Epidemiological Profile of Trisomy 21 in Senegal
Article Information
Yaay Joor Koddu Biigé DIENG1*, Seydi Abdou BA1, Djénéba Fafa CISSE2, Aminata MBAYE1, Ndèye Fatou SOW3, Guillaye DIAGNE2, Awa KANE1, Madjiguène KANE1, Grace Neema SIKULI1, Papa Moctar FAYE1, Amadou Lamine FALL1, Ousmane NDIAYE1
1Centre Hospitalier National D’Enfants Albert Royer, SN, Av. Cheikh Anta Diop, Dakar, Senegal
2Centre Hospitalier National de Pikine, Dakar, Senegal
3Hôpital Dalal Jamm, Dakar, Senegal
*Corresponding Author: Yaay Joor Koddu Biigé DIENG, National Children's Hospital Albert Royer, Dakar, Senegal.
Received: 21 January 2024; Accepted: 26 January 2024; Published: 14 February 2024
Citation: Yaay Joor Koddu Biigé DIENG, Seydi Abdou BA, Djénéba Fafa CISSE, Aminata MBAYE, Ndèye Fatou SOW, Guillaye DIAGNE, Awa KANE, Madjiguène KANE, Grace Neema SIKULI, Papa Moctar FAYE, Amadou Lamine FALL, Ousmane NDIAYE. Epidemiological Profile of Trisomy 21 in Senegal. Fortune Journal of Health Sciences. 7 (2024): 56-59.
View / Download Pdf Share at FacebookAbstract
Introduction: Trisomy 21 (T21) is the most common genetic cause of learning disabilities and congenital malformations in humans. The genetic background is well established and maternal age is the main risk factor implicated. The aim of this study was to describe the epidemiological profile of T21 in the Senegalese population.
Patients and method: This was a 7-year retrospective observational study, from November 2016 to October 2023. We considered all cases of T21 in the cohort of the genetic consultation at the Albert Royer Children's Hospital. The data collected were analysed using SPSS 21.
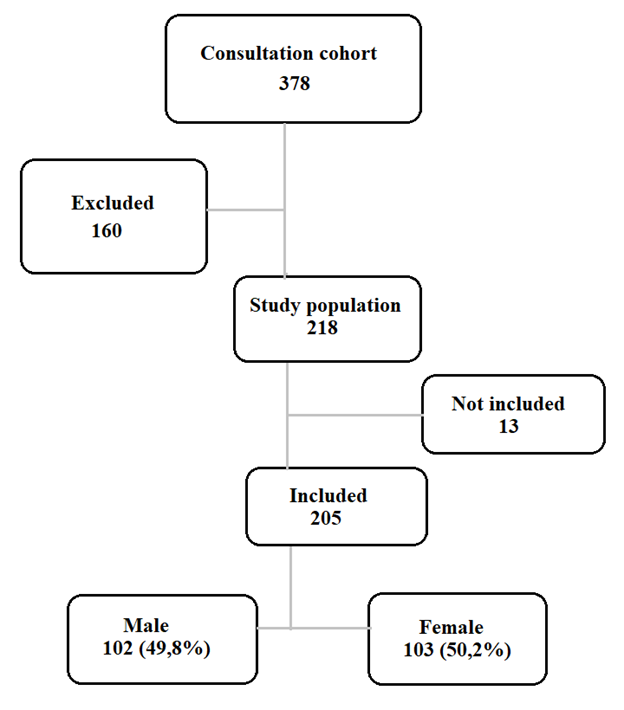
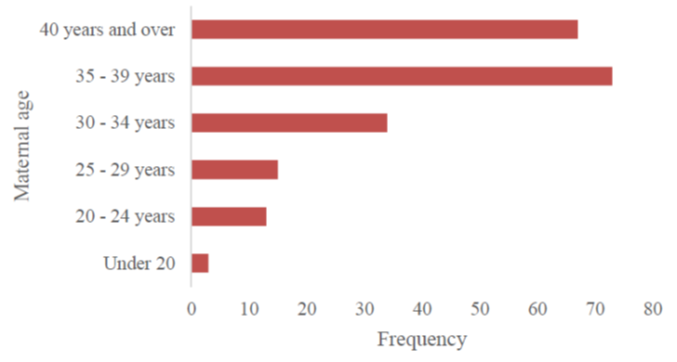
Results: We collected 218 cases of T21 among the 378 patients seen at the consultation. According to the inclusion criteria, 205 cases of patients with T21 were selected. The sex ratio was 0.99. Consanguinity was found in 17.1% of patients and T21 in 2.9%. At least one spontaneous abortion was found in 27.8% of the mothers. The median age of the mothers at conception was 37 years. The 35-39 age group was the most represented (35.6%). A deep organ malformation was found in 70.9% of cases, dominated by cardiopathies (60.9%), the most frequent of which were: atrioventricular canal (44%), atrial septal defect (21.1%), persistent ductus arteriosus (18.3%) and interventricular septal defect (15.6%).
