Efficacy of High Frequency Oscillatory Ventilation and Co-Administered Inhaled Nitric Oxide and Intravenous Sildenafil in Treating Persistent Pulmonary Hypertension of Newborn in Congenital Diaphragmatic Hernia
Article Information
Dr. Rajiv Parapurath MD1, Dr. Nalinikanta Panigrahy MD, DrNB2, Dr. Madan Samuel DM, MS, DPS3*
1Head of Neonatology, Department of Neonatology, NMC Specialty Hospital Group, Dubai, UAE
2Consultant Neonatologist, Rainbow Children’s Hospital, Banjara Hills, Hyderabad, India
3Consultant and Head of Paediatric Surgery and Newborn Services, NMC Specialty Hospital Group, Dubai, UAE
*Corresponding Authors: Dr. Madan Samuel DM, MS, DPS, Consultant and Head of Paediatric Surgery and Newborn Services, NMC Specialty Hospital Group, Dubai, UAE.
Received: 28 June 2025; Accepted: 04 July 2025; Published: 11 July 2025
Citation: Rajiv Parapurath, Nalinikanta Panigrahy, Madan Samuel. Efficacy of High Frequency Oscillatory Ventilation and Co-Administered Inhaled Nitric Oxide and Intravenous Sildenafil in Treating Persistent Pulmonary Hypertension of Newborn in Congenital Diaphragmatic Hernia. Journal of Pediatrics, Perinatology and Child Health. 9 (2025): 126-136.
View / Download Pdf Share at FacebookAbstract
Objective: To prospectively evaluate efficacy of co-administered intravenous sildenafil and inhaled nitric oxide (iNO) in treating severe persistent pulmonary hypertension of newborn (PPHN) in congenital diaphragmatic hernia (CDH) neonates on high frequency oscillatory ventilation (HFOV).
Methods: Of 106-neonates, 41 with severe-PPHN on HFOV were treated with intravenous sildenafil and iNO (HSN). Twenty-nine with moderate- PPHN on conventional mechanical ventilation (CMV) and iNO (MNO), and 36 with no-PPHN on CMV and inotropes. Primary (60-days) outcomes analysed were survival, treatment failure, and adverse events. Secondary (60-days) outcomes were ventilation free days (VFD) at 60-days, neonatal intensive care unit free days (NICUFD) at 60-days. Variables are presented as true mean ± one standard deviation.
Results: Survival in neonates on HSN was 76% versus 83% on MNO (p = 0.862). Overall survival was 80%. The treatment failure rate was 24% in neonates on HSN. In comparison treatment failure rates were 48% in MNO and 25% in CMV. Statistically, infants with adverse events were equivocal among the 3-groups. Infants treated preoperatively by HSN had less VFD at 60-days, 16 ± 9 versus 44 ± 21 MNO (p = 0.001). Few NICUFD at 60-days were observed in HSN 6 ± 5 versus 32 ± 15 MNO (p=0.001). Three-dimensional echocardiography revealed that biventricular dysfunction was the primary factor contributing to death in this cohort of CDH-neonates.
Conclusions: Intravenous sildenafil and iNO achieved 76% survival in CDH-neonates with severe-PPHN on HFOV. Less VFD at 60-days and NICUFD at 60-days was observed in neonates treated with HSN.
Keywords
Congenital diaphragmatic hernia; Inhaled nitric oxide; Neonates; Persistent pulmonary hypertension of newborn; Sildenafil
Congenital diaphragmatic hernia articles; Inhaled nitric oxide articles; Neonates articles; Persistent pulmonary hypertension of newborn articles; Sildenafil articles
Article Details
Abbreviations: CMV: Conventional Mechanical Ventilation; CPAP: Continuous Positive Airway Pressure; HFOV: High Frequency Oscillatory Ventilation; HSN: High Frequency Oscillatory Ventilation + intravenous sildenafil + iNO; HFD: Hospital Free Days at 60-days; iNO: Inhaled Nitric Oxide; MAP: Mean airway pressure; MNO: Conventional Mechanical Ventilation + iNO; NICUFD: Neonatal Intensive Care Unit Free Days at 60-days; NICU: Neonatal Intensive Care Unit; OSI: Oxygen Saturation Index; PPHN: Persistent Pulmonary Hypertension of newborn; VFD: Ventilation Free Days at 60-days. 95% CI: 95% Confidence Interval
Trial Registration No: 02-6250020: 09/2014.
1. Introduction
Congenital Diaphragmatic Hernia occurs in approximately 1 in 3,000 to 5,000 live births, with an estimated 26% to 32% mortality in tertiary care centres [1-4]. In CDH-neonates, poor outcome is often linked to a combination of lung hypoplasia, abnormal pulmonary vasculature, and varying degrees of left ventricular dysfunction. Pulmonary vasculature in CDH is decreased with arterioles exhibiting increased muscularisation [5]. Preaciner and intraaciner arterioles exhibit significant muscular hyperplasia and thickening of arterial media and adventitia [5]. Abnormal arterial muscularisation increases the susceptibility of developing PPHN (68% to 79%), resulting in considerable morbidity and mortality [6-8]. Current therapeutic options are selective pulmonary vasodilators, such as, inhaled nitric oxide (iNO) and phosphodiesterase type-5 inhibitor, sildenafil (Pfizer. Inc). Both pharmaceutical agents increase intracellular cyclic guanosine monophosphate through their respective physiological pathways causing pulmonary arterial vasodilatation [8,9]. Sildenafil and iNO are also known to cause bronchodilation, while demonstrating anti-inflammatory, and anti-proliferative properties [10,11]. Sildenafil and iNO are proven to clinically perform synergistically improving oxygenation index, reversing right-to-left shunt ratio over ductus arteriosus, reducing mean duration of ventilation and need for extracorporeal membrane oxygenation [6,9,11-13].
The purpose of the study was to evaluate the efficacy of intravenous sildenafil and iNO in treating severe-PPHN in CDH-neonates. Studied primary (60-days) outcomes were survival, adverse events and treatment failure. Secondary (60-days) outcomes were VFD at 60-days, NICUFD at 60-days and hospital free days (HFD) at 60-days.
