Endovascular Approach to Ruptured Sphenopalatine Artery: A Case Report and Literature Review
Article Information
Ram Saha1, Abu Bakar Siddik2, Masum Rahman3, Samar Ikram3, Cecile Riviere-cazaux4, Abdullah Alamgir5, Badrul Alam Mondal6, Quazi Deen Mohammad6, Sirajee Shafiqul Islam7*
1Department of Neurology, Virginia Commonwealth University, VA, USA
2Department of Pain Medicine, Mayo Clinic, Jacksonville, Florida, USA
3Department of Neurological Surgery, Mayo Clinic, MN, USA
4Mayo Clinic Alix school of medicine, Rochester, MN, USA
5Department of Neurosurgery, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh
6Department of Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh
7Department of Interventional Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh
*Corresponding Author: Dr. Sirajee Shafiqul Islam, Associate Professor, Department of Interventional Neurology, National Institute of Neurosciences & Hospital, Dhaka, Bangladesh
Received: 14 May 2021; Accepted: 25 May 2021; Published: 31 May 2021
Citation:
Ram Saha, Abu Bakar Siddik, Masum Rahman, Samar Ikram, Cecile Riviere-cazaux, Abdullah Alamgir, Badrul Alam Mondal, Quazi Deen Mohammad, Sirajee Shafiqul Islam. Endovascular Approach to Ruptured Sphenopalatine Artery: A Case Report and Literature Review. Journal of Spine Research and Surgery 3 (2021): 037-044.
View / Download Pdf Share at FacebookAbstract
Epistaxis is a rare complication following the endonasal approach of skull base surgery. Conservative methods like anterior and posterior nasal packing can be useful, but when these fail, a neuro-interventional technique can be used as a last-resort measure in cases of severe bleeding. The authors identify a 22-year-old female patient with recurrent epistaxis following resection of skull-base chordoma through an endonasal approach. An endovascular catheter digital subtraction angiogram identified the cause of epistaxis as a rupture of the left sphenopalatine artery branch of the left external carotid artery. A large dissecting aneurysm in the right intracerebral artery was also incidentally found. The unique co-occurrence of vascular problems was successfully managed by neuro-interventional techniques.
Keywords
Epistaxis, Sphenopalatine Artery, Dissecting Aneurysm, Endovascular, Angiogram
Epistaxis articles Epistaxis Research articles Epistaxis review articles Epistaxis PubMed articles Epistaxis PubMed Central articles Epistaxis 2023 articles Epistaxis 2024 articles Epistaxis Scopus articles Epistaxis impact factor journals Epistaxis Scopus journals Epistaxis PubMed journals Epistaxis medical journals Epistaxis free journals Epistaxis best journals Epistaxis top journals Epistaxis free medical journals Epistaxis famous journals Epistaxis Google Scholar indexed journals Sphenopalatine Artery articles Sphenopalatine Artery Research articles Sphenopalatine Artery review articles Sphenopalatine Artery PubMed articles Sphenopalatine Artery PubMed Central articles Sphenopalatine Artery 2023 articles Sphenopalatine Artery 2024 articles Sphenopalatine Artery Scopus articles Sphenopalatine Artery impact factor journals Sphenopalatine Artery Scopus journals Sphenopalatine Artery PubMed journals Sphenopalatine Artery medical journals Sphenopalatine Artery free journals Sphenopalatine Artery best journals Sphenopalatine Artery top journals Sphenopalatine Artery free medical journals Sphenopalatine Artery famous journals Sphenopalatine Artery Google Scholar indexed journals Dissecting Aneurysm articles Dissecting Aneurysm Research articles Dissecting Aneurysm review articles Dissecting Aneurysm PubMed articles Dissecting Aneurysm PubMed Central articles Dissecting Aneurysm 2023 articles Dissecting Aneurysm 2024 articles Dissecting Aneurysm Scopus articles Dissecting Aneurysm impact factor journals Dissecting Aneurysm Scopus journals Dissecting Aneurysm PubMed journals Dissecting Aneurysm medical journals Dissecting Aneurysm free journals Dissecting Aneurysm best journals Dissecting Aneurysm top journals Dissecting Aneurysm free medical journals Dissecting Aneurysm famous journals Dissecting Aneurysm Google Scholar indexed journals Endovascular articles Endovascular Research articles Endovascular review articles Endovascular PubMed articles Endovascular PubMed Central articles Endovascular 2023 articles Endovascular 2024 articles Endovascular Scopus articles Endovascular impact factor journals Endovascular Scopus journals Endovascular PubMed journals Endovascular medical journals Endovascular