Epicardial ligation of the left atrial appendage: Long-term results in patients with high stroke and bleeding risk
Article Information
Karin Nentwich1,3*, Nuki Kazaishvili1, Elena Ene1, Artur Berkovitz1, Julian Müller5, Sebastian Barth2,3, Thomas Deneke4
1Department of Invasive electrophysiology, Campus Bad Neustadt, 97616 Bad Neustadt/Saale, Germany
2Department of Cardiology and imaging, Campus Bad Neustadt, 97616 Bad Neustadt/Saale, Germany
3Department of Cardiology, Philipps-University Marburg, 35043 Marburg, Germany
4Department of Cardiology, Clinic Nuernberg Campus south, 90471 Nuernberg, Germany
5Department of Cardiology, Heart center Bad Krozingen, 79189 Bad Krozingen, Germany
*Corresponding Author: Karin Nentwich, Department of Invasive electrophysiology, Campus Bad Neustadt, 97616 Bad Neustadt/Saale, Germany.
Received: 13 April 2025; Accepted: 21 April 2025; Published: 27 June 2025
Citation: Karin Nentwich, Nuki Kazaishvili, Elena Ene, Artur Berkovitz, Julian Müller, Sebastian Barth, Thomas Deneke. Epicardial ligation of the left atrial appendage: Long-term results in patients with high stroke and bleeding risk. Journal of Surgery and Research. 8 (2025): 299-304
View / Download Pdf Share at FacebookAbstract
Introduction: Left atrial appendage (LAA) closure may be performed in atrial fibrillation (AF) patients with contraindications for anticoagulation and high stroke risk. Multiple trials have proven non inferiority of endocardial LAA occlusion in terms of total stroke events versus oral anticoagulation, while epicardial LAA exclusion at the time of open heart surgery has led to a decrease in embolic stroke events. In our center percutaneous LAA ligation using the Lariat device is the primary technique for LAA closure since long-term antithrombotic medication is not needed. Long-term safety and efficacy of percutaneous epicardial ligation appears favorable, but there is a lack of evidence of stroke prevention. We present follow-up data up to 6 years of a large single center group of AF patients with contraindications to oral anticoagulation (OAC) therapy undergoing the LARIAT procedure.
Method: 125 patients out of 211 screened patients (59%) were eligible for percutaneous epicardial ligation between December 2015 and August 2023. Standardized follow up (FUP) with transesophageal echo (TOE) was performed at 6 weeks, 12 weeks and 12 months. Clinical (death, hemorrhagic stroke, embolic event, readministration of anticoagulation) FUP was performed every 12 months thereafter. If possible, TOE data after 12 months were documented.
Results: LAA ligation using the LARIAT system was successful in 118 (50,4% male) of 125 patients (93%). Mean age was 74.3 years, mean CHA2DS2VASC score 4,1, mean HASBLED score was 3. Procedure associated major complication was recorded in 1 patient with LAA laceration and need for surgical revision, procedure associated minor complications were recorded in 3 patients. 6 weeks FUP was available in 92 patients, 12 weeks FUP in 66 patients and 12 months FUP in 40 patients. 6 patients developed pericarditis, 7 patients developed devicerelated thrombus (1 patient had a stroke), 110 patients had complete LAA closure and 7 patients had a documented gap of a mean of 2 mm (all ≤ 3 mm). During long term clinical FUP after 12 months (mean 37,7 ± 18 months) 20 patients died (6,7% per year), 4 patients had an embolic event (1 peripheral embolic event, 3 strokes) meaning a stroke rate of 0,98%/y. In 13 patients anticoagulation was restarted for different reasons. In 74 patients T0E was available mean 32 months after ligation documenting no late development of thrombus or gap.
Conclusion: Long-term follow-up of percutaneous epicardial ligation of the LAA shows high LAA closure efficacy and safety. No late ligationassociated complications were noted, no new thrombus or gap formation could be detected after 3 months. Percutaneous epicardial ligation of the LAA results in an absolute risk reduction of 3%/y and a relative risk reduction of 76 % for a CHA2DS2VASC 4 AF population.
