Epidemiology, Treatment, and Evolution of Glioblastoma in a Low-Income Country: Moroccan Experience
Article Information
Asmaa Naim1,2*, Hamza ouazzani1, Soumya Rafii2, Abdessamad Azhari1, Abdallah Badou2,3
1Mohammed VI University of Health Sciences (UM6SS), Casablanca, Morocco
2Immunology and of Molecular Biology Cellular and Molecular Pathology Laboratory Faculty of Medicine and Pharmacy, University Hassan II, Casablanca, Morocco
3Mohammed VI Center for Research and Innovation, Rabat, Morocco, Mohammed VI University of Health Sciences, Casablanca, Morocco
*Corresponding Author: Asmaa Naim, University Mohamed VI of health of Sciences; Faculty of Medicine and Pharmacy University Hassan II of Casablanca, Morocco.
Received: 30 December 2022; Accepted: 18 January 2023; Published: 24 January 2023
Citation:
Asmaa Naim, Hamza ouazzani, Soumya Rafii, Abdessamad Azhari, Abdallah Badou. Epidemiology, Treatment, and Evolution of Glioblastoma in a Low-Income Country: Moroccan Experience. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 16-20.
View / Download Pdf Share at FacebookAbstract
Purpose: Glioblastoma is the most aggressive and frequent primary brain tumor in adults. Its prognosis remains dismal despite breakthroughs in all therapy modalities. This study was out to present the epidemiological, clinical, therapeutic and evolutionary characteristics of glioblastomas in our center as a low-income nation.
Patients and Methods: The study was carried out at University hospital in collaboration with two departments: Neurosurgery and Cancer therapy Center between June 1st, 2016, and June 30th, 2021. Two qualified physicians retrieved all of the data from the hospital's medical records. All clinical, paraclinical, and evolutionary characteristics were collected. Utilizing an Excel data file, the data was extracted, coded, and analyzed using GraphPad Prism 6.
Results: The study concerned 35 patients, their median age was 60,6 years [32-87] with a male predominance (sex ratio M/F=2,18), Clinical signs varied; motor impairment was present in 60% of patients, intracranial pressure syndrome in 48.5%, higher function problems in 37.1% and seizures in 20% of cases. Complete resection, partial excision, and biopsy were the surgical techniques used in 34%, 40%, and 26% of the cases, respectively. Twenty-two patients received radiotherapy with concomitant chemotherapy according to the STUPP protocol in 15 cases. The evolution at 3 months could be characterized in 28 patients and was marked by 14.2% good response, 37.1% stability, 20% progression and 8.5% death. Patients with Glioblastoma with good KPS score>70 and a low ki67 index who received total resection, followed by radio-chemotherapy, had longer median survival, which was 12 months on average.
Conclusion: Our epidemiological findings are comparable to previously published data; however, in o
Keywords
<p>Epidemiology; Evolution; Glioblastoma; Treatment; Survival</p>
Epidemiology articles; Evolution articles; Glioblastoma articles; Treatment articles; Survival articles
Epidemiology articles Epidemiology Research articles Epidemiology review articles Epidemiology PubMed articles Epidemiology PubMed Central articles Epidemiology 2023 articles Epidemiology 2024 articles Epidemiology Scopus articles Epidemiology impact factor journals Epidemiology Scopus journals Epidemiology PubMed journals Epidemiology medical journals Epidemiology free journals Epidemiology best journals Epidemiology top journals Epidemiology free medical journals Epidemiology famous journals Epidemiology Google Scholar indexed journals Evolution articles Evolution Research articles Evolution review articles Evolution PubMed articles Evolution PubMed Central articles Evolution 2023 articles Evolution 2024 articles Evolution Scopus articles Evolution impact factor journals Evolution Scopus journals Evolution PubMed journals Evolution medical journals Evolution free journals Evolution best journals Evolution top journals Evolution free medical journals Evolution famous journals Evolution Google Scholar indexed journals Glioblastoma articles Glioblastoma Research articles Glioblastoma review articles Glioblastoma PubMed articles Glioblastoma PubMed Central articles Glioblastoma 2023 articles Glioblastoma 2024 articles Glioblastoma Scopus articles Glioblastoma impact factor journals Glioblastoma Scopus journals Glioblastoma PubMed journals Glioblastoma medical journals Glioblastoma free journals Glioblastoma best journals Glioblastoma top journals Glioblastoma free medical journals Glioblastoma famous journals Glioblastoma Google Scholar indexed journals Treatment articles Treatment Research articles Treatment review articles Treatment PubMed articles Treatment PubMed Central articles Treatment 2023 articles Treatment 2024 articles Treatment Scopus articles Treatment impact factor journals Treatment Scopus