free journals Endovascular best journals Endovascular top journals Endovascular free medical journals Endovascular famous journals Endovascular Google Scholar indexed journals Angiogram articles Angiogram Research articles Angiogram review articles Angiogram PubMed articles Angiogram PubMed Central articles Angiogram 2023 articles Angiogram 2024 articles Angiogram Scopus articles Angiogram impact factor journals Angiogram Scopus journals Angiogram PubMed journals Angiogram medical journals Angiogram free journals Angiogram best journals Angiogram top journals Angiogram free medical journals Angiogram famous journals Angiogram Google Scholar indexed journals sphenopalatine artery articles sphenopalatine artery Research articles sphenopalatine artery review articles sphenopalatine artery PubMed articles sphenopalatine artery PubMed Central articles sphenopalatine artery 2023 articles sphenopalatine artery 2024 articles sphenopalatine artery Scopus articles sphenopalatine artery impact factor journals sphenopalatine artery Scopus journals sphenopalatine artery PubMed journals sphenopalatine artery medical journals sphenopalatine artery free journals sphenopalatine artery best journals sphenopalatine artery top journals sphenopalatine artery free medical journals sphenopalatine artery famous journals sphenopalatine artery Google Scholar indexed journals neurological articles neurological Research articles neurological review articles neurological PubMed articles neurological PubMed Central articles neurological 2023 articles neurological 2024 articles neurological Scopus articles neurological impact factor journals neurological Scopus journals neurological PubMed journals neurological medical journals neurological free journals neurological best journals neurological top journals neurological free medical journals neurological famous journals neurological Google Scholar indexed journals vascular anatomy articles vascular anatomy Research articles vascular anatomy review articles vascular anatomy PubMed articles vascular anatomy PubMed Central articles vascular anatomy 2023 articles vascular anatomy 2024 articles vascular anatomy Scopus articles vascular anatomy impact factor journals vascular anatomy Scopus journals vascular anatomy PubMed journals vascular anatomy medical journals vascular anatomy free journals vascular anatomy best journals vascular anatomy top journals vascular anatomy free medical journals vascular anatomy famous journals vascular anatomy Google Scholar indexed journals sphenopalatine artery articles sphenopalatine artery Research articles sphenopalatine artery review articles sphenopalatine artery PubMed articles sphenopalatine artery PubMed Central articles sphenopalatine artery 2023 articles sphenopalatine artery 2024 articles sphenopalatine artery Scopus articles sphenopalatine artery impact factor journals sphenopalatine artery Scopus journals sphenopalatine artery PubMed journals sphenopalatine artery medical journals sphenopalatine artery free journals sphenopalatine artery best journals sphenopalatine artery top journals sphenopalatine artery free medical journals sphenopalatine artery famous journals sphenopalatine artery Google Scholar indexed journals Chordomas articles Chordomas Research articles Chordomas review articles Chordomas PubMed articles Chordomas PubMed Central articles Chordomas 2023 articles Chordomas 2024 articles Chordomas Scopus articles Chordomas impact factor journals Chordomas Scopus journals Chordomas PubMed journals Chordomas medical journals Chordomas free journals Chordomas best journals Chordomas top journals Chordomas free medical journals Chordomas famous journals Chordomas Google Scholar indexed journals
Article Details
1. Introduction
Skull base surgery poses a somewhat low risk of neurovascular injury regardless of the surgical approach taken, although this is especially true when endoscopy is used. We present a unique case, a young female who has a postoperative course complicated by both a right ICA dissecting aneurysm and a ruptured left sphenopalatine artery after a skull-base chordoma resection. This co-occurrence of vascular complications allows us to explore the topic of neurovascular injuries based on a nasopharyngeal approach. Ensuring adequate hemostasis in skull-base surgeries is extremely important to limit the rate of complications intra-operatively and post-operatively, given the delicate and complex neurological and vascular anatomy at the base of the skull.
