Emergency Surgical Conversion Following Device Embolization During Transcatheter Aortic Valve Implantation: A Case Report
Article Information
BARRY Misbaou1*, CHABRY-HUN Yuthiline1, Mesut GUN2, HARMOUCHE Majid1, CAUS Thierry1
1Department of Cardiac Surgery, Amiens Picardie University Hospital Center, 1 Rue du Professeur Christian CABROL, 80054 Amiens, Cedex1, France.
2Department of Cardiology, Amiens Picardie University Hospital Center, 1 Rue du Professeur Christian CABROL, 80054 Amiens, Cedex1, France.
*Corresponding author: Misbaou BARRY, Heart-Chest-Vascular Surgery, Center, University Hospital Center Amiens Picardie University Hospital Center, 1 Rue du Professeur Christian CABROL, 80054 Amiens, Cedex1, France. Tel: +00 33 32 20 87 324.
Received: 19 May 2022; Accepted: 31 May 2022; Published: 27 June 2022
Citation: BARRY Misbaou, CHABRY-HUN Yuthiline, Mesut GUN, HARMOUCHE Majid, CAUS Thierry. Emergency Surgical Conversion Following Device Embolization During Transcatheter Aortic Valve Implantation: A Case Report. Cardiology and Cardiovascular Medicine 6 (2022): 308-314.
View / Download Pdf Share at FacebookAbstract
Surgical conversion following embolization of a TAVI device is very rare. We report the case of a 78-year-old woman who underwent a TAVI procedure. This procedure failed due to migration of the prosthesis, which is embedded at the foot of the TABC in the ascending aorta. This patient had an emergency surgical conversion. We want to draw attention to the fact that the management of the TAVI patient by the surgical team in case of conversion should be rapid and the surgical intervention should be short.
Keywords
Conversion; Embolization; Device; Surgery
Article Details
Abreviation:
TAVI: Transcatheter Valve Implantation; ECG: Electrocardiogram; PR: Time from onset of atrial activation (P wave) to onset of ventricular myocardial activation; mmHg: millimeter of mercury ; B2: the second heart sound; LVH: Left Ventricular Hypertrophy; Segment ST: the ST-segment; V1, V2, V5, V6: the electrodes; Onde T: Twave; Ao : Aorta; Vmax: maximum velocity; IP: Permeability index; LVEF: Left Ventricular Ejection Fraction; MR: Mitral Regurgitation; mm: millimeter; TABC: Brachiocephalic Artery Trunk; ECC: Extracorporel Circulation; mn: minute
1. Introduction
Transcatheter Aortic Valve Implantation (TAVI) is nowadays recognized as an effective solution for all patients who are at risk to conventional surgery for aortic stenosis [1]. Despite the growing experience of cardiologists in the implantation of these TAVI devices, a number of technical limitations associated with the TAVI procedure are known; one of these technical limitations is the inability to reposition the implant in case of suboptimal deployment [2]. In this suboptimal deployment, when there is an underexpansion or migration of the prosthesis, it is difficult to have an optimal result of the procedure without resorting to conventional surgery. In this case, it is a migration of the prosthesis into the ascending aorta. We want to draw attention to the fact that the management of the TAVI patient by the surgical team in case of conversion must be rapid and the surgical procedure must be short (clamping time, perfusion time and gesture).
2. Material and Method
We report the case of a 78-year-old female patient, active, living at home with her husband, with a history of recent hyperparathyroidism and as risk factor a hypertension with dyslipidemia. She weighs 79 kg for 163 cm height. She complained of class 3 dyspnea. The clinical examination revealed a blood pressure of 134/76 mmHg, a saturation of 96% on room air, a heart rate of 76/min, a systolic murmur at the aortic focus with an abolished B2, radiating to the neck vessels. The Electrocardiogram (ECG) showed a sinus rhythm at 72/min, PR at 160ms, electrical Left Ventricular Hypertrophy (electrical LVH), ST segment suspended in V1, V2, steep in V5 and sub-shifted in V6 with diphasic T waves.
Transthoracic Echocardiography (TTE) showed a tight aortic stenosis with a mean VgAo gradient of 93 mmHg, Vmax of 6 m/s, PI of 0.15, VAS of 0.43 cm2 or 0.2 cm2/m2, concentric LVH, subaortic septal bulge of 17 mm non obstructive. Left Ventricular Ejection Fraction (LVEF) at 65%. Hypokinesia of the inferior wall, minimal mitral Regurgitation (minimal RM). Coronary angiography shows a dominant right network, free of any significant stenosis. No indication for revascularization before aortic replacement. A pre-TAVI scan showed an aortic annulus with a perimeter of 71.4 mm. Given this picture of calcified, tight, symptomatic aortic stenosis, the cardiologists decided to perform an endocavitary aortic valve replacement.