Conclusion: Maternal age, the main risk factor identified for T21, seems to present a different profile, notably younger in our series.
Keywords
Trisomy 21; Epidemiological profile; Maternal age; Senegal
Article Details
1. Introduction
Trisomy 21 (T21) or Down's syndrome is the most common genetic cause of intellectual disability and congenital malformations in the human population. It is the most common chromosomal anomaly, with an incidence of around 1/600 newborns [1,2]. It is now more than 60 years since the genetic context was identified. The study of genetic markers has shown that T21 results in 90% of cases from an error during maternal meiosis [2,3]. Two risk factors for non-disjunction have been observed in young women: the absence of genetic recombination between homologous chromosomes and the presence of an exchange in the telomeric position [4,5]. As maternal age increases, non-disjunctions linked to pericentromeric exchanges appear [5-8]. In addition, it is well known that recurrence is greater in young mothers and that the incidence increases considerably with advanced maternal age. However, the biological basis of the effect of maternal age remains largely unknown. The biological reasons underlying this spectrum of clinical and experimental observations are still unknown and constitute major biological enigmas. Recently, it has been suggested that the situation is highly complex and depends on multifactorial characteristics. Over the last five decades, numerous studies have been devoted to the search for the origin of the common type of T21 characterized by an extra free chromosome 21 [4,8]. Until now, the only risk factor for T21 has been advanced maternal age. Based on two observations: the non-existence of major studies in this area in black African populations and the proportion of young women who are mothers of children with Down's syndrome, we conducted this study, the main objective of which is to study the epidemiological profile of maternal age and cardiac anomalies in T21 in a black African population.
2. Patients and methods
We conducted this retrospective observational study (on patient follow-up records) over a period from November 2016 to October 2023, i.e. duration of 07 years. It focused on patients seen at the genetics consultation at the Albert Royer Children's Hospital, which is the paediatric referral centre for the country and the sub-region, and at the same time the only one to offer this medical genetics consultation. This consultation is organised by appointment once a week for 45 minutes to an hour per family. We considered all cases of T21 in the consultation cohort during the study period (with or without cytogenetic confirmation). We did not include patients whose records were unusable, in particular incomplete for certain criteria such as maternal age and sex, patient age and clinical description consistent with the pathology.
Data were collected using a standardised individual form (Google forms), which collected the following information about the patient: identity, date of birth, sex, parental consanguinity; about the mother: age at the time of conception and history of abortion; and about trisomy: cytogenetic confirmation and karyotype results where applicable, associated organ malformations and type of heart disease where applicable. Data were analysed using SPSS 21.
3. Results
We identified 218 cases of T21 among the 378 patients seen at the genetic consultation, i.e. 57.7% of the cohort. According to the inclusion criteria, 205 cases of patients with T21 were selected. The sex ratio was 0.99.
In the family history, parental consanguinity was found in 17.1% of patients and T21 in 2.9%, although no cases were found in the patient's siblings.
As regards the mothers, more than a quarter (27.8%) had had at least one spontaneous abortion before the patient's conception. The median age of the mothers at the time of conception was 37, with an average of 36. The 35-39 age group was the most represented (35.6%).
When specified (for 148 patients), a deep organ malformation (cardiac, digestive, urinary, ENT) was found in 70.9%. Heart disease was the most common, accounting for 60.9% of malformations and 59% of patients. Among these cardiopathies, atrioventricular canal was the most common (44%), followed by atrial septal defect (21.1%), persistent ductus arteriosus (18.3%) and ventricular septal defect (15.6%).