journals Treatment PubMed journals Treatment medical journals Treatment free journals Treatment best journals Treatment top journals Treatment free medical journals Treatment famous journals Treatment Google Scholar indexed journals Survival articles Survival Research articles Survival review articles Survival PubMed articles Survival PubMed Central articles Survival 2023 articles Survival 2024 articles Survival Scopus articles Survival impact factor journals Survival Scopus journals Survival PubMed journals Survival medical journals Survival free journals Survival best journals Survival top journals Survival free medical journals Survival famous journals Survival Google Scholar indexed journals brain tumor articles brain tumor Research articles brain tumor review articles brain tumor PubMed articles brain tumor PubMed Central articles brain tumor 2023 articles brain tumor 2024 articles brain tumor Scopus articles brain tumor impact factor journals brain tumor Scopus journals brain tumor PubMed journals brain tumor medical journals brain tumor free journals brain tumor best journals brain tumor top journals brain tumor free medical journals brain tumor famous journals brain tumor Google Scholar indexed journals Cancer therapy articles Cancer therapy Research articles Cancer therapy review articles Cancer therapy PubMed articles Cancer therapy PubMed Central articles Cancer therapy 2023 articles Cancer therapy 2024 articles Cancer therapy Scopus articles Cancer therapy impact factor journals Cancer therapy Scopus journals Cancer therapy PubMed journals Cancer therapy medical journals Cancer therapy free journals Cancer therapy best journals Cancer therapy top journals Cancer therapy free medical journals Cancer therapy famous journals Cancer therapy Google Scholar indexed journals intensity-modulated radiotherapy articles intensity-modulated radiotherapy Research articles intensity-modulated radiotherapy review articles intensity-modulated radiotherapy PubMed articles intensity-modulated radiotherapy PubMed Central articles intensity-modulated radiotherapy 2023 articles intensity-modulated radiotherapy 2024 articles intensity-modulated radiotherapy Scopus articles intensity-modulated radiotherapy impact factor journals intensity-modulated radiotherapy Scopus journals intensity-modulated radiotherapy PubMed journals intensity-modulated radiotherapy medical journals intensity-modulated radiotherapy free journals intensity-modulated radiotherapy best journals intensity-modulated radiotherapy top journals intensity-modulated radiotherapy free medical journals intensity-modulated radiotherapy famous journals intensity-modulated radiotherapy Google Scholar indexed journals anti-angiogenic target articles anti-angiogenic target Research articles anti-angiogenic target review articles anti-angiogenic target PubMed articles anti-angiogenic target PubMed Central articles anti-angiogenic target 2023 articles anti-angiogenic target 2024 articles anti-angiogenic target Scopus articles anti-angiogenic target impact factor journals anti-angiogenic target Scopus journals anti-angiogenic target PubMed journals anti-angiogenic target medical journals anti-angiogenic target free journals anti-angiogenic target best journals anti-angiogenic target top journals anti-angiogenic target free medical journals anti-angiogenic target famous journals anti-angiogenic target Google Scholar indexed journals therapeutic articles therapeutic Research articles therapeutic review articles therapeutic PubMed articles therapeutic PubMed Central articles therapeutic 2023 articles therapeutic 2024 articles therapeutic Scopus articles therapeutic impact factor journals therapeutic Scopus journals therapeutic PubMed journals therapeutic medical journals therapeutic free journals therapeutic best journals therapeutic top journals therapeutic free medical journals therapeutic famous journals therapeutic Google Scholar indexed journals
Article Details
1. Introduction
Adult Glioblastomas (GBM) are the most prevalent and malignant primary brain tumors [1]. Imaging advances, notably with new magnetic resonance imaging modalities, and the incorporation of molecular biology into categorization have resulted in improved treatment management [2]. Glioblastoma is an excellent illustration of a multidisciplinary consultation, whose core tenet is surgery with macroscopically full surgical resection coupled with radiotherapy [3]. Indeed, breakthroughs in neurosurgery, most notably awake surgery, and radiation with novel methods like as intensity-modulated radiotherapy, and chemotherapy with new anti-angiogenic target medicines have improved prognosis. However, because glioblastoma relapses often, the outlook is still bleak [4]. Our research intends to assess glioblastoma's epidemiological, therapeutic, and prognostic trends in light of our local experience.