3. Results
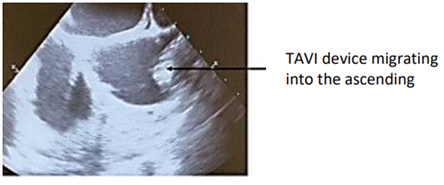
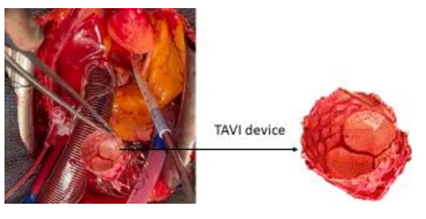
The TAVI procedure was unsuccessful due to migration of the prosthesis, which was embedded at the foot of the TAB in the ascending aorta. The patient was referred directly to the operating room for surgical management Figure1.
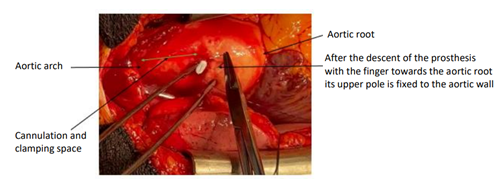
After a median stereotomy and opening of the pericardium, the TAVI prosthesis is perceived very high to palpation, clearly straddling the origin of the TABC. It can descend with the finger towards the aortic root, but it is immediately pushed back downstream during the following systole. It can be stabilized in an intermediate position by securing its superior pole to the aortic wall with a transfixing point Figure 2.
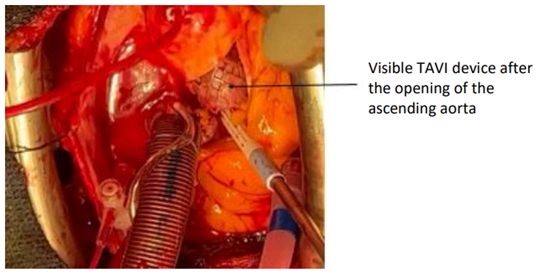
It is then possible to perform an aortic cannulation at the foot of the TABC and to install a conventional bypass graft between the aorta and the right atrium. After aortic clamping and initiation of retrograde cardioplegia through the coronary sinus, we performed a long oblique aortotomy astride the body of the prosthesis (corevalve) Figure3.
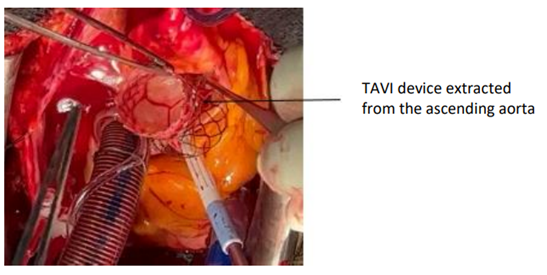
After releasing the prosthesis from its attachment point, it can be extirpated through the aortotomy Figure 4 and Figure 5.
The native valve is then exposed, which is a type I bicuspid with a massively calcified coronary and noncoronary valve. We performed additional cardioplegia through the coronary ostia. Then, we performed valve leaflet excision, deincrustation, and repair of the valve annulus before solid implantation of a number 23mm Perimount prosthesis. Closure of the aorta in two planes with 4/0 on felt splint. Easy stop of the ECC in sinus rhythm, careful purging and aortic decamping, decannulation and neutralization of circulating heparin. Verification of hemostasis, osteosynthesis of the sternum and closure of the wall on drains and ventricular electrodes.