2. Methods
2.1 Study Design
Study prospectively evaluated 123 consecutive neonates with antenatally diagnosed diaphragmatic hernia between January 2015 and December 2020. All CDH-neonates were treated according to institutionalised protocols. The study was conducted at tertiary level III-neonatal intensive care unit without paediatric extracorporeal membrane oxygenation. Consent was obtained for treatment from neonates’ parents/carer. Institution’s internal review board, research and ethics committee (No.02-6250020: 09/2014), approved study protocol, which confirmed with provisions of Declaration of Helsinki 1995 (Revised Edinburgh 2000).
2.2 Parameters Assessed
Primary diagnosis and continuous monitoring of PPHN was performed by three-dimensional-echocardiography and preductal oxygenation saturation index (OSI) by pulse oximetry until there was complete resolution of PPHN or death.
Three-dimensional-echocardiography diagnosed and regularly monitored the progression of PPHN. Basic measurements utilized to define PPHN were velocity of tricuspid regurgitation jet and tricuspid annular plane systolic excursion, and/or abnormal right ventricular dilatation, interventricular septum deviation, and right ventricular dysfunction, with or without left ventricular dysfunction.
Preductal oxygenation saturation index (OSI) by pulse oximetry (Formula estimating OSI: Mean Airway Pressure (MAP: cmH2O) x Fraction of inspired oxygen (FiO2) x 100 ÷ SpO2) [14], was used to confirm the diagnosis and for continuous monitoring of PPHN. Neonates were categorized as severe-PPHN (OSI: 26 - 38); moderate-PPHN (OSI: 10 - 25) and no-PPHN (OSI: 0 - 9) [14]. Classification determined the mode of ventilation. Optimal-CMV for moderate-PPHN or no-PPHN, and HFOV for severe-PPHN.
Cranial ultrasound scan for significant intracranial bleeds was performed prior to or immediately after treatment commencement.
Exclusions included CDH-neonates requiring extracorporeal membrane oxygenation, severe cardiac anomalies, life threatening multiple congenital malformations, severe renal, skeletal and orthopaedic anomalies, severe skeletal and thoracic deformities and chromosomal anomalies. Anterior Morgagni hernias (n = 4) and diaphragmatic eventration (n = 1) were excluded, as they were stable and underwent delayed repair. No fetuses had undergone fetal surgery.
Ventilation
CDH-neonates were intubated immediately after birth along with placement of nasogastric tube on continuous suction. Primary treatment was optimal-CMV (SLE6000, GE.UK.) with permissible hypercapnia. The objective was to maintain preductal saturations: 85% - 95%, post ductal saturations: ≥ 70% and arterial PaCO2: 45 - 60 mmHg (permissive hypercapnia). Organ perfusions were monitored by pH: > 7.2, lactate levels: < 5mmol/L and urinary output: 1 - 2 ml/Kg/Hour. Oxygen toxicity was avoided by decreasing FiO2 guided by SpO2. CMV maintained ventilatory rate of 40 - 60/minute with peak inspiratory pressures (PIP): 22 - 24 cmH2O, and positive end-expiratory pressure (PEEP): 4 - 6 cmH2O.
Neonates were commenced on HFOV (SLE6000, GE.UK.) when PIP was > 24 cmH2O and OSI ≥ 26. Initial HFOV settings adopted were MAP: 12 - 14 cmH2O, frequency: 6 - 8 Hertz (Hz), amplitude (Δp): 30 - 40 cmH2O to achieve chest vibrations, and inspiration/expiration rate of 1:1.
Selective Pulmonary Vasodilators
iNO was initiated when OSI ≥ 10. Response to treatment (30-minutes) was evaluated by increases in PaO2: ≥ 20 mmHg, fall in OSI: ≤ 10 with decreased FiO2. iNO (20 ppm) was commenced for maximum duration of 96-hours [11]. Following successful treatment, iNO was gradually weaned [11]. Nitrogen dioxide (NO2) and methaemoglobin levels were regularly assessed. Methaemoglobin level was measured prior to initiation of iNO and subsequently, 60-minutes after commencement. Thereafter, methaemoglobin level was evaluated daily.
Intravenous sildenafilwas started at 32 ± 2 minutes when there was no response to iNO, with an increasing OSI ≥ 10. Response (60-minutes) was evaluated by increases in PaO2: ≥ 20 mmHg; fall in OSI: ≤ 10 and decrease in FiO2. Intravenous sildenafil was administered as loading dose of 0.4 mg/kg over 3-hours, followed by a continuous infusion of 1.6 mg/Kg/day [9]. Based on clinical response, therapy was continued for a maximum period of 14-days. Sildenafil was gradually weaned when OSI: < 5, SpO2: 85% - 95%, FiO2: 0.3. Gradual dose reduction of sildenafil to 50% occurred over 12-hours, followed by further dose reductions and stoppage after 18-to-24-hours. Sildenafil infusion was reinitiated if there was increase in OSI: > 10, SpO2: < 85%, FiO2: > 0.5.
Mean blood pressure (≥ 50th percentile) was maintained with dobutamine 10 - 20 mcg/Kg/minute and dopamine 10 mcg/Kg/minute. Neonates were sedated with midazolam (60 mcg/Kg/hour) and fentanyl (1 - 5 mcg/Kg/hour). Intravenous fluids, total parenteral nutrition and antibiotic therapy completed standardised care. Intravenous milrinone (phosphodiesterase type-3 inhibitor. Auro Pharma. Inc.) 50 µg/Kg infusion over 60-minutes followed by maintenance dose of 0.33 to 0.99 µg/Kg/minute was the other inotropic-vasodilator used. Echocardiography determined usage of milrinone. Hypovolaemia was corrected by 0.9% saline or ringer lactate at 10 - 20 mL/Kg boluses. Standardized treatment protocols for CDH care were in accordance with relevant NICU guidelines, regulations, and product characteristic recommendations. All infants were evaluated at completion of treatment/discharge by: (1). Cranial ultrasound scan at discharge. (2). Three-dimensional-echocardiography prior to discharge.