2. Material and Methods
2.1 Study Design and Settings
This retrospective descriptive study was conducted between June 1st, 2016, and June 30th, 2021 at the International University Hospital Cheikh Khalifa in Casablanca, Morocco.
2.2 Participants and Eligibility Criteria
The inclusion criteria were as follows: a) freshly diagnosed, histologically verified Glioma grade IV; b) tumor site in the brain; c) anatomopathological report in our center; and d) treatment by our multidisciplinary team. All patients who didn't meet the conditions of the study were turned away due to the exclusion criteria.
2.3 Data Collection
All data were extracted from hospital medical records using standardized data collection forms. Data collected included sociodemographic, clinical (age, sex, clinical stage, performance status….), paraclinical, therapeutic and evolutive parameters.
2.4 Statistical Analyses
The data extraction was carried out via an Excel datasheet, coded, and analyzed using GraphPad prism 6.
2.5 Ethical Considerations
The present study was done through the ethical aspects respecting all the conditions of confidentiality, anonymity, and self-determination of participants.
3. Results
During our investigation, we collected 35 cases of glioma Grade IV, with the main clinical features presented in table 1. Glioblastoma was detected histologically in 97.2% of patients, whereas Gliosarcoma was discovered in one case (2.8%). Immunohistochemistry was done on 24 of our patients (68.5%) and revealed a positive IDH status in one patient, GFAP and OLIG 2 expression was negative in one patient but positive in the others, a high cell proliferation index of more than 20% was noted in 13 patients, and a positive anti-p53 antibody in 71.4% of the cases. In our dataset, all our patients did computed tomography (CT) coupled with magnetic resonance imaging MRI in 30 cases.
|
Number of patients |
35 (100%) |
|
Median age, years (range) |
60,6 [32-87] |
|
Gender |
|
|
Male |
24 (68,5%) |
|
Female |
11(31,4%) |
|
Median time to the diagnosis, months (range) |
3,6 [0,25-10] |
|
Median KPS at diagnosis (range) |
76 [20-100] |
|
Main symptoms |
|
|
Headache |
7 (20%) |
|
Intracranial hypertension syndrome |
17(48,5%) |
|
Sensory-motor deficit |
26 (74%) |
|
Seizures |
7(20%) |
|
Tumor localization |
|
|
Right hemisphere |
19 (54,2%) |
|
Left hemisphere |
16 (45,7%) |
|
Main lobe localization |
|
|
Frontal |
6 (17%) |
|
Temporal |
7 (20%) |
|
Parietal |
9 (25%) |
|
Occipital |
4 (11,5%) |
|
Others |
9 (26,5%) |
|
Median Tumor size, cm (range) |
5,11[2,8-8,4] |
|
Primary treatment |
|
|
Gross total resection |
12 (34%) |
|
Subtotal resection |
14 (40%) |
|
Stereotactic Biopsy |
9 (26%) |
|
Treatment modalities |
|
|
Radiotherapy |
22(62,5%) |
|
Chemotherapy |
15 (42,8%) |
Table 1: Clinical characteristics of Glioma Grade IV patients enrolled in the study and their therapeutic management.
The progression of our patients was marked by surgical problems in 7 cases, including hydrocephalus in 2 cases, infectious complications in 2 cases, and thromboembolic complications in 3 cases. We documented 3 hematological toxicities as post-chemotherapy side effects. The brief carcinologic history was characterized by lesion stability in 13 cases (37.1%), regression of lesions by at least >50% in 5 patients (14.2%), advancement in 7 patients (20%), and mortality in 3 patients (8.5%). Seven of our patients had unresolved follow-up issues. Only 25 individuals were followed up on after a median overall survival of 12 months; there were 19 (76%) fatalities and 6 (24%) patients were still living: two are now in complete remission, two had lesion stability, one has tumor progression, and one does not get radiological follow-up owing to palliative treatment because of medullary aplasia. In order to highlight the impact of primary treatment in the overall survival we perform overall survival Kaplein-Meier based on the therapy received by the patients involved in our study (Figure 1 a,b,c).