Dissecting carotid aneurysms of the internal cerebral artery are extremely rare, occurring in 2.5 to 3 people out of 100,000 [1-3]. However, they are a common cause of cerebral infarction in patients younger than 40 years old [3, 4]. One study has found that dissecting intracranial aneurysms of non-traumatic origin are more common than previously expected [5]. The exact etiology behind dissecting ICA aneurysms is unknown, although these have been linked to trauma and underlying arterial pathologies, causing a sudden tear in the intimal layer of the artery [3, 6]. These dissecting aneurysms most commonly occur in the extracranial portion of the ICA rather than the ICA (70 to 80% of cases), and they are associated with vertebral artery dissection in up to 20% of cases [3, 4]. The typical presentation of this is an ipsilateral headache or neck pain, delayed ischemic symptoms from the affected hemisphere or retina, Horner’s syndrome, lower cranial nerve palsies, and pulsatile tinnitus [2, 3]. The prognosis is unpredictable, with death occurring following neurological sequelae in 15% of cases [3].
Most epistaxes originate in the area of Kiesselbach and do not require as aggressive of an intervention. However, in 10% of cases, these bleeds originate in the posterior nasal area, including the sphenopalatine artery (SP artery), and require more serious methods of management [7, 8]. Traditional methods of cauterization and packing are often highly unsuccessful in cases such as these. Sphenopalatine arterial electrocoagulation has been found to be an effective way of stopping these bleeds [9], and endoscopic control of the SP artery via ligation has been successful in controlling significant epistaxis of the posterior nasal area [8, 10].
In regards to endoscopic skull-base surgery, in a cohort of 330 patients, 3% of patients experienced postoperative epistaxis, most of which were resolved by nasal packing [11]. Thus, we hope that this unique co-occurrence of two vascular abnormalities will allow us to explore the topic of neurovascular complications in skull-base surgeries, especially in regard to managing dissecting carotid aneurysms and endovascular approaches to sphenopalatine arteries.
2. Case Report
A 22-year-old female was referred to the Department of Neuro-intervention from the department of neurosurgery for evaluation of recurrent nasal bleeding following surgical excision of a skull-base chordoma through the endonasal approach. An endovascular catheter digital subtraction angiogram was performed through a right femoral puncture. Her right intracranial artery injection revealed a large dissecting aneurysm (16mm in length by 11mm, 3 mm wide at its neck) at the cavernous segment. Injection of her left extracranial artery demonstrated active bleeding from multiple branches of the spheno-palatine artery of the branch of the left maxillary artery. A digital occlusion test was performed by compressing the right common carotid artery (CCA) and the anterior communicating artery (AcomA) was patent, sufficiently supplying the contralateral right anterior circulation. After this discovery, the following procedures were performed to manage these findings:
Procedure 1: A 6F Envoy guiding catheter was placed at the horizontal petrous segment of the RICA. A microcatheter (Echelon 10, ev3, USA) over the micro guidewire (Traxcess .014”, Microvention, USA) was navigated by guiding the catheter into the large aneurysm. A detachable GDC coil (16mmx30cm, Stryker, USA) was deployed into the aneurysm and a distal loop was left at the neck.
Procedure 2: the 6F Envoy guiding catheter was then placed at the left external carotid artery (LECA). A microcatheter (Echelon 10, ev3, USA) over the micro guidewire (Traxcess .014”, Microvention, USA) was navigated by guiding the catheter into the sphenopalatine artery. A 25% (NBCA + lipiodol) emulsion was then injected slowly through a microcatheter by a 1cc syringe. This was withdrawn once there was adequate embolization to stop the bleeding.