Stabilization and Surgical Repair
Following successful completion of treatment, babies were transferred from HFOV to CMV. Weaning-off HFOV occurred when preductal SpO2: ≥ 95%. Weaning from CMV was performed by modifying PIP-PEEP to achieve PaCO2: ≥ 45 mmHg.
Surgical repair of diaphragm was performed when babies were on CMV with OSI: < 5, FiO2: ≤ 0.3, systolic blood pressure 60 - 80 mmHg, diastolic blood pressure 35 - 45 mmHg and preductal SpO2: 85% - 95%. At repair organs were well perfused with lactate < 3 mmol/L and urine output ≥ 2 ml/Kg/Hour. Following repair, infants were weaned-off CMV to continuous positive airway pressure (CPAP). They were acclimatised to room air following a stabilisation period on standard flow nasal cannula oxygen.
2.3 Objective
Response to treatment was measured by incremental decline of OSI leading to discontinuation of pulmonary vasodilators and ventilatory support. Primary endpoint was to define survival at discharge, and failure of medical treatment of PPHN in CDH-neonate leading to death at end of 60-days or prior to discharge. Secondary outcomes studied were VFD at 60-days, NICUFD at 60-days, HFD at 60-days.
2.4 Statistics
Statistical analysis was performed using Statistical Package for Social Sciences software (SPSS 21 for Windows, SPSS Inc., Chicago, Ill., USA). Continuous variables are presented as true mean (µ) ± one standard deviation. Depending on distribution, Student’s t test or Mann Whitney U test was used to compare continuous variables between MNO and HSN. Categorical variables are reported as observational frequency (N, %) and analysed using Pearson’s chi-squared test. Survival was estimated using Kaplan Meier estimates. Multivariate logistic regression analysis to determine independent associations of variables, such as gender, side of defect, adverse events, and OSI with outcomes. P value <0.05 was considered statistically significant. Sensitivity and specificity of treatment modalities MNO and HSN were calculated from optimal areas under constructed receiver-operating characteristic curve (AUROC). To summarise net effect of pulmonary vasodilators on survival and ventilation duration, ventilator-free days (VFD) at 60-days, neonatal intensive care unit-free days (NICUFD) at 60-days and hospital free-days (HFD) at 60-days were calculated using following formula: VFD = 0 if subject dies within 60-days of ventilation. VFD = 60 - x, if successfully extubated from ventilation, x-days from initiation. VFD = 0 if subject is ventilated for > 60-days. NICUFD and HFD at 60-days were calculated in similar manner [15].
3. Results
3.1 Demographics
Antenatal ultrasound scans (100%) and maternofoetal magnetic resonance scans (78%) identified 123-fetuses with posterolateral (Bochdalek) diaphragmatic hernia. Seven (6%) stillbirths, and 5 (4%) elective termination of pregnancy for fetal anomaly ensued. Cohort comprised of 111 (90%) livebirths. Birth by lower segment caesarean section occurred in 80 (72%) and 31 (28%) by vaginal delivery. Five (4%) were excluded due to chromosomal aberration. Cytogenetic analysis confirmed normal male [46, XY: N = 73 (69%)] or female [46, XX: N = 33 (31%)] karyotype in 106-neonates with gender ratio (Male-to-Female = 2:1). Consanguinity occurred in 6 (6%).
Antenatal scans were performed at mean gestational age 23-weeks (Table 1). There was no statistically significant association between observed to expected lung to head ratio (O/E LHR) and treatment modalities HSN and MNO. Mean O/E LHR in neonates on HSN was 43.5 ± 14.3 versus 42.6 ± 12.3 MNO (p = 0.886) (Table 1). In comparison, O/E LHR was lower in non-survivors. Survivors 44.5 ± 13.2 versus 23.5 ± 1.6 non-survivors (p = 0.001). There was no significant association between survival and gestational age (p = 0.721).
|
Characteristics |
CMV CDH: No-PPHN (OSI: 0-9) N = 36 |
MNO CDH: M-PPHN (OSI: 10–25) N = 29 |
HSN CDH: S-PPHN (OSI: 26–38) N = 41 |
|
Antenatal Ultrasound Scan and Birth Attributes |
|||