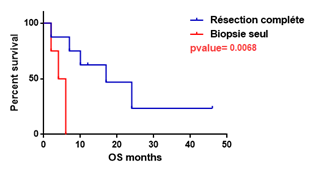
Figure 1-a: Kaplein-Meier estimate of overall survival in patients treated by complete resection versus those with Biopsy alone.

Figure 1-a: Kaplein-Meier estimate of overall survival in patients treated by complete resection versus those with Biopsy alone.
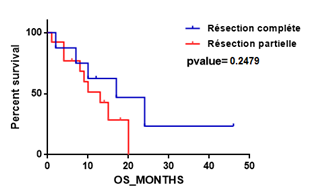
Figure 1-b: Kaplein-Meier estimate of overall survival in patients with complete versus partial resection.
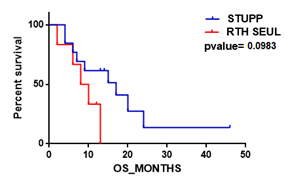
Figure 1-c: Kaplein-Meier estimate of overall survival in our patients receiving radiotherapy with Temozolomide versus radiotherapy alone.
4. Discussion
4.1 Epidemiology
Glioblastoma patients survival in Morocco is a topic that hasn't been studied all that much. Indeed, there is no national cancer registry, and regional registries do not have glioblastoma-specific descriptive epidemiological data. However, according to some regional academic studies, glioblastoma accounts for 47.6% of intracranial tumor malignancies with frequency of 0.94% (0.48% in 2016 and 1.37% in 2017) in all cancers [5]. The median age in our study was 60, 6 years, which is consistent with international publications, and we had a male/female sex ratio of 2.18, which is greater than what has been documented in regional and global literature [1-6].
4.2 Clinical Features
The median time to the diagnosis in our study is a little longer than the average published studies [7]. This could be explained by delayed of consultation and ongoing self-medication. We found a significant prevalence of headache in our study (68,5%), but we also found that 48,5% of patients had intracranial pressure syndrome, which is consistent with previously published data that ranges from 28% to 57% [8,9]. According to the literature, the occurrence of a motor deficiency at the time of diagnosis is a crucial prognostic marker, but its prevalence varies. Chang's team states that, in their experience, 35.9% of cases [8], but other team’s record up to 45% [9]. In our study, the motor deficiency was discovered in 60% cases, which might explain our patients' poor prognosis. Performance status, which restricts therapy options, is a crucial factor in the management of GBM. Patients with poor prognosis account for 17.1% of patients in our experience as opposed to 11% as described in the literature [10,11].
4.3 Paraclinical Features
The studies differed in their data regarding tumor location, although the majority found a primary parietal location [5,10-12]. Our study is in agreement with these data regarding the site brain. However, we found some differences about the focality and the median size. Indeed, we observed higher multifocality (17.1%) compared to 11,7% and 14%, respectively, in American and European experiences [8,10]. This discovery might be the cause of our patients' adverse evolution. Similarly, the median size in our study was 5,11cm, although in other articles it does not surpass 4,5cm, which explains a substantial part of our patients' bad prognosis. The general histological features in our investigation were comparable to those reported in the literature [13]. However, no conclusions about molecular characteristics could be drawn in our study, because just a few patients did immunohistochemistry investigation and only one patient had an evaluated MGMT status. This absence of data is due to our patient’s socioeconomic status and the non-reimbursement of the molecular investigation by insurance companies in our nation.