Keywords
Atrial fibrillation, Transesophageal echocardiography, Left atrial appendage
Article Details
Abbreviations
AF - Atrial fibrillation
EUMDR – European Medical Device Regulation
FUP – Follow - Up
ICU – intensive care unit
LAA – left atrial appendage
NOAK – new oral anticoagulants
OAC – oral anticoagulants
TOE – transesophageal echocardiography
Introduction
Atrial fibrillation is common in elderly patients with a rising incidence in the western population. It is associated with an increased risk for embolic events and anticoagulation has been proven to reduce thrombus formation in the left atrial appendage (LAA) thereby preventing stroke and embolic events [1]. First observations of possible benefits of occlusion of the LAA were published 2005 [2]. Meanwhile percutaneous occlusion of the left atrial appendage has been established in selected patient cohorts with contraindication for anticoagulation and high stroke risk. Non inferiority to warfarin for total stroke (embolic and hemorrhagic) has been shown for endocardial occlusion in several large studies [3-5]. Epicardial LAA exclusion at the time of open heart surgery has demonstrated significant decrease in embolic strokes and mortality [6,7]. Percutaneous epicardial ligation of the LAA using the LARIAT System (AtricureÒ) was FDA approved in 2014 and received EUMDR approval in 2023 with the intended clinical benefit of reduction in thromboembolic events when used in patients with atrial fibrillation who are intolerant to or contraindicated for long-term oral anticoagulation therapy [8]. After effective LAA ligation any form of anticoagulation or antithrombotic treatment is dispensable as no device is implanted endocardially. Epicardial ligation using the LARIAT system is the primary approach for LAA occlusion in our institution, as several patients do not tolerate any form of anticoagulation due to contraindications related to their underlying disease resulting in recurrent bleedings. We present our TOE and clinical long-term FUP data after percutaneous epicardial LAA ligation in an elderly, high embolic stroke risk group of AF patients.
Method
Patient selection
From December 2015 until July 2023 211 patients with atrial fibrillation and CHADSVASC >1 were screened for epicardial ligation with an absolute contraindication to oral anticoagulation like bleeding complication, arteriovenous malformations, amyloid angiopathy or with electrical isolation of the LAA after multiple left atrial ablations. All patients received a cardiac CT scan for evaluation of morphology and size of the LAA. 125 were eligible for epicardial ligation (59% of screened patients. Of the patients not eligible for LAA ligation 63 patients (see table 1) were treated with a percutaneous endocardial device and 23 patients refused any LAA occlusion.
|
Clinical contraindications n=41 (66%) |
Anatomical contraindications n=22 (34%) |
|
History of cardiac surgery 37 (58 %) |
Superiorly or backwards orientated LAA with the anterior lobe behind the pulmonary trunk 19 (30%) |
|
Renal failure with dialysis 6 (9,5%) |
Left rotated heart 0 |
|
Pectus excavates 0 |
LAA width > 50 mm 2 |
|
History of thoracic radiation 0 |
Multiple lobes with different orientations and wider distance than 50 mm 0 |
|
NYHA IV classification 0 |
Adipositas BMI 1 (1,6%) |
|
Planned cardiac surgery with surgical LAA resection 2 (3%) |
Thrombus in LAA 1 (1,6 %) |
|
Adhesions 2 (3%) |
Table 1: Screening failures for epicardial ligation of the LAA with LARIAT in our cohort.
Procedure
After having given informed consent the patients were put under analgosedation with continuous propofol infusion combined with boli of piritramide. After puncturing the pericardium and introducing a soft tip in the pericardial space, transseptal puncture is performed under TOE guidance [9]. Identifying the anterior lobe in the TOE combined with the angiogram of the LAA an endocardial wire with a magnet at its end is placed in the LAA anterior lobe. Via epicardial access a second magnet attached wire is introduced and connected to the endocardial placed magnet. Over the connected magnet wires the snare is advanced over the LAA and positioned at the LAA neck. The optimal closing position is confirmed by angiogram, balloon insufflation and TOE guidance. After closing down of the snare complete capture of the LAA is confirmed by TOE. The suture is released from the snare. After 2 tightenings of the suture with a tension device, the result of the ligation is confirmed by TOE for central gap or missed lobes. The suture is cut and a pigtail drain is placed in the pericardial space. The patient is monitored in the ICU for 24 hours. For prevention of pericarditis treatment with colchicine was started before the procedure and continued for 6 weeks. All patients were discharged without any form of anticoagulation, except the ones with known coronary heart disease being treated with 100 mg acetyl acid/d.