|
Gestational age at Antenatal Scan (weeks: µ ± SD) |
23.7 ± 1.7 |
23.7 ± 1.8 |
23.2 ± 1.2 |
|
Observed to Expected Lung to Head Ratio (O/E LHR: µ ± SD) |
44.2 ± 13.7 |
40.4 ± 9.9 |
43.5 ± 14.4 |
|
Liver in Thorax (N: %) |
12: 33% |
16: 55% |
27: 66% |
|
Demographics |
|||
|
Male (N: %) |
21: 58% |
20: 76% |
32: 78% |
|
Female (N: %) |
15: 42% |
9: 24% |
9: 22% |
|
Left / Right / Bilateral (N) |
29 / 7 / 0 |
21 / 8 / 0 |
34 / 6 / 1 |
|
Gestational Age at Birth (weeks: µ ± SD) |
37.5 ± 1.8 |
37.6 ± 1.7 |
37.3 ± 1.6 |
|
Birth weight (grams: µ ± SD) |
3198 ± 390 |
3111 ± 341 |
3123 ± 362 |
|
Birth Weight (Z-scores) |
0.16 |
0.13 |
0.15 |
|
Pulmonary Vasodilators, Inotropes and Ventilation Parameters |
|||
|
iNO: N = 70: 66% |
0 |
29 |
41 |
|
Sildenafil: N = 41: 39% |
0 |
0 |
41 |
|
Milrinone: N = 57: 54% |
20: 56% |
16: 55% |
21: 51% |
|
Dopamine: N = 79: 75% |
27: 75% |
22: 76% |
30: 73% |
|
Dobutamine: N = 25: 24% |
9: 25% |
7: 24% |
9: 22% |
|
Epinephrine: N = 20: 19% |
6: 17% |
5: 17% |
9: 22% |
|
Total Ventilation Days |
6 ± 1 |
13 ± 2 |
40 ± 5 |
|
Total CPAP Days |
5 ± 2 |
7 ± 1 |
6 ± 2 |
|
Oxygen Therapy Days |
5 ± 1 |
8 ± 1 |
8 ± 2 |
|
Outcome Measures |
|||
|
Survival (N: %) |
30: 83% |
24: 83% |
31: 76% |
|
VFD at 60-days (µ ± SD) |
50 ± 15 |
44 ± 21 |
16 ± 9 |
|
NICUFD at 60-days (µ ± SD) |
40 ± 12 |
32 ± 15 |
6 ± 5 |
|
HFD at 60-days (µ ± SD) |
25 ± 9 |
5 ± 4 |
2 ± 2 |
|
CMV and HFOV: Ventilatory Settings |
|||
|
CMV (N = 65: 61%) |
Value: µ ± SD |
HFOV (N=41: 39%) |
Value: µ ± SD |
|
PIP (cm H2O) |
22 ± 1 |
MAP (cm H2O) |
13 ± 1 |
|
PEEP (cm H2O) |
5 ± 1 |
Amplitude (Δp: cm H2O) |
35 ± 2 |
|
Ventilation Rate (breaths/minute) |
50 ± 8 |
Frequency (Hz: cm H2O) |
7 ± 1 |
|
Abbreviations: CDH: Congenital Diaphragmatic Hernia. CPAP: Continuous positive airway pressure. CMV: Conventional Mechanical Ventilation. HFOV: High Frequency Oscillatory Ventilation. HFD: Hospital Free Days at 60-days. MAP: Mean Airway Pressure. NICUFD: Neonatal Intensive Care Unit Free Days at 60-days. PIP: Peak Inspiratory Pressure. PEEP: Positive End Expiratory Pressure. VFD: Ventilation free days at 60-days. Note: Oxygen therapy by standard flow nasal cannula. |
|||
Table 1: Demographics, Ventilation and Outcome.
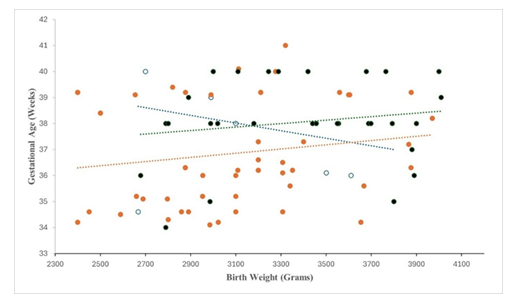
Near-term neonates were born at mean gestational age 37.5 ± 1.8 weeks and mean birth weight 3146 ± 364 grams (Figure 1). Combined chest and abdominal X-ray revealed left-CDH: 84 (79%), right-CDH: 21 (20%), and bilateral-CDH: 1 (1%) (Table 1). Survival in left-CDH was 67% versus 13% right-CDH (p = 0.001). CDH-neonates with bilateral-hernia and diaphragmatic agenesis survived.
Metabolic screening tests were normal. Total parenteral nutrition was commenced at day 3 ± 0.5 and continued until feeds were established. Post-surgical repair of diaphragm, enteral feeds was started at day 2 ± 1. Postoperative chest tube placement was not practiced.
3.2 Treatment Response
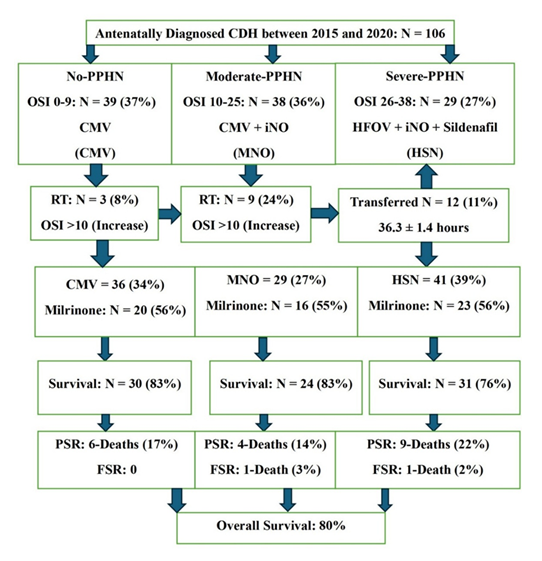
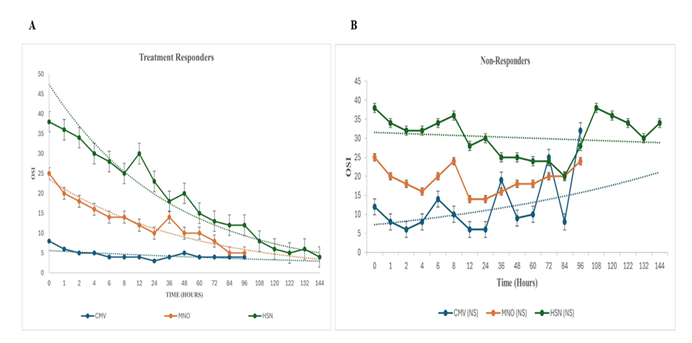
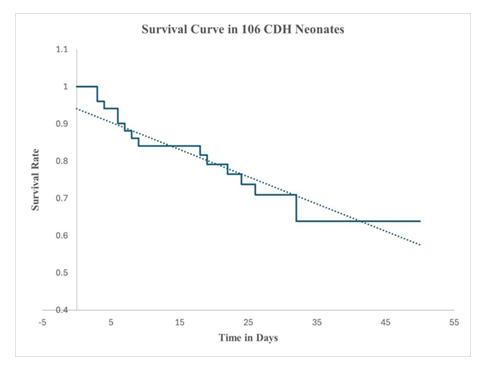
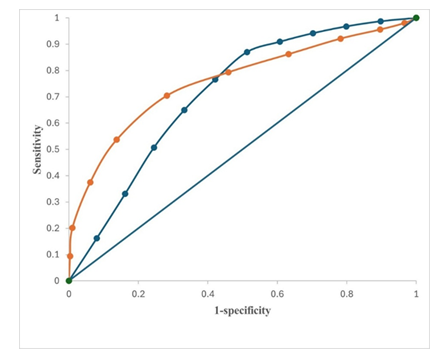
CDH-neonates with Severe-PPHN: OSI: 38 ± 1, ensued in 41-neonates (Figure 2). Twenty-nine were transitioned to HFOV from delivery room CMV at 0.9 ± 0.3 hours and commenced on intravenous sildenafil and iNO (HSN). Twelve babies, refractory to treatment were transferred to HSN at 38.5 ± 2.4 hours (Figure 2). Sustained incremental decline in OSI was observed in treatment-responders (Figure: 3A). Comparing treatment responders (Figure 3A) and non-responders (Figure 3B); at 6-hours there was 36% versus 10% OSI decline (p = 0.001), at 36-hours 47% versus 34% OSI decrease (p = 0.03), at 72-hours 55% versus 21% OSI reduction (p = 0.001), with complete resolution of PPHN in treatment-responders at 144-hours in 31 (76%). Seventy-six percent survived (Figure 4). HSN in resolving severe-PPHN had 80% sensitivity and 74% specificity (AUROC = 0.803. 95% CI: 0.774 - 0.863) (Figure 5).