4.4 Treatment Features
The extent of resection varies greatly in the literature. Complete resection rates varied from 26 percent in the Combs study [14], 39%in STUPP [15], to 75% in the Belghali study [5]. While the partial resection rate in Belghali [5] ranges from 12.5% to 26% in Sinning [16] and 44% in STUPP [15] The percentage of biopsies, however, varies from 15% [5, 15-16] to 50% [17]. In our analysis, the biopsy rate is somewhat higher (26%) and the total resection rate is lower (34%), compared to the primary published studies. This finding can be explained by the low-performance status at the time of diagnosis, as well as the report's location and/or size. Indeed, a thorough surgery is conditioned firstly by the operability of the patient (general state and comorbidity) and secondly by the resectability of a tumor (the size of the lesion and its location). In terms of oncological treatment, 42% of patients in our research got STUPP regimens, which is somewhat lower than the results from the Stupp or Bauchet studies, which were 58, 3% and 50%, respectively [7,15]. The smaller sample size compared to these two studies may help to explain the outcome. In concordance of Stupp protocol, our patients get concomitant chemoradiation and adjuvant chemotherapy throughout 6 cycles of Temozolomide. In our study, two patients underwent bevacizumab-based chemotherapy for recurrence at a dosage of 10 mg/kg every 15 days. The literature on recurrence therapy supports this approach [18].
4.5 Evolution and Prognosis
Many prognostic variables have been identified in the literature. Indeed, Lacroix et al. discovered that age is a significant prognostic factor in their analysis of 416 patients, as with young patients aged 45 years was 14 months compared to outliving senior patients aged >65 years, only 7 months [20]. The findings of our investigation support this conclusion. In addition, Liang et al. found that performance status is related to median survival in a study of 335 patients. In comparison to a KPS of 80%, where the median survival is 8.8 months, if the KPS is >70-80%, the median survival is 11.2 months [19]. Our findings are consistent with the literature since patients with a KPS score >70 had a longer median survival (13.7 months) than those with a KPS of 70 (8.5 months). It should also be highlighted that Ki67 levels are linked to survival [19]. Based on the criterion that has already been established in the literature, we demonstrate increased survival when the ki67 is less than 20 (12.2 months) as opposed to a ki67 of more than 20. (8.5 months). Other immunohistochemistry markers, such as IDH status, were published in the literature, and the researchers concluded that the IDH mutation was of substantial value in the prognosis of glioblastoma after controlling for other variables that might impact survival, such as clinical status or surgical resection extent [21]. Due to the poor representativeness of this subgroup and the fact that only 16 patients in our series had IDH1 status testing, we are unable to make any conclusions in this area. Of those 16 patients, only one had an IDH mutated. The quality of the surgical resection is the main predictive variable associated with survival. Indeed, survival following full resection can exceed 16.9 months, but it drops to 5 months after the biopsy [22]. Our results are consistent with the literature, with a median survival of 15.3 months following total resection, 10.5 months following partial resection, and only 5 months following biopsy. Adjuvant therapy is also an important component of therapeutic management. Several studies found an improvement in median survival after radio-chemotherapy using the STUPP protocol. The findings in our series are consistent with the literature, with a median survival of 14 months following combined radio-chemotherapy, similar to the STUPP study. We reported a lower median survival after radiation alone (9 months) which confirmed the beneficence of the association with chemotherapy as mentioned in the literature. It's critical to emphasize that MGMT predicts a better outcome after radiation and temozolomide. Patients with low MGMT expression respond to temozolomide more effectively (55%) than patients with high MGMT expression (7%) [23]. It has also been found that methylated MGMT patients who underwent radio chemotherapy with TMZ had improved progression-free survival and overall survival when compared to radiation alone [24]. In our investigation, just one patient underwent MGMT profiling. Despite the lack of MGMT methylation in this case and the restricted therapeutic choices in our environment, the patient underwent Temozolomide-based chemotherapy and was in full remission after a 19-month follow-up. It should be mentioned that in this case, we performed a complete resection, highlighting the crucial role of a high-quality surgical resection.
4.6 Study Limits
The authors are aware that firstly the study did not provide enough data on molecular parameters due to a lack of a local operational molecular biology platform and patients’ limited financial resources for external help. Secondly, the study is monocentric and has a limited follow-up.
5. Conclusion
While we wait for tailored treatments based on the incorporation of molecular characteristics into medicines, the only guarantee of a better prognosis for GBM remains early diagnosis and management at reference centers by a multidisciplinary team of neuro-oncology experts, particularly in low-income countries.
References
- Arora RS, Alston RD, Eden TOB, et al. Age–incidence patterns of primary CNS tumors in children, adolescents, and adults in England. Neuro-Oncology. 1 août 11 (2009): 403-413.
- Ostrom QT, Cioffi G, Waite K, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro-Oncology 23 (2021): iii1-105.
