaps explain the intriguing clinical and morphologic overlaps and differences between SEF and LGFMS. LGFMS is by far the more extensively analyzed subtype of the two [14]. The differential diagnosis of SEF may be broad especially in small biopsies; because of its epithelioid morphology. It includes other soft tissue tumors such as epithelioid hemangioendothelioma, clear cell sarcoma, ossifying fibromyxoid tumors as well as carcinoma and melanoma. Immunostains are predominantly useful for excluding other neoplasms in the differential diagnosis. Some cases show indistinguishable from LGFMS, areas. In addition, the immunophenotype of SEF might be similar to that of LGFMS [17]. In fact, the shared and distinct genetic features could perhaps explain the intriguing morphologic and clinical overlaps and differences between SEF and LGFMS. Around 95% of the cases show a FUS-CREB3L2 fusion gene, typically on the basis of a balanced translocationt (7;16) (q33;p11) [14].
The prognosis of SEF is generally poor. According to the literature, local relapse have been reported in 30 to 50 % of SEF patients; between two to five years after initial diagnosis [18]. Due to the rarity of the condition, no standard treatment protocols have been reported [7]. To the best of our knowledge, the only study recording the outcome of a consecutive series of patients (13 cases) with SEF treated at a single referral center; has been conducted by Chew. W et al. [11]. 11 patients underwent radical resection for localized disease with microscopically involved resection margins R1. All of patients experienced tumor relapse. In only 1 case where R0 resection was achieved, no evidence of relapse has been reported (follow-up of 105 months). Palliative chemotherapy was established in 7 cases among the 10 with metastatic disease. The median progression-free survival post 1st line chemotherapy was 2.7 months. Thus, sensitivity to chemotherapy regimens widely used for soft tissue sarcomas (doxorubicin, Ifosfamide, gemcitabine, docetaxel) appears to be limited [11]. Moreover, preoperative or postoperative radiation as used in other soft tissue sarcomas also should be considered in cases which cannot be excised with clear margins [7].
3. Conclusion
In summary, we have reported the clinical and histopathologic features of a case of SEF as SEF is an extremely rare cancer. Up to present, no standard efficient treatment regimens have been clearly identified. There is a need for a further understanding of SEF’s biology to improve its poor prognosis and collecting a sizable patient cohort remains essential to review treatment outcomes.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
All authors read and approved the final manuscript.
Funding
No funding source was needed
References
- Antonescu CR, Rosenblum MK, Pereira P, et al. Sclerosing Epithelioid Fibrosarcoma. The American Journal of Surgical Pathology 25 (2001): 699-709.
- Ossendorf C, Studer GM, Bode B, et al. Sclerosing epithelioid fibrosarcoma: case presentation and a systematic review. Clin Orthop Relat Res 466 (2008):1485-1491.
- Smith PJ, Almeida B, Krajacevic J, et al. Sclerosing epithelioid fibrosarcoma as a rare cause of ascites in a young man: a case report. J Med Case Rep 2 (2008): 248.
- Grunewald TG, von Luettichau I, Weirich G, et al. Sclerosing epithelioid fibrosarcoma of the bone: a case report of high resistance to chemotherapy and a survey of the literature. Sarcoma (2010): 431-627.
- Folk GS, Williams SB, Foss RB, et al. Oral and maxillofacial sclerosing epithelioid fibrosarcoma: report of five cases [published correction appears in Head Neck Pathol. Head Neck Pathol 1 (2007): 13-20.
- Wang X, Wang J. Primary sclerosing epithelioid fibrosarcoma of the kidney: Report of two additional cases with a clinicopathological and molecular cytogenetic study, Experimental and Molecular Pathology 107 (2019): 179-183.
- Popli A, Mahajan R, Rustagi T, et al. Sclerosing Epithelioid Fibrosarcoma of the Coccyx: A Case Report and Review of Literature. Cureus 10 (2018): e2407.
- Luo Y, Hu W, Wu H, et al. ¹?F-fluorodeoxyglucose PET/CT features and correlations with histopathologic characteristics in sclerosing epithelioid fibrosarcoma. Int J Clin Exp Pathol 7 (2014): 7278-7285.
- Antonescu CR, Rosenblum MK, Pereira P, et al. Sclerosing epithelioid fibrosarcoma: a study of 16 cases and confirmation of a clinicopathologically distinct tumor. Am J Surg Pathol 25 (2001): 699-709.
- Meis-Kindblom JM, Kindblom LG, Enzinger FM. Sclerosing epithelioid fibrosarcoma. A variant of fibrosarcoma simulating carcinoma. Am J Surg Pathol 19 (1995): 979-993.
- Chew W, Benson C, Thway K, et al. Clinical Characteristics and efficacy of chemotherapy in sclerosing epithelioid fibrosarcoma. Med Oncol 35 (2018): 138.
- Wojcik JB, Bellizzi AM, Dal Cin P, et al. Primary Sclerosing Epithelioid Fibrosarcoma of Bone. The American Journal of Surgical Pathology 38 (2014): 1538-1544.
- Patterson JW, Tchernev G, Chokoeva AA, et al. Sclerosing epithelioid fibrosarcoma. Wiener Medizinische Wochenschrift 167 (2016): 120-123.
- Elsa Arbajian, Florian Puls, Cristina R, et al. In-depth Genetic Analysis of Sclerosing Epithelioid Fibrosarcoma Reveals Recurrent Genomic Alterations and Potential Treatment Targets Clin Cancer Res 23 (2017): 7426-7434.
- Perez D, Fullmer JM, Naous R. A rare case of TLE1-positive sclerosing epithelioid fibrosarcoma expanding the differential diagnosis of TLE1-positive tumors: a case report. AME Case Rep 3 (2019).
- Stockman David L, Ali Siraj M, He Jie, et al. Sclerosing Epithelioid Fibrosarcoma presenting as intra-abdominal “sarcomatosis” with a novel EWSR1-CREB3L1 gene fusion. Human Pathology (2014).
- Doyle LA, Wang WL, Dal Cin P, et al. MUC4 Is a Sensitive and Extremely Useful Marker for Sclerosing Epithelioid Fibrosarcoma. The American Journal of Surgical Pathology 36 (2012): 1444-1451.
- Leisibach P, Weder W, Soltermann, A, et al . Primary Sclerosing Epithelioid Fibrosarcoma of the Lung in a Patient with Lynch Syndrome. Lung 190 (2012): 691-695.