CDH-neonates with Moderate-PPHN: OSI: 24 ± 1 occurred in 38-neonates. They were ventilated on CMV with iNO (MNO). In 29 (76%), response to treatment occurred at 4-hours, with significant decline in OSI by 53% in both responders-to-treatment and non-responders (Figure 3A /B). At 36-hours it was equivocal between responders and non-responders, 38% versus 31% (p = 0.062). However, at 48-hours, OSI was 66% versus 38% (p = 0.001) between responders and non-responders. At 96-hours complete resolution occurred in 24 (83%) versus 5 (17%) deaths (p = 0.001) (Figure 3A/B). Nine (24%) babies were refractory to MNO-treatment with OSI: 17 ± 2 at 36-hours. They were transitioned to HSN (Figure 2). Survival was 83%. MNO resolved moderate-PPHN with 73% sensitivity and 69% specificity (AUROC = 0.73. 95% CI: 0.69 - 0.78) (Figure 5).
Comparative analysis demonstrated infants treated preoperatively by HSN had few VFD at 60-days, 16 ± 9 versus 44 ± 21 MNO (p = 0.001). NICUFD at 60-days was less in HSN 6 ± 5 versus 32 ± 15 MNO (p = 0.001). HFD at 60-days was equivalent between HSN 2 ± 2 versus 5 ± 4 MNO (p = 0.056).
CDH-neonates without PPHN: OSI: 7 ± 2 occurred in 39-neonates (37%). They were commenced and maintained on CMV and inotropes (Figure 2, Table 1). Survival was 83% with 25% treatment failure (Figure 2).
Comparative analysis showed VFD and NICUFD at 60-days was statistically equivocal between CMV and MNO [VFD: CMV 50 ± 15 versus 44 ± 21 MNO (p = 0.741), and NICUFD: CMV 40 ± 12 versus 32 ± 15 MNO (p = 0.665)]. Less VFD at 60-days and NICUFD at 60-days was observed in HSN versus CMV [VFD: HSN 16 ± 9 versus 50 ± 15 CMV (p = 0.001). NICUFD: HSN 6 ± 5 versus 40 ± 12 CMV (p = 0.001)]. HFD at 60-days was less in HSN 2 ± 2 versus 25 ± 9 CMV (p = 0.001) and similarly, MNO 5 ± 4 versus 25 ± 9 CMV (p = 0.001).
Figure 2: Outcome Flowchart of 106 Antenatally Diagnosed Posterolateral (Bochdalek) Congenital Diaphragmatic Hernia (CDH); Flowchart demonstrating categorisation of CDH-neonates as defined by OSI and outcome measures of all 106 antenatally diagnosed posterolateral (Bochdalek) CDH. Abbreviations: CMV: Conventional Mechanical ventilation. FSR: Following Surgical Repair. HFOV: High Frequency Oscillatory Ventilation. HSN: High Frequency Oscillatory Ventilation + Sildenafil + iNO. iNO: Inhaled Nitric Oxide. MNO: Conventional Mechanical Ventilation + iNO. PSR: Prior to Surgical Repair. RT: Refractory to Treatment.
Figure 3: A: Scatter chart shows OSI of responders-to-treatment (N = 85: 80%), which included neonates on CMV without selective pulmonary vasodilators (N = 30), MNO (N = 24) and HSN (N = 31). iNO, 20 ppm was used for a maximum period of 96-hours. Intravenous sildenafil was utilized for a maximum period of 336-hours. Response to treatment occurred at 30-minutes, which was sustained and improved incrementally by 96-hours and 144-hours, respectively. Mean values with exponential trendlines B: Scatter chart illustrates OSI of 21 (20%) non-responders who died from PPHN and biventricular dysfunction on three-dimensional-echocardiography. Mean values with exponential trendlines.
|
Adverse Events (AEs) All 106 - Neonates |
CDH: CMV OSI: 0-9 N = 36: 34% |
CDH: MNO OSI: 10-25 N = 29: 27% |
CDH: HSN OSI: 26-38 N = 41: 39% |
|
Infants with AEs |
24: 67% |
21: 72% |
28: 68% |
|
Number of AEs |
40 |
47 |
69 |
|
AEs Successfully Managed |
34: 85% |
42: 89% |
59: 86% |
|
AEs and Death |
6: 15% |
5: 11% |
10: 14% |
|
Packed Red Blood Cell Transfusion |
13: 36% |
10: 35% |
15: 37% |
|
Pneumothorax |
8: 22% |
8: 28% |
11: 27% |
|
Hypovolaemia |
4: 11% |
8: 28% |
8: 20% |
|
Hypotension |
4: 11% |
6: 21% |
9: 22% |
|
Hypokalaemia (K+: < 3.3 mmol/L) |
5: 14% |
6: 21% |
8: 20% |
|
Thrombocytopenia Platelets: <100,000 per mm3 |
5: 14% |
6: 21% |
6: 15% |
|
Sepsis |
2: 6% |
1: 4% |
3: 7% |
|
Bradycardia |
0 |
2: 7% |
4: 10% |
|
Drug Withdrawal Syndrome |
0 |
0 |
3: 7% |
|
Treatment Failure (N: %) |
9: 25% |
14: 48% |
10: 24% |
|
Mortality (N: %) |
6: 17% |
5: 17% |
10: 24% |
|
Treatment failure was defined as failure of medical treatment leading to death and/or transfer to HSN. Normal K+: 3.7 to 5.9 mmol/L. |
|||
Table 2: Adverse Events.