7 days after this procedure, a new angiogram showed that the RICA dissecting aneurysm was almost completely embolized. The nasal bleeding had stopped as well. The patient’s general condition was improved enough to be discharged home and she was advised for a follow-up visit and appointment with a neuro-oncologist.
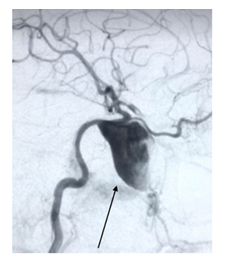
Figure 1A: angiography of right internal carotid artery showed a dissecting aneurysm at cavernous segment.
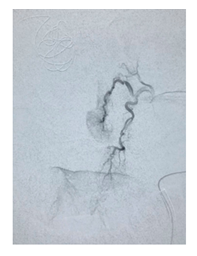
Figure 1B: Bleeding per left sphenopalatine artery revealed by angiogram. Contrast is passing the tissue.
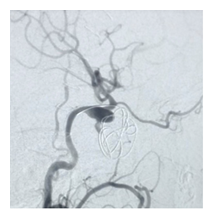
Figure 2A: After seven days of coiling check angiogram showed the dissecting aneurysm was almost embolized.
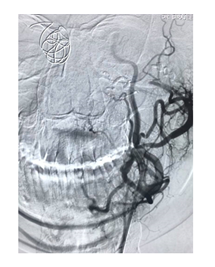
Figure 2B: Post embolization by NBCA liquid embolic agent. Injection showed no bleeding point.
3. Discussion
Chordomas are a rare form of cancer belonging to the sarcoma family affecting 1 in a million individuals per year. It is a slow growing, locally invasive tumor, commonly arising from remnants of the notochord [12, 13]. Our patient suffered from a skull base chondroma and an endonasal approach was used for tumor resection. Outcomes, such as tumor recurrence or complication rates are directly related to the expertise of the skull base surgeon and the team [14]. Epistaxis after endoscopic skull base surgery is a frequent complication, with devastating consequences due to hemorrhage. Post-operative epistaxis remains a common finding and the patient is at risk of deteriorating from hemorrhage. Like our patient, bleeding from large-caliber vessels such as the sphenopalatine and carotid artery is frequently seen. After anterior nasal packing alone was unsuccessful, our patient was injected with a 25% (NBCA + lipiodol) emulsion through a microcatheter using a 1cc syringe. NBCA is a vinyl monomer of alkyl 2-cyanoacrylate family that polymerizes on contact with ionic solutions such as normal saline, contrast dye or blood. When used in its purest form, polymerization is immediate. Addition of Ethiodol oil increases viscosity, prolonging polymerization duration, yet still providing necessary radiopacity for monitored administration using x-ray guidance. The ratio of NBCA Glue to Ethiodol is dependent on lesion type, degree of bleeding, flow characteristics of the vessel to be embolized and transit time from catheter tip to target [15, 16]. Vessel perforation during microcatheter placement is a common complication and can be reduced using a flow-directed microcatheter [16]. Injecting the mixture slowly also prevents risk of venous occlusion, and it is imperative that skilled personnel administer the mixture.
The use of a NBCA glue for endovascular management is relatively new with only a few cases reported in literature. Using the NBCA glue is a fast, easy technique and helps avoid multiple catheter manipulation when only one vessel needs to be embolized. In higher concentrations, it provides adequate sealing of larger blood vessels, with minimal risk of penetration into arterioles [17]. A 2004 study published in the Journal of Vascular Surgery studying the effects of NBCA for control of acute arterial hemorrhage reports that NBCA embolization was able to stop arterial bleeding in patients where previous coiling or particulate embolization had failed [18]. Furthermore, a 2017 study studying the efficacy of pre-embolizing a meningioma using a 13% dilute NBCA-lipiodol mixture noted that 56% (18 cases) of patients experienced a 50% reduction of T1-weighted MR imaging (T1-W1), further reflecting the therapeutic benefits of using an NBCA-lipiodol mixture for embolization [19]. Our patient did not experience any recurring bleeding after the procedure which used NBCA, and her follow-up visit after 7 days was unremarkable. Our patient’s case is unique because not only did her angiogram reveal a ruptured sphenopalatine artery, but a large dissecting aneurysm at the cavernous segment was also present. The presence of two vascular abnormalities made it challenging to operate surgically and required skilled expertise to navigate through its management. The increased use of imaging techniques in clinical practice have led to a higher detection rate of intracranial aneurysms, and dissection of the extracranial portion of the internal carotid artery is most frequently reported [20]. A commonly accepted classification of aneurysms is small (4-10 mm), large (10-25 mm) and giant (> 25 mm).