In all the patients for whom cytogenetic confirmation was possible (55 = 26.8%), the karyotype found T21 in its free and homogeneous form.
4. Discussion
In our countries with very limited resources, universal health coverage is very low. For most of the population, the burden of healthcare costs still falls on patients' families. The heavy burden of mortality and the precariousness of the technical platform leave little room for certain aspects of medical practice. Antenatal screening for accessible genetic anomalies, including T21, and management of developmental delay are among the aspects that are not yet taken into account in health priorities.
Our series represents more than half of the medical genetics consultation cohort, with 57.7% of cases. Although known to be the most common viable chromosomal anomaly in humans, this frequency alone does not seem sufficient to assess the extent of the problem in our setting. In fact, we note on the one hand the existence of other specialised consultations in the facility, the absence of a single, centralised patient file, and the cost of care borne by families quite often limit the multidisciplinary care on offer. As a result, many patients are only seen in the organ speciality or specialities concerned, except in the event of a therapeutic impasse associated with developmental delay, or in the event of a family case. Furthermore, our series, compiled from the cohort of programmed outpatients, does not take into account cases of T21 who died during hospitalisation or before being transferred to our facility. Although little is known about its frequency in our countries, it remains much higher than the figures in the West [2,3,10] due to the timidity of antenatal screening, which is not systematic, and also to the non-existence of abortion, which is illegal in our practice except where there is a vital maternal risk.
In our series, we noted a balanced sex ratio of 0.99, whereas the main trend in the literature is towards an imbalance in favour of boys [8,9], with the exception of the foetopathological series following abortions for T21, where a slight female predominance was found [10]. In fact, meta-analysis of 55 publications providing the sex ratio, this male predominance was found in all studies with a high rate of cytogenetic confirmation of more than 30%, unlike our series [9].
Nearly one pregnancy in six (17.1%) in our series was the result of a consanguineous union, but no case of trisomy was found in the patients' siblings. This is supported by cytogenetics, which found the accidental form of T21 (non-hereditary) in all patients tested, as widely reported in the literature [3,6,7].
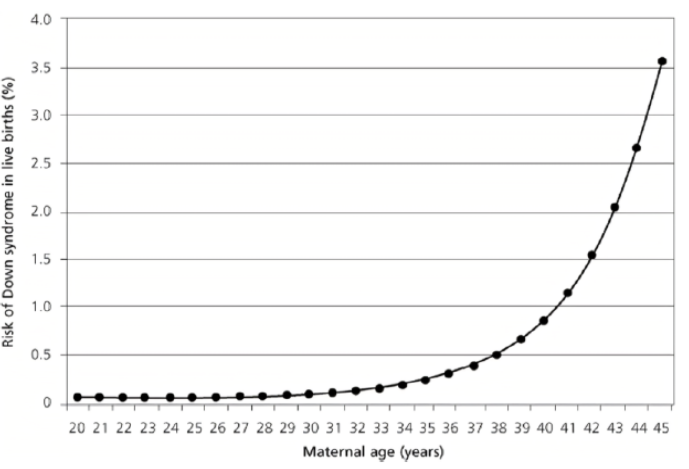
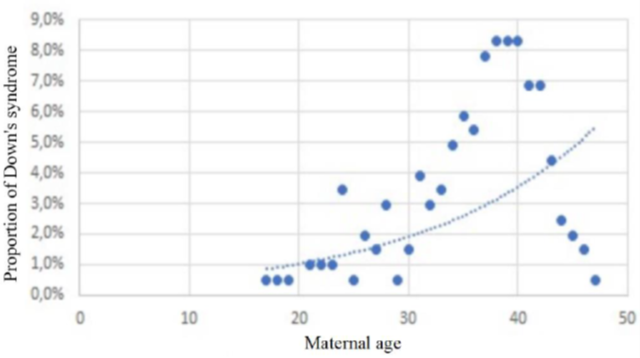
More than a quarter (27.8%) of the mothers had had at least one spontaneous abortion prior to conception. The median age of the mothers at the time of conception was 37 years, which is close to the average in our series (36 years). The 35-39 age group, the most represented (35.6%), cannot be correlated with fertility in this population. In fact, the Senegal Demographic and Health Survey for 2023 reports a peak in fertility in the 25-29 age group [11]. This discrepancy helps to maintain advanced maternal age as a strong predisposing factor for the occurrence of T21. However, as correlated by Hetch and Hook (Figure 3) [3,12], this theoretical exponential evolution observed in large samples of Caucasian populations does not maintain the same speed in Figure 4, which is representative of our series; This is probably due to the drastic drop in the birth rate over the age of 39 in Senegal as a whole [11], whereas in France, for example, the overall prevalence has risen from 14/10,000 in 1978 to 23/10,000 live births in 2005, at the same time as the average age of mothers has increased from 26 to 30 over the study period [3].