Survival probability at 24-hours was 100%. Between 3rd and 10th day, 87% survived. By the 11th and 21st day, 85% were alive (Figure 4). Late survival probability between 22nd and 32nd postnatal days was 80% (Figure 4). There were no further deaths at mean discharge 50 ± 15 days. Survival in CDH-neonates with moderate-PPHN on MNO was 83% versus 76% with severe-PPHN on HSN, which was not statistically significant (p = 0.862). Usage of milrinone, dopamine, dobutamine and epinephrine was equivocal among neonates on CMV, MNO and HSN (Table 1).
Multivariate logistic regression analysis demonstrated there was an increased risk of mortality in CDH-neonates with (1). PPHN (OR: 1.968, 95% CI: 1.72 - 2.26. p = 0.012); (2). Left ventricular and biventricular dysfunction on three-dimensional-echocardiography (OR:1.899, 95% CI: 1.55 - 2.15. p = 0.013); (3). Preoperative pneumothorax (OR: 2.911, 95% CI: 2.66 - 3.19. p = 0.011), and (4). Packed red blood cell transfusion (OR: 2.771, 95% CI: 2.44 - 2.98. p = 0.010).
There were no serious adverse events that warranted cessation of selective pulmonary vasodilator treatment (Table 2). Severe-PPHN associated with biventricular dysfunction on three-dimensional-echocardiography resulted in 19 (90%) deaths. Sepsis and left ventricular dysfunction caused 2 (10%) casualties.
Surgical Repair of CDH
Surgical repair of diaphragm was significantly delayed in infants on HSN 19 ± 2 versus 3 ± 1 days on MNO (p = 0.001) (Table 3). Major gastrointestinal anomalies, which underwent concomitant correction were: (1). Oesophagostomy and gastrostomy for long-gap oesophageal atresia; (2). ligation of tracheoesophageal fistula and esophagoesophagostomy for tracheoesophageal fistula and oesophageal atresia; (3). Duodenoduodenostomy for duodenal atresia; (4). Jejunojejunostomy for jejunal atresia; (5). Correction of caecal volvulus, (6). Correction of small bowel volvulus following lysis of inflammatory bands, and (7). Colostomy for rectal atresia. Type of diaphragmatic defect and hernial repair are summarized in Table 3 [16]. Majority underwent synthetic patch repair (83%). Two deaths, which occurred post-surgery were related to sepsis and left ventricular dysfunction.
|
SURGICAL INTERVENTION (N = 87: 82%). DIAPHRAGMS REPAIRED (N = 88) |
|||
|
Surgical Repair |
CDH: CMV No-PPHN OSI: 0-9 N = 30 |
CDH: MNO M-PPHN OSI: 10–25 N = 25 |
CDH: HSN S-PPHN OSI: 26–38 N = 32 |
|
Time at Surgery (Days: µ ± SD) |
2.4 ± 0.6 |
4.3 ± 0.2 |
19.1 ± 2.2 |
|
Primary Diaphragmatic Muscle Repair (N = 12: 14%) |
10 |
2 |
0 |
|
Synthetic Mesh Repair (N = 72: 83%) |
20 |
23 |
29 |
|
Primary Combined Latissimus Dorsi and Serratus Anterior Flap Repair [6] (N = 3: 3%) |
0 |
0 |
3 |
|
Congenital Diaphragmatic Agenesis |
0 |
0 |
3 |
|
Bilateral Diaphragmatic Hernias |
0 |
0 |
1 |
|
Associated Gastrointestinal Anomalies. (N = 22: 25%) |
4 |
6 |
12 |
|
Post Surgical Survival Rate (%) |
100 |
96 |
97 |
|
Defect Size of Diaphragms at Hernia Repair (N = 88) |
|||
|
Type A (N = 17: 19%) |
15 |
2 |
0 |
|
Type B (N = 39: 44%) |
12 |
14 |
13 |
|
Type C (N = 27: 31%) |
3 |
9 |
15 |
|
Type D (N = 5: 6%) |
0 |
0 |
5 |
|
Note: Type A: Defect surrounded by diaphragmatic muscle [16]. Type B: Defect has <50% of the chest wall devoid of diaphragm [16]. Type C: Defect has >50% of the chest wall lacking diaphragm [16]. Type D: Complete or near complete absence of the diaphragm [Diaphragmatic Agenesis] [6,16]. One child had bilateral diaphragmatic hernia, hence 88-diaphragmatic repairs. |
|||
Table 3: Surgical Management.