The most challenging aneurysms to treat include wide neck dissecting aneurysms, like the one our patient had [21]. Her aneurysm measured 16 x 11 x 3 mm and was treated using the Guglielmi detachable coil (GDC) embolization method. Inserting coils within the aneurysm halts blood flow, preventing further hemorrhage through the weakened vessel wall. This method supersedes surgery due to ease with which the platinum coil is inserted. Common practice involves using the transfemoral approach under general anesthesia [21]. The GDC coil is platinum and its malleable property allows it to effortlessly deform within the aneurysm. Over the last decade, advancements in GDC technology have also improved this method of treatment. Surgeons now have a variety of coil sizes to use and multi-dimensional coils for safer placement are also available [21]. A 2006 article looking into the safety of coil embolization reported a low complication risk during surgery, ranging between 8-18% compared to surgical clipping which had an overall risk of 23% [22]. Furthermore, the study reports a 3% reduction in morbidity among those who underwent coil embolization compared to surgical clipping over a course of 7 years. The survival benefit remained consistent over time with a log-rank of p=0.03 [22]. Notably, the outcome of coil embolization is independent of age for both ruptured and unruptured aneurysms, whereas surgical clipping indicated a better prognosis in patients > 65 years of age [22]. However, it is important to note that long term angiographic evidence in regards to long term occlusion and recurrence status with GDC use is lacking, and while surgical clipping has high procedural risks, its long term benefits have been well established in literature. Common complications of coil embolization include thrombo-embolic events (2.5-14.5%), coil malposition (14.6%) and coil collapse (8%) [21].
Furthermore, morphology of the aneurysm and its location also play a pivotal role in determining long term therapeutic benefits of coil embolization [21]. A review article published in JAMA Neurology recommends using a dome-to-neck ratio when using embolization coils [21]. According to Dovey et. al, it is mathematically logical to deduce that a small neck aneurysm with a width < 5 would be easier to pack than a wide neck. Furthermore, the study states that embolizing with GDC is relatively more challenging in aneurysms < 4 mm and less efficacious in aneurysms > 10 mm [21]. In their study, patients with a dome-to-neck ratio of < 2 had a 58% occlusion rate whereas those with a ratio of > 2 demonstrated an 80% occlusion rate using GDC [21]. With a rare tumor occurrence, multiple vascular abnormalities and risk of life-threatening hemorrhage, our patient’s condition was critical. Based on our patient’s age, risk factors and radiological results, we aimed to provide her with a treatment offering maximal therapeutic benefit and with this case report, we hope to share our insight with those who may be faced with similar challenges.
4. Conclusion
Bleeding from the sphenopalatine artery and dissecting aneurysm of an intracranial artery following endonasal resection of chordoma is an infrequent complication. Early diagnosis and adequate neuro-interventional techniques, like GDC coiling and embolization, can appropriately manage those conditions. We recommend early imaging and prompt neurological intervention for persistent nasal bleeding following endonasal resection of tumors in cases when more conservative techniques are not sufficient for controlling epistaxis.
Conflict of Interest
All authors declare no conflict of interest.
Disclosures
The authors have no financial conflicts of interest to declare.
Human/Animal Rights
Patient provided informed written consent to use the any relevant information for research and publications.
Acknowledgments
We want to acknowledge and thank CMSR (center for medical study and research) foundation.
References
- Sami Tahir Al Kindi, Abdulaziz Bakathir, Faisal Al Azri, et al. Dissecting aneurysm of the internal carotid artery as a complication of facial bone trauma. Oman medical journal 34 (2019): 70.