Similarly, it has already been shown that 20% of cases of T21 are unrelated to maternal age. By modelling the prevalence in the general population solely on the risk associated with maternal age, an approximation bias is introduced by applying a risk model to the entire population in only 80% of cases. This approximation is made in all existing publications on the subject using this methodology [3, 12].
In the search for organ malformations (cardiac, digestive, urinary, ENT), at least one was found in 70.9% of cases. As reported in the literature [13-15], cardiac disease was the most frequent in our series; similarly, in terms of the heart, atrioventricular canal remained the most frequent cardiac defect in our series (44%), where we also reported a high proportion of persistent ductus arteriosus (18.3%), as found in the large American series [9], but not in Europe [14,15].
5. Conclusion
Maternal age, the main risk factor identified for T21, seems to present a different profile, notably younger in our series drawn from a black African population.
References
- Hook EB. The impact of aneuploidy upon public health: mortality and morbidity associated with human chromosome abnormalities. In: Dellarco VL, Voytek PE, Hollaender A, eds. Aneuploidy: etiology and mechanisms (proceedings of a symposium on aneuploidy: etiology and mechanisms, helds. March 25-29, 1985, in Washington, DC). Basic Life Sciences 36 (1985): 7-33.
- Turleau C, Vekemans M. Trisomie 21: 50 ans entre médecine et science. Med Sci (Paris). 26 (2010): 267-272.
- Rousseau T, Amar E, Ferdynus C, et al. Variations de prévalence de la trisomie 21 en population française entre 1978 et 2005. Journal de Gynecologie Obstetrique et Biologie de la Reproduction 39 (2010): 290-229
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum Genet 70 (1985): 11-17.
- Lamb NE, Freeman SB, Savage-Austin A, et al. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nature Genet 14 (1996): 400-405.
- Khoshnood B, De Vigan C, Vodovar V, et al. A population-based evaluation of the impact of antenatal screening for Down’s syndrome in France, 1981-2000. Br J Obstet Gynaecol 111: 485-490.
- Gaulden ME. Maternal age effect: the enigma of Down syndrome and other trisomic conditions. Mutat Res 296 (1992): 69-88.
- Penrose L. The relative effects of paternal and maternal age in mongolism. J Genet 27 (1933): 219-224.
- Kovaleva NV. Sex ratio in Down syndrome. Tsitologiia i Genetika. 36 (2002): 54-69.
- Grangé G, Tantau J, Acuna N, et al. Fréquence des malformations associées à la trisomie 21. J Gynecol Obstet Biol Reprod 35 (2006): 477-482.
- ANSD et ICF. Enquête Démographique et de Santé du Sénégal 2023: Rapport des indicateurs-clés. Dakar, Sénégal et Rockville, Maryland, USA : ANSD et ICF (2023).
- Hecht CA, Hook EB. The imprecision in rates of Down’s syndrome by 1-year maternal age intervals: a critical analysis of rates used in biochemical screening. Prenat Diagn 14 (1994): 729-738.
- Torfs CP, Christianson RE. Anomalies in Down syndrome individuals in a large population-based registry. Am J Med Genet 77 (1998): 431-438.
- Hyett J, Moscoso G, Nicolaides K. Abnormalities of the heart and great arteries in first trimester chromosomally abnormal fetuses. Am J Med Genet 69 (1997): 207-216.
- Kallen B, Mastroiacovo P, Robert E. Major congenital malformations in Down syndrome. Am J Med Genet 65 (1996): 160-166.