4. Discussion
CDH is a complex anomaly with diverse structural variances of the lung and abnormalities of the heart due to compression from herniated viscera. Co-administered intravenous sildenafil and iNO was effective in treating severe-PPHN (OSI: 26 - 38), which reduced OSI in first 4-hours of commencing therapy. OSI declined consistently with complete resolution of PPHN by 144-hours. In this group of critically ill neonates, PPHN mortality was 24%, which compared with reported overall mortality of 26% to 32% [1-4,9,17,18]. Usage of concentration targeted intravenous sildenafil dose of 0.4 mg/kg, followed by 1.6 mg/kg/day continuous infusion achieved appropriate sildenafil plasma levels that was reflected in sustained incremental reduction in OSI [9,17-19]. Sildenafil with iNO decreased pulmonary vascular resistance and increased cardiac output improving OSI by reversing left-to-right shunting across the ductus arteriosus [9]. Sildenafil probably reduced degradation of cGMP produced by iNO, thereby, working synergistically to improve cardiac output, and reducing pulmonary hypertension. Milrinone (50%) and inotropes (75%) were required to maintain normal systemic blood pressure, and for preventing ventilation/perfusion mismatch [17-19]. Eleven percent of infants refractory to iNO responded to intravenous sildenafil [18]. Adverse events were overcome with timely usage of milrinone and inotropes, although, this usage was proportionally symmetrical among all 106-neonates.
Low MAP HFOV was effective in achieving maximum lung inflation, which avoided overdistension [6,11,20-22]. HSN adopted lung protective low MAP ventilation, which was lower than VICI trial but in accordance with Wild et al. [20] and Semama et al. [22] achieving 76% survival in CDH-neonates with severe-PPHN [4,20,22]. It was the preferred rescue mode of ventilation when CMV/MNO had failed. HSN had less VFD at 60-days, NICUFD at 60-days and HFD at 60-days, respectively. In the absence of paediatric extracorporeal membrane oxygenation multimodal treatment with standardized protocols targeting pulmonary hypertension and following lung protection strategy during ventilation led to 76% survival.
CDH-neonates with moderate-PPHN benefited from optimal low-barotrauma CMV and iNO, achieving adequate alveolar recruitment and ventilation. Early commencement of inotropes and milrinone increased cardiac output, maintained adequate mean blood pressure, and enhanced oxygen delivery to tissues. Survival was 83% with OSI falling by 50% in the first 4-hours of treatment and reaching complete resolution by 96-hours. Positive response and high survival have been previously reported, although, in this group, milrinone could have contributed as an inotrope and pulmonary vasodilator [23-25]. Study postulates that pulmonary vasoconstriction and ventilation-perfusion mismatching responded to iNO and associated left ventricular hypoplasia with potential pulmonary venous hypertension was resolved by milrinone. iNO failure rate was 35% with 24% being rescued with HSN. In near-term CDH-neonates with moderate-PPHN, iNO did not reduce the risk of death in 17%, or use of HSN in 24%. Deaths occurred from biventricular dysfunction coupled with aberrant pulmonary vascular development, which dulled nitric oxide signalling pathway.
Antenatally diagnosed CDH without PPHN (37%) were treated by optimal low-barotrauma CMV and early inotropes. This protocol led to early hernia repair (58-hours) with 83% survival. In low-risk CDH-neonates low-barotrauma CMV with early inotropes significantly increases survival in antenatally diagnosed, O/E LHR of 44.2 with type A and B diaphragmatic defects [16]. Timing of surgery was dependent on haemodynamic and ventilatory stability in eighty-seven operated CDH-neonates. This resulted in early or delayed diaphragmatic hernia repair, ensuring 98% post-operative survival.
Limitation of study was lack of randomization. Unique strength of this prospective observational study was that clinically sildenafil and nitric oxide acted synergistically in treating severe-PPHN in critically ill CDH-neonates on HFOV. Sensitivity and specificity of HSN was 80% and 74% respectively, ensuring 76% survival in this high-risk group. Preoperative pneumothorax was a strong predictor of mortality. Frequent blood sampling resulted in administration of packed red blood cell transfusion to prevent anaemia and improve oxygenation. Release of immune mediators following transfusion can cause further pulmonary damage in hypoplastic lungs of CDH-neonates, increasing risk of mortality by 3-fold. Lastly, milrinone acted as an inotropic-vasodilator in >50% of CDH-neonates in this study, which may have improved left ventricular function and promoted pulmonary vasodilation in conjunction with sildenafil and iNO.
5. Conclusion
Congenital diaphragmatic hernia is a complex anomaly whose management has gradually improved with technological advances in ventilation and development of new selective pulmonary vasodilators. Multimodal therapy (HSN) achieved 76% survival with less VFD at 60-days and NICUFD at 60-days in CDH-neonates with severe-PPHN. Intravenous sildenafil usage in CDH neonates will be further validated in CoDiNOS trial [6]. Overall survival of CDH-neonates with or without PPHN was 80%. Despite the need for inotropes and milrinone, efficacy of HSN in treating severe-PPHN in CDH-neonates seems promising in NICUs without paediatric extracorporeal membrane oxygenation.
Advances in Knowledge
- • Three-dimensional-echocardiography and non-invasive, validated, preductal oxygenation saturation index by pulse oximetry diagnosed and monitored the progression of PPHN in CDH-neonates.
- • Co-administered intravenous sildenafil and inhaled nitric oxide acted synergistically on CDH-neonates with severe- PPHN on high frequency oscillatory ventilation achieving 76% survival. Overall survival of CDH-neonates with or without PPHN was 80%.
- • Less VFD at 60-days, NICUFD at 60-days and HFD at 60-days was observed in neonates treated with HSN.
- • There were no serious adverse events that warranted cessation of selective pulmonary vasodilator treatment.
Application to Patient Care
- • Tertiary level III neonatal intensive care units without paediatric extracorporeal membrane oxygenation would benefit from multimodal treatment protocol of HFOV and co-administered intravenous sildenafil and iNO on CDH-neonates with severe-PPHN.
- • Three-dimensional-echocardiography was an accurate diagnostic tool, which diagnosed and regularly monitored the progression of PPHN in CDH-neonates.
- • Low mean airway pressure HFOV was effective in achieving maximum lung inflation.
- • Study outcomes can be used in counselling parents and carers of these critically ill neonates.
Statements and Declarations
Contributors’ Statements and Declarations
All authors (RP, NP and MS) contributed to the study concept and design. Material preparation, data collection and analysis were performed by RP, NP and MS. The first draft of the manuscript was written by Dr. Madan Samuel and all authors commented on previous versions of the manuscript. All authors read and approved of the final manuscript. They approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding: The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests: The authors have no relevant financial or non-financial interests to disclose.