Follow up
Transesophageal FUP was scheduled at 6 and 12 weeks and 12 months. Clinical FUP (death, embolic event/stroke or readministration of anticoagulation) was documented during all TOE follow-up sessions and once every 12 months afterwards. The data are expressed as mean ± standard deviation (SD) for continuous variables or as numbers and percentages for categorical variables. Kaplan Meier Curve was calculated with SPSS 2023.
Results
Procedure
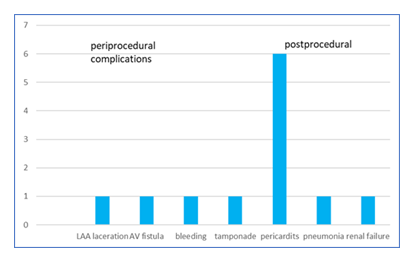
Percutaneous epicardial ligation using the Lariat system was attempted in 125 patients (64 males, 61 females) and successfully completed in 118 patients (94 %). Mean age was 74,3 ± 9 y (range 40-87y), mean CHA2DS2-VASc score was 4,1 (range 2-6), mean HAS-BLED score 3,3. More details of the patients’ characteristics see in table 2. Mean procedure time was 82,3 ± 24 min, mean radiation time 17 ± 6,8 min. 4 patients showed pericardial adhesions preventing epicardial ligation (all terminal renal failure under dialysis), 1 patient developed a LAA thrombus during the procedure, in 1 patient epicardial puncture failed due to extreme obesity, 1 patient was treated with a hybrid approach due to a very large LAA with epicardial ligation of the large anterior lobe and implantation of an endocardial device in the residual posterior lobe. Acute procedural (0-5 day) adverse events occurred in 12 patients (1 major complication (0.9%) occurred with laceration of the LAA requiring emergency surgical closure of the LAA with Atriclip and 11 minor complications (9,4%) figure 1).
|
Patients’ characteristics |
n = number (%) |
|
Gender (male) |
59 (50,4 %) |
|
Mean age (y) |
74,3 ± 9 |
|
CHADSVASC score |
4,1 |
|
HASBLED score |
3,3 |
|
Ejection fraction (%) |
61 ± 9 |
|
Coronary heart disease |
44 (37,6 %) |
|
Prior pulmonary vein isolation |
11 (9,4 %) |
|
Prior stroke |
27 (23 %) |
Table 2: Patients’ characteristics.
TOE and clinical FUP
6 weeks TOE FUP was available in 93 patients (79%). In 7 patients (7,5%) a central gap of 2 mm median was detected, 5 of them at the beginning of the learning curve. An additional 6 patients with no evidence of LA to LAA communications had a documented thrombus and were retreated with anticoagulation. 2 of them showed spontaneous contrast in the TOE. 1 patient presented thrombus on a central gap of 2 mm. 1 patient developed a pericardial effusion and was put on steroids.
3 patients died in the first 6 weeks (2 patients due to cerebral bleeding 3 months after the initial bleeding, 1 patient due to COVID).
12 weeks TOE FUP was available 65 patients (55%). Central gaps were still present in 4 patients (1 patient developed a stroke with a gap of 2 mm) and in 3 patients the leakage was closed. 1 additional patient developed a thrombus on the 2 mm central leak.
4 patients had documented thrombus: 4 thrombi resolved completely under anticoagulation and 3 thrombi partially, 1 patient denied control-TOE. 1 patient who missed the 6-week FUP showed thrombus in TOE at 12 weeks.
2 patients died (one patient with cerebral bleeding, one patient with unknown circumstances).
12 months TOE FUP was available in 40 patients (33%) with a central gap detected in 1 patient, 4 previous gaps were closed and in 2 patients no TOE FUP is available.