4. Comments
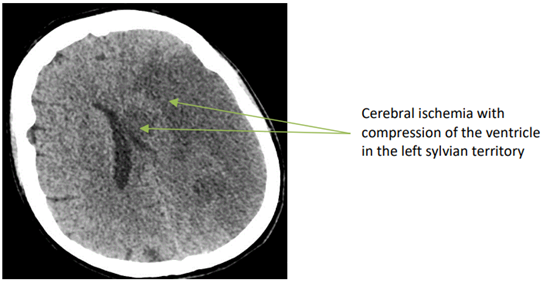
Since Professor Alain Cribier and his team revolutionized the management of aortic stents by performing the first Transcatheter Aortic Bioprosthesis (TAVI) implantation in humans in 2002, the indications have continued to expand. Emergency conversion from TAVI to conventional surgery is a rare event. Complications requiring conversion are TAVI device extracted from the ascending aorta 6 unpredictable. The main reasons for these complications are most likely related to inappropriate anatomy, poor prosthesis fit, and errors during implantation. In a series of 1775 patients undergoing TAVI between 2001 and 2016, Arsalan et al. [3] reported a conversion to sternotomy of 2.1% or 32 patients. Of these 32 converted patients, there were 5 annular ruptures, 9 device embolizations, and 15 tamponades. Hein et al. [4] reported an emergency conversion from TAVI to conventional cardiac surgery that was required in 24 of 1975 patients or 1.2%. The main causes of conversion in this series were aortic annulus rupture, aortic perforation or thoracic aortic dissection, prosthesis embolization, myocardial perforation, severe aortic insufficiency, and coronary obstructions. In this series, the mean time interval between TAVI abortions and surgery was 19 minutes. Four of 24 patients (16.7% died during the initial surgery, seven of 24 (29.2%) within 72 hours, and 30-day mortality was 45.8%. Seiffert et al. [5] presented a series of 458 patients who underwent TAVI, of whom 13 patients or 2.8% required urgent conversion to surgery. Complications included device embolization/migration 17%, aortic regurgitation 12%, and root rupture 5%. Sternotomy and surgical hemostasis were performed in 5 patients or 13% with left ventricular perforation and cardiac tamponade. Coronary obstruction representing 15% required emergency percutaneous coronary intervention in 6 patients. In the series by Liang et al. [6], of 2102 TAVI patients, 51 (2.5%) developed catastrophic cardiac events. Causes included cardiac perforation and tamponade (n=19 or 37.3%), acute left ventricular failure (n=10 or 19.6%), coronary artery obstruction (n=10 or 19.6%), aortic root rupture (n+7 or 13.7%), and device embolization (n=5 or 9.8%. Considering the potentially fatal complications, some authors have found a 30-day survival rate of more than 50% (3). For other authors, emergency conversion from TAVI to surgery is a rare event resulting in a mortality of about 45% after 30 days [4]. Moritz Seiffert's team found a mortality rate of 38.5% in patients requiring surgical conversion [5]. It should be noted that although the immediate mortality rate in patients requiring conversion, more than half of the patients survived the first 30 days. In this case, the lack of cardiac surgery could have resulted in a fatal outcome. TAVI patients converted to surgery should have very rapid management with a very short surgical time. Mani Arsalan et al published in their series of 32 converted patients, an average procedure time of 193 min and an average infusion time of 89 min [3]. The management of our patient was rapid, with the entire team on site, with the hybrid room and the cardiac surgery block rooms in the same location. We noted a procedure time of approximately 100 min, with a perfusion time of 70 min and a clamping time of 50 min. The main advantage of the active presence of a fully trained interdisciplinary team (cardiology and cardiac surgery) during the TAVI procedure is the possibility of immediate treatment of all possible complications. The immediate postoperative evolution of our patient was marked by the development of cerebral ischemia in the entire sylvian left territory Figure 6.
References
- Zajarias A, Cribier AG. Outcomes and safety of percutaneous aortic valve replacement. J Am Coll Cardiol. 53 (2009): 1829-1836.
- Vahanian A, Alfieri O, Al-Attar N. et al. Transcatheter valve implantation for patients with aortic stenosis: A position statement from the European Association of Cardio-Thoracic Surgery (EACTS) and the European Society of Cardiology (ESC), in collaboration with the European Association of Percutaneous Cardiovascular Interventions (EAPCI). Eur Heart J 11 (2008): 1463-147
- Arsalan M, Kim WK, Van Linden A, et al. Predictors and outcome of conversion to cardiac surgery during transcatheter aortic valve implantation. Eur J Cardiothorac Surg. 54 (2018): 267-272.
- Hein R, Abdel Wahab M, Sievert H, et al. Outcome of patients after emergency conversion from transcatheter aortic valve implantation to surgery. EuroIntervention 9 (2013): 446-451
- Seiffert M, Conradi l, Baldus S, et al. Severe intraprocedural complications after transcatheter aortic valve implantation: calling for a heart team approach. Eur J Cardiothorac Surg 44 (2013): 478-484.
- Liang Y, Dhoble A, Pakanati A, et al. Catastrophic cardiac events during transcatheter aortic valve replacement. Can J Cardiol 37 (2021): 1522-1529.