Conflict of Interest: The authors declare no competing interest.
Data Availability: The data that support the findings of this study are not publicly available due to privacy reasons and in accordance with consent provided by parents/carers on the use of confidential data.
References
- Paoletti M, Raffler G, Gaffi MS, et al. Prevalence and risk factors for congenital diaphragmatic hernia: a global view. J Pediatr Surg 55 (2020): 2297-307.
- Politis MD, Bermejo-Sanchez E, Canfield MA, et al. Prevalence and Mortality among Children with Congenital Diaphragmatic Hernia: A Multi-Country Analysis. Ann Epidemiol 56 (2021): 61-69.
- Gupta VS, Harting MT, Lally PA, et al. Mortality in Congenital Diaphragmatic Hernia: A Multicenter Registry Study of Over 5000 Patients Over 25 Years. Ann Surg 277 (2023): 520-527.
- Snoek KG, Capolupo I, van Rosmalen J, et al. Conventional mechanical ventilation versus high frequency oscillatory ventilation for congenital diaphragmatic hernia: a randomised clinical trial (the VICI-trial). Ann Surg 263 (2016): 867-874.
- Kool H, Mous D, Tibboel D, et al. Pulmonary vascular development goes awry in congenital lung abnormalities. Birth Defects Res C Embryo Today 102 (2014): 343-358.
- Samuel M, Parapurath R. Primary Combined Latissimus Dorsi, and Serratus Anterior Flap Repair of Right-Sided Congenital Diaphragmatic Agenesis in a Neonate. Sultan Qaboos University Med J 16 (2016): e96-e100.
- Putnam LR, Tsao K, Morini F, et al. Evaluation of variability in inhaled nitric oxide use and pulmonary hypertension in patients with congenital diaphragmatic hernia. JAMA Pediatr 170 (2016): 1118-1194.
- Cochius-den Otter S, Schaible T, Greenough A, et al. The CoDiNOS trial protocol: an international randomised controlled trial of intravenous sildenafil versus inhaled nitric oxide for the treatment of pulmonary hypertension in neonates with congenital diaphragmatic hernia. BMJ Open 9 (2019): e032122.
- Cochius-den Otter SCM, Kipfmueller F, de Winter BCM, et al. Pharmacokinetic modelling of intravenous sildenafil in newborns with congenital diaphragmatic hernia. Eur J Clin Pharmacol 76 (2020): 219-227.
- Wharton J, Strange JW, Møller GM, et al. Antiproliferative Effects of Phosphodiesterase Type 5 Inhibition in Human Pulmonary Artery Cells. Am J Respir Crit Care Med 172 (2005): 105-113.
- Parapurath R, Samuel M. Efficacy of Inhaled Nitric Oxide and Intragastric Sildenafil in Treatment of Persistent Pulmonary Hypertension of Newborn (PPHN) on High Frequency Oscillatory Ventilation (HFOV). Adv Pediatr Res 10 (2023): e1000068.
- Yang MJ, Russell KW, Yoder BA. Congenital diaphragmatic hernia: a narrative review of controversies in neonatal management. Transl Pediatr 10 (2021): 1432-1447.
- Rafat N, Schaible T. Extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Front Pediatr 7 (2019): e336.
- Muniraman HK, Song AY, Ramanathan R, et al. Evaluation of Oxygen Saturation Index compared with Oxygenation Index in neonates with hypoxemic respiratory failure. Jama Netw Open 2 (2019): e191179.
- Yehya N, Harhay MO, Curley MAQ, et al. Reappraisal of ventilatory-free days in critical care research. Am J Respir Crit Care Med 7 (2019): 828-836.
- Baschat AA, Desiraju S, Bernier ML, et al. Management advances for congenital diaphragmatic hernia: integrating prenatal and postnatal prospectives. Transl Pediatr 13 (2024): 643-662.
- Kipfmueller F, Schroeder L, Berg C, et al. Continuous intravenous sildenafil as an early treatment in neonates with congenital diaphragmatic hernia. Pediatr Pulmonol 53 (2018): 452-460.
- Bialkowski A, Moenkemeyer F, Patel N. Intravenous sildenafil in the management of pulmonary hypertension associated with congenital diaphragmatic hernia. Eur J Pediatr Surg 25 (2015): 171-176.
- Noori S, Friedlich P, Wong P, et al. Cardiovascular effects of sildenafil in neonates and infants with congenital diaphragmatic hernia and pulmonary hypertension. Neonatology 91 (2007): 92-100.
- Wild KT, Mathew L, Ades AM, et al. Association between initial ventilation mode and hospital outcomes for severe congenital diaphragmatic hernia. J Perinatol 44 (2024): 1353-1358.
- Fuyuki M, Usui N, Taguchi T, et al. Prognosis of conventional vs. high-frequency ventilation for congenital diaphragmatic hernia: a retrospective cohort study. J Perinatol 41 (2021): 814-823.
- Semama C, Vu S, Kyheng M, et al. High-frequency oscillatory ventilation versus conventional ventilation in the respiratory management of term neonates with a congenital diaphragmatic hernia: a retrospective cohort study. Eur J Pediatr 181 (2022): 3899-3906.
- Yang MJ, Fenton S, Russell K, et al. Left-sided congenital diaphragmatic hernia: can we improve survival while decreasing ECMO? J Perinatol 40 (2020): 935-942.
- Lawrence KM, Monos S, Adams S, et al. Inhaled Nitric Oxide Is Associated with Improved Oxygenation in a Subpopulation of Infants with Congenital Diaphragmatic Hernia and Pulmonary Hypertension. J Pediatr 219 (2020): 167-172.
- Datin-Dorriere V, Walter-Nicolet E, Rousseau V, et al. Experience in the management of eighty-two newborns with congenital diaphragmatic hernia treated with high-frequency oscillatory ventilation and delayed surgery without the use of extracorporeal membrane oxygenation. J Intensive Care Med 23 (2008): 128-135.