In 2 patients with a previous thrombus, thrombus could be re-detected (after withdrawal of the reinitiated anticoagulation for thrombus detected at 6 weeks FUP (resolved at 12 weeks FUP). No new thrombus formation could be detected at 12 months FUP, 3 patients under NOAC showed no recurrence of thrombus.
2 patients died, one due to worsening heart failure and one due to sepsis.
Long-term TOE FUP
In the long-term FUP TOE (performed for different reasons after mean 32 months) was available in 74 patients: in 73 cases LAA closure was complete without apparent thrombus or gap, including the 7 patients with a previous detected gap at the 6 weeks TOE. No new gap or thrombus formation could be detected.
Long-term clinical follow-up
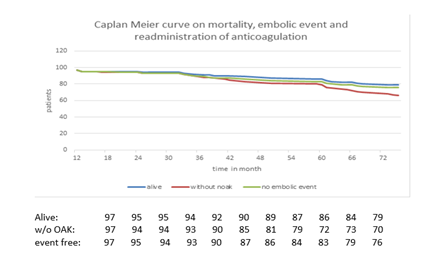
99 patients underwent long-term FUP of 38±18 months (13 to 74 months). Non-procedural related deaths occurred in 20 patients (19,8%) producing a mortality rate of 20,2%, a mean rate of 6,5% per year (figure 2).
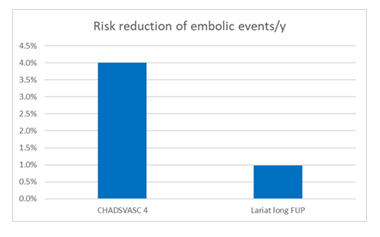
3 patients developed an embolic event, resulting in an incidence of 0,98% per year (figure 3) and an absolute risk reduction of 3,02%/y. 2 patients after stroke TEE showed a good long-term result after epicardial ligation. 1 patient with a peripheral embolic event had documented thrombus on TOE.
13 patients were retreated with anticoagulation therapy due to different reasons like acute coronary heart syndromes, valve implantation, stroke etc. (summarized in figure 2).
Discussion
Our data show an acute success rate of LAA closure of 94% with a low acute major complication rate of 0,8% in a high-risk patient cohort. Pericarditis as a minor complication occurred in 4%. Short-term FUP revealed 7.5% thrombus and 7.5% gaps associated with a low clinical event rate (1 stroke in a patient with a gap of 2 mm). There were no new thrombus formation or gaps detected after 6 weeks. In our long-term FUP on TOE and clinical data of patients undergoing epicardial LAA exclusion we observed an event rate of 0,98% per year. According to CHAsDS2VASC score of 4 with an estimated embolic event rate of 4% per year these data demonstrate a relative risk reduction of embolic events of 76%. Non-procedural death occurred in 20 patients (19.8%) meaning a mortality rate of 6,5% per year (see table 3).
In the 5-years data of Prevail and Protect AF trial 49 events (stroke and systemic embolism) occurred (6,6%, annual rate of 1,3%) [10]. The latest results of Watchman flex in 1 year showed an 1 year mortality of 10, 8% and stroke rate of 1%/y [11]. In a cohort of 1047 patients treated with the Amplatzer Cardiac Plug the observed thromboembolism rate was 2,3% [12]. However, the latest results of the LAAOS III trial [6] documented an annual rate of embolism of 1,2% in patients with surgical LAA occlusion and anticoagulation in a cohort with indication for cardiac surgery, but no indication for LAA occlusion. Our cohort has a higher CHAsDS2VASC score than Protect AF (CHAsDS2VASC score 3,5) and higher age than in the trials Protect AF (72 y) and LAAOS III (71 y). It can be concluded our long-term results after epicardial ligation with an embolic event rate of 0,98% demonstrates a high efficacy in an elderly, high risk group.
The latest trends for stroke prevention are half dose NOAC combined with LAA occlusion. [6,13]. It remains unclear whether half NOAC would be applicable to our patient cohort with very strict contraindications for anticoagulation like cerebral amyloid angiopathy or angiodysplasia as the HASBLED score in our cohort of 3.3 is higher than in each trial mentioned before. In contrast the possibility of abstinence of any anticoagulation after epicardial ligation of the LAA is the strength of this approach in these high risk patients.