- Zetterling M, Carlström C, Conrad P. Review article-Internal carotid artery dissection. Acta neurologica scandinavica 101 (2000): 1-7.
- Guillon Bt, Lévy C, Bousser M G. Internal carotid artery dissection: an update. Journal of the neurological sciences 153 (1998): 146-158.
- Thanvi B, Munshi S K, Dawson S L, et al. Carotid and vertebral artery dissection syndromes. Postgraduate medical journal 81 (2005): 383-388.
- Ohkuma H, Suzuki S, Ogane K. Dissecting aneurysms of intracranial carotid circulation. Stroke 33 (2002): 941-947.
- O'Connell BK, J Towfighi, R W Brennan, et al. Dissecting aneurysms of head and neck. Neurology 35 (1985): 993-993.
- Neil Alexander Krulewitz, Megan Leigh Fix. Epistaxis. Emergency medicine clinics of North America 37 (2019): 29-39.
- Beatriz Agreda, Ángel Urpegui, José Ignacio Alfonso, et al. Ligation of the sphenopalatine artery in posterior epistaxis. Retrospective study of 50 patients. Acta Otorrinolaringologica (English Edition) 62 (2011): 194-198.
- Behrooz Gandomi, Mohammad Hosein Arzaghi, Bijan Khademi, et al. Endoscopic cauterization of the sphenopalatine artery to control severe and recurrent posterior epistaxis. Iranian journal of otorhinolaryngology 25 (2013): 147.
- Abdelkader M, Leong S, White P. Endoscopic control of the sphenopalatine artery for epistaxis: long-term results. The Journal of laryngology and otology 121 (2007): 759.
- Christopher F Thompson, Marilene B Wang, Brandon J Kim, et al. Incidence and management of epistaxis after endoscopic skull base surgery. ORL 74 (2012): 315-319.
- Dahlin DC, Maccarty Chordoma C S. A study of fifty-nine cases. Cancer 5 (1952): 1170-1178.
- Brian P Walcott, Brian V Nahed, Ahmed Mohyeldin, et al. Chordoma: current concepts, management, and future directions. The lancet oncology 13 (2012): e69-e76.
- Sumin Geng, Junting Zhang, Li?Wei Zhang, et al. Diagnosis and microsurgical treatment of chondromas and chondrosarcomas of the cranial base. Oncology letters 8 (2014): 301-304.
- Sheu A, Ang M, Fang A, et al. Off-label intravascular uses of embolic glue: how we avoid sticky situations. Journal of Vascular and Interventional Radiology 3 (2016): S206-S207.
- Tamatani S, Koike T, Ito Y, et al. Embolization of arteriovenous malformation with diluted mixture of NBCA. Interventional Neuroradiology 6 (2000): 187-190.
- Ankit A Mahadevia, Kieran J Murphy, Rick Obray, et al. Embolization for intractable epistaxis. Techniques in vascular and interventional radiology 8 (2005): 134-138.
- John William Kish, Michael D Katz, Victoria Marx M, et al. N-butyl cyanoacrylate embolization for control of acute arterial hemorrhage. Journal of Vascular and Interventional Radiology 15 (2004): 689-695.
- Hiroyuki Ohnishi, Shigeru Miyachi, Kenichi Murao, et al. Infiltrated embolization of meningioma with dilute cyanoacrylate glue. Neurologia medico-chirurgica 57 (2017): 44-50.
- Keiko Irie, Makoto Negoro, Motoharu Hayakawa, et al. Treatment of a spontaneous intracranial dissecting aneurysm with stent-assisted coil embolization. Neuroradiology 45 (2003): 825-829.
- Zach Dovey, Mukesh Misra, John Thornton, et al. Guglielmi detachable coiling for intracranial aneurysms: the story so far. Archives of Neurology 58 (2001): 559-564.
- Secretariat MA. Coil embolization for intracranial aneurysms: an evidence-based analysis. Ontario health technology assessment series 6 (2006): 1.
