Efficacy of Cetuximab and 4-PBA Combination Therapy in Human Oral Squamous Cell Carcinoma Cells
Article Information
Akira Noguchi*, Takeshi Nishida, Hideki Hatta, Kohji Takagi, Toshiko Kakiuchi, Shinichi Tanaka, Takashi Minamisaka, Takahiko Nakajima, Johji Imura
Department of Diagnostic Pathology, Faculty of Medicine, Academic Assembly, University of Toyama, Toyama City, Japan
*Corresponding Author: Akira Noguchi, Department of Diagnostic Pathology, Faculty of Medicine, Academic Assembly, University of Toyama, 2630 Sugitani, Toyama City, Toyama, 930-0194, Japan
Received: 23 November 2021; Accepted: 02 December 2021; Published: 20 December 2021
Citation: Akira Noguchi, Takeshi Nishida, Hideki Hatta, Kohji Takagi, Toshiko Kakiuchi, Shinichi Tanaka, Takashi Minamisaka, Takahiko Nakajima and Johji Imura. Efficacy of Cetuximab and 4-PBA Combination Therapy in Human Oral Squamous Cell Carcinoma Cells. Journal of Cancer Science and Clinical Therapeutics 5 (2021): 562-575.
View / Download Pdf Share at FacebookAbstract
Cetuximab is a powerful anti-neoplastic agent that can inhibit cell growth in oral squamous cell carcinomas (OSCCs). Unfortunately, there are cases with unfavorable outcomes. Because few studies have focused on the combined effects of cetuximab and histone deacetylase (HDAC) inhibitors, we aimed to evaluate the antitumor effect of cetuximab in combination with HDAC inhibitors in human OSCC cell lines, and investigate the mechanism of apoptosis enhancing activity thereof. We used human OSCC cell lines treated with cetuximab and several HDAC inhibitors. The WST assay and ApoToxGlo™ Triplex Assay determined cell survival. We employed the TdTmediated dUTP-biotin nick end labeling method to detect apoptosis. We used western blotting to examine the histone acetylation status, ER stress markers, and epidermal growth factor receptor (EGFR) signaling pathways. Our results show that cetuximab in combination with 4-phenyl butyric acid (4-PBA) remarkably decreases cell growth in vitro. In addition, the combined treatment resulted in increased EGFR mRNA expression and promoted the activation of ERK. The combination treatment induced apoptosis at a significantly higher frequency than did either agent alone. In conclusion, the combination of cetuximab and 4-PBA is more effective against human OSCC cells, allowing for potential clinical applicability of combination treatment in OSCC therapies.
Keywords
Oral squamous cell carcinoma; Combination therapy; Tetuximab; Histone deacetylase inhibitors; Cancer; Epigenetics
Oral squamous cell carcinoma articles; Combination therapy articles; Tetuximab articles; Histone deacetylase inhibitors articles; Cancer articles; Epigenetics articles
Oral squamous cell carcinoma articles Oral squamous cell carcinoma Research articles Oral squamous cell carcinoma review articles Oral squamous cell carcinoma PubMed articles Oral squamous cell carcinoma PubMed Central articles Oral squamous cell carcinoma 2023 articles Oral squamous cell carcinoma 2024 articles Oral squamous cell carcinoma Scopus articles Oral squamous cell carcinoma impact factor journals Oral squamous cell carcinoma Scopus journals Oral squamous cell carcinoma PubMed journals Oral squamous cell carcinoma medical journals Oral squamous cell carcinoma free journals Oral squamous cell carcinoma best journals Oral squamous cell carcinoma top journals Oral squamous cell carcinoma free medical journals Oral squamous cell carcinoma famous journals Oral squamous cell carcinoma Google Scholar indexed journals Combination therapy articles Combination therapy Research articles Combination therapy review articles Combination therapy PubMed articles Combination therapy PubMed Central articles Combination therapy 2023 articles Combination therapy 2024 articles Combination therapy Scopus articles Combination therapy impact factor journals Combination therapy Scopus journals Combination therapy PubMed journals Combination therapy medical journals Combination therapy free journals Combination therapy best journals Combination therapy top journals Combination therapy free medical journals Combination therapy famous journals Combination therapy Google Scholar indexed journals Tetuximab articles Tetuximab Research articles Tetuximab review articles Tetuximab PubMed articles Tetuximab PubMed Central articles Tetuximab 2023 articles Tetuximab 2024 articles Tetuximab Scopus articles Tetuximab impact factor journals Tetuximab