Epicardial ligation could be attempted successfully in 94% after careful evaluation with a CT scan. These results are comparable with Protect AF trial (91%), Prevail trial (95,1%), NCDR registry (93%) and the Flex trial (99%). [3,4,11,14]. Beside the common contraindications for epicardial ligation [15] we identified certain conditions like history of dialysis, extreme obesity, or thrombus formation that may preclude epicardial ligation. In 4 patients the procedure has to be aborted due to adhesions, all patients suffered from terminal renal failure with chronic dialysis.
In all patients acute complete closure of the LAA could be achieved with no gap detected with intraprocedural TOE. However, during early FUP 7 patients developed a central gap (5 of them during learning curve with a lower tension power in very fragile patients as recommended) from 1 mm to 3 mm (median 2 mm). 1 patient developed symptomatic stroke, and 1 patient developed a thrombus, that resolved as well as the gap under anticoagulation, 3 gaps closed spontaneously. In the 1 y FUP 1 residual gap could be detected, no new central gap could have been detected over the whole FUP interval.
The corresponding complication after endocardial LAA occlusion is the meaning of peri device leakages (PDL). Higher PDLs are observed for the Watchman device compared to the Amulet device. [16,17], their clinical meaning had been assumptive until recently. Successful occlusion had been defined arbitrarily with a PDL< 3 mm until < 5 mm [3,4,18,19]. In the NCDR registry the gap rate was 1,8 %, defined > 5 mm, any gap was detectable in 25% of patients [14]. Latest data show association of any gap size with stroke [20,21], so even smaller gaps might not be benign. A comparison between endocardial LAA occlusion with Watchman and epicardial ligation with Lariat showed significant lower incidence of leakages in the lariat group [22]. This confirms findings of another study with endothelization of gaps after epicardial ligation with the LARIAT system in most patients [23], as well as it can be reported in our study. Central leaks are easier to be closed with plug devices in contrast to the eccentric located gaps after endocardial closure. [24-26]. It can be summarized acute and long-term efficacy of epicardial ligation is comparable to endocardial device implantation in respect to closure- and event rate.
Regarding to safety aspects we had 1 major complication with laceration of the LAA requiring surgical closure of the LAA (0,9%) and 2 minor complications (AV fistula without intervention and prolonged bleeding from the subxiphoid puncture site treated with a deep suture) periprocedural. We observed no complication related to the epicardial puncture. Lakkireddy et al. [27] reported a major complication rate of 10% reducing to a rate of 2,1% by changing to the micropuncture needle. In Protect AF acute complication rate was 8%, going down to 2,2% in Prevail. The ACP trial [12] reports of 4,97% major complications.
Until discharge 8 patients developed pericarditis requiring steroids (4%), in 1 patient the pericardial drain clotted requiring change of the pigtail catheter, 1 patient developed a pneumonia and 1 patient showed up with decline in renal function due to colchicine therapy. The incidence of pericarditis could be reduced by starting colchicine therapy even before the procedure to 1,58% [27]. This could not be achieved in our cohort mostly due to incompliance of the patients because of side effects. By changing early to a low dose steroid regime with progressive dose reduction we could significantly reduce the incidence of pericarditis (tables 3,4).