- Baba M, Adali N. Analyse de survie des patients atteints de glioblastome au Maroc. Revue d’Épidémiologie et de Santé Publique. Mai 70 (2022): S105.
- Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a « state of the science » review. Neuro-Oncology. 1 juill 16 (2014): 896-913.
- Belghali MY, Ba-M’hamed S, Admou B, et al. Caractéristiques épidémiologiques, cliniques, thérapeutiques et évolutives des patients atteints de glioblastome cérébral?: série de cas pris en charge au centre d´oncohématologie du Centre Hospitalier Universitaire Mohammed VI de Marrakech en 2016 et 2017. Pan Afr Med J [Internet]. (2021): 39.
- Tamimi AF, Tamimi I, Abdelaziz M, et al. Epidemiology of Malignant and Non-Malignant Primary Brain Tumors in Jordan. Neuroepidemiology 45 (2015): 100-108.
- Bauchet L, Mathieu-Daude H, Fabbro-Peray P, et al. Oncological patterns of care and outcome for 952 patients with newly diagnosed glioblastoma in 2004. Neuro-Oncology. 1 juill 12 (2010): 725-735.
- Chang SM. Patterns of Care for Adults With Newly Diagnosed Malignant Glioma. JAMA. 2 févr 293 (2005): 557.
- Omuro A. Glioblastoma and Other Malignant Gliomas: A Clinical Review. JAMA. 6 nov 310 (2013): 1842.
- Études des glioblastomes incidents de mai 2006 à mai 2007, Angers–NiceGlioblastoma incident studies from May 2006 to May 2007 in Angers and Nice, France. Neurochirurgie (2010): 499-502.
- Rasmussen BK, Hansen S, Laursen RJ, et al. Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the Danish Neuro-Oncology Registry. J Neurooncol. déc 135 (2017): 571-579.
- Yuile P, Dent O, Cook R, et al. Survival of glioblastoma patients related to presenting symptoms, brain site, and treatment variables. Journal of Clinical Neuroscience. août 13 (2006): 747-751.
- Sommerlath VN, Buergy D, Etminan N, et al. Molecular features of glioblastomas in long-term survivors compared to short-term survivors—a matched-pair analysis. Radiat Oncol. déc 17 (2022): 15.
- Combs SE, Gutwein S, Schulz-Ertner D, et al. Temozolomide Combined with Irradiation as Postoperative Treatment of Primary Glioblastoma Multiforme: Phase I/II Study. Strahlenther Onkol. juin 181 (2005): 372-377.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med. 10 mars 352 (2005): 987-996.
- Sinning M, Frelinghuysen M, Gallegos M, et al. Outcome of patients with primary glioblastoma in Chile: single-center series. ecancer [Internet]. (2021): 15.
- Chaichana KL, Parker SL, Olivi A, et al. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas: Clinical article. JNS. août 111 (2009): 282-292.
- Seystahl K, Wick W, Weller M. Therapeutic options in recurrent glioblastoma—An update. Critical Reviews in Oncology/Hematology. mars 99 (2016): 389-408.
- Liang J, Lv X, Lu C, et al. Prognostic factors of patients with Gliomas – an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer. déc 20 (2020): 35.
- Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, the extent of resection, and survival. Journal of Neurosurgery. août 95 (2001): 190-198.
- Bleeker FE, Atai NA, Lamba S, et al. The prognostic IDH1 R132 mutation is associated with reduced NADP+-dependent IDH activity in glioblastoma. Acta Neuropathol. avr 119 (2010): 487-494.
- Stummer W, van den Bent MJ, Westphal M. Cytoreductive surgery of glioblastoma as the key to successful adjuvant therapies: new arguments in an old discussion. Acta Neurochir. juin 153 (2011): 1211-1218.
- Chinot OL, Barrié M, Fuentes S, et al. Correlation Between O 6 -Methylguanine-DNA Methyltransferase and Survival in Inoperable Newly Diagnosed Glioblastoma Patients Treated With Neoadjuvant Temozolomide. JCO 25 (2007): 1470-1475.
- Rivera AL, Pelloski CE, Gilbert MR, et al. MGMT promoter methylation is predictive of response to radiotherapy and prognostic in the absence of adjuvant alkylating chemotherapy for glioblastoma. Neuro-Oncology 12 (2010): 116-121.