Thrombus formation is also of major concern. The incidence of thrombus in our cohort is high at 7,6%, all detected at the 6 weeks FUP TOE, with only 1% in the long-term interval in a patient who missed his initial FUP. There were no late thrombus detected in patients with no thrombus detected at the 6 week TOE and complete LAA closure. All patients were discharged without any anticoagulation except the ones with coronary heart disease. They were put on 100 mg ASA. Though the rate is higher compared to other trials [22,28], clinical embolic complication is acceptable with 1 stroke after 12 weeks (0,85%). The incidence of DRT with endocardial devices has been reported to be 3,8 to 7,2% [29,30]. Its clinical association with embolic events is well established [31]. Identifying risk factors for DRT is important for stroke prevention. Several risk factors had been identified for the endocardial devices like clinical characteristics (age, prior stroke, coagulopathy, low ejection fraction, renal failure, vascular diseases, permanent atrial fibrillation, low velocity in the LAA), procedural factors (LAA diameter, implant depth, procedural complications) and post-procedure therapy (antiplatelets, DOAC) [31,32]. Risk factors for percutaneous epicardial ligation have not been identified yet. Some of the risk factors for endocardial devices might also be relevant for the Lariat system like LA size or presence of smog, postprocedural anticoagulation regime (see table 5). In the long-term FUP no new thrombus formation could be documented after 6 weeks. In contrast new DRT development after endocardial LAA closure can be detected in the longer FUP (DRT 0,8% after 6 weeks, 1,8% after 6 months and 1,7% after 1 year) [33]. In our cohort female gender, high CHAsDS2VASC and higher age seem to be associated with higher risk for thrombus formation. Its clinical meaning has to be evaluated as at least in this small cohort no embolic event occurred. For epicardial ligation it seems to be crucial to identify thrombus in the very early state for prevention of embolic events.
|
Procedures’ characteristics |
n = number (%) |
|
Mean procedure time (min) |
82,3 ± 24 |
|
Mean radiation time (min) |
17 ± 6,8 |
|
Major complication |
1 (0,8%) |
|
Minor complication |
2 (1,6%) |
|
Complication during first 12 h |
1 (0,8%) |
|
Complication until discharge |
8 (6,4%) |
Table 3: Procedures characteristics.
|
6 weeks FUP |
12 weeks FUP |
12 months FUP |
13-75 months FUP |
|
|
n=93 |
n=65 |
n = 39 |
n=99 |
|
|
Gaps |
7 (7,5%) |
4 (7,2 %)* |
1 (2,5 %)* |
0* |
|
Thrombus |
7 (7,5 %) |
3 (4,6 %)* |
2 (5,12 %)* |
1 (1 %)* |
|
Death |
3 (3,2 %) |
2 (2,1 %) |
2 (5,12 %) |
20 (5%) |
|
Embolic event |
0 |
1 (1,5 %) |
0 |
3 (3%) |
* no new thrombus or gap detected.
Table 4: Findings depending on the FUP timing
|
Patient |
Age (y) |
Gender |
CHAsDS2VASC |
Other RF |
Thrombus resolved |
Terminal anticoagulation regime |
|
Patient 1 |
84 |
F |
5 |
Gap 2 mm |
+ |
0 |
|
Patient 2 |
72 |
F |
4 |
Smog |
n.a. |
NOAK |
|
Patient 3 |
76 |
F |
5 |
Lupus AC + |
+ |
NOAK |
|
Patient 4 |
81 |
M |
4 |
- |
+ |
ASA |
|
Patient 5 |
80 |
F |
6 |
Gap 2 mm |
+ |
ASA |
|
Patient 6 |
68 |
F |
4 |
Smog |
+ |
0 |
|
Patient 7 |
83 |
F |
5 |
- |
+ |
NOAK |
|
Patient 8 |
69 |
F |
3 |
Long PD |
+ |
0 |
Table 5: Characteristics of patients with thrombusformation (AC anticoagulans, PD procedure duration).
Limitations
This observational study is a retrospective analysis of data in a real-world scenario with all its limitations due to the compliance of the patients like missed FUPs or medication intake or lack of motivation for additional FUPs. Some patients needed further cardiologic treatment like valve repair, acute coronary syndromes or cardiac surgery with change of the anticoagulation regime. Some patients took part in heart failure programs and received special heart care and with maybe better outcomes.
Conclusion
This study demonstrates high LAA closure efficacy and safety of epicardial ligation with LARIAT with no development of new gaps or DRTs at 12 months or later, including a high rate of endothelization of gaps and resolution of thrombus. Close FUP in the first 12 weeks is essential for driving an optimal anticoagulation regimen. Effective risk reduction for stroke and embolic event can be noted over more than 5 years especially in patients with very high bleeding risk and high stroke risk.
Ethics
This study is a retrospective clinical study that had been approved by our local ethics committee. It follows the ethical standards of the Declaration of Helsinki from 1964 and later amendments. No specific national laws had been involved.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Van Dellen D, Burnapp L, Citterio F, et al. Pre-emptive live donor kidney transplantation-moving barriers to opportunities: An ethical, legal and psychological aspects of organ transplantation view. World J Transplant 11 (2021): 88-98.
- Pavlakis M, Kher A. Pre-emptive kidney transplantation to improve survival in patients with type 1 diabetes and imminent risk of ESRD. Semin Nephrol 32 (2012): 505-511.
- Nashan B, Abbud-Filho M, Citterio F. Prediction, prevention, and management of delayed graft function: where are we now? Clin Transplant 30 (2016): 1198-1208.
- Kim DW, Tsapepas D, King KL, et al. Financial impact of delayed graft function in kidney transplantation. Clin Transplant 34 (2020): e14022.
- Helanterä I, Mengel M. Revisiting acute T cell-mediated rejection in kidney allografts. Am J Transplant 22 (2022): 681-682.
- Huh J, Baines L, Talbot D, et al. Severe anti-thymocyte globulin-induced cytokine release syndrome in a renal transplant patient. Anaesth Rep 9 (2021): 16-19.
- Knaus HA, Rottner T, Baumann CK, et al. Cytokine Release Syndrome during Antithymocyte Globulin/Anti-T Lymphocyte Globulin Serotherapy for Graft-versus-Host Disease Prophylaxis before Allogeneic Hematopoietic Stem Cell Transplantation: Incidence and Early Clinical Impact According to American Society of Transplantation and Cellular Therapy Grading Criteria. Transplant Cell Ther 28 (2022): 260.e1-260.e9
- Boomer JS, To K, Chang KC, et al. Immunosuppression in patients who die of sepsis and multiple organ failure. JAMA 306 (2011): 2594-2605.
- Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med 383 (2020): 2255-2273.
- Jarczak D, Nierhaus A. Cytokine Storm-Definition, Causes, and Implications. Int J Mol Sci 23 (2022): 11740.
- Monard C, Abraham P, Schneider A, et al. New Targets for Extracorporeal Blood Purification Therapies in Sepsis. Blood Purif 52 (2023): 1-7.
- Moriyama K, Nishida O. Targeting Cytokines, Pathogen-Associated Molecular Patterns, and Damage-Associated Molecular Patterns in Sepsis via Blood Purification. Int J Mol Sci 22 (2021): 8882.
- Mitzner S, Kogelmann K, Ince C, et al. Adjunctive Hemoadsorption Therapy with CytoSorb in Patients with Septic/Vasoplegic Shock: A Best Practice Consensus Statement. J Clin Med 12 (2023): 7199.
- El Karoui K, Fervenza FC, De Vriese AS. Treatment of IgA Nephropathy: A Rapidly Evolving Field. J Am Soc Nephrol 35 (2024): 103-116.
- Becker S, Lang H, Vollmer Barbosa C, et al. Efficacy of CytoSorb®: a systematic review and meta-analysis. Crit Care 27 (2023): 215.
- Klinkmann G, Koball S, Reuter DA, et al. Hemoperfusion with CytoSorb®: Current Knowledge on Patient Selection, Timing, and Dosing. Contrib Nephrol 200 (2023): 17-24.
- Wang X, Guo Z, Chai Y, et al. Application Prospect of the SOFA Score and Related Modification Research Progress in Sepsis. J Clin Med 12 (2023): 3493.
- Acharya S, Lama S, Kanigicherla DA. Anti-thymocyte globulin for treatment of T-cell-mediated allograft rejection. World J Transplant 13 (2023): 299-308.
- Friesecke S, Stecher SS, Gross S, et al. Extracorporeal cytokine elimination as rescue therapy in refractory septic shock: a prospective single-center study. J Artif Organs 20 (2017): 252-259.
- Nakamura K, Okazaki T, Tampo A, et al. The polymyxin-B direct hemoperfusion OPTimal Initiation timing with Catecholamine PMX-OPTIC study: A multicenter retrospective observational study. Artif Organs 18 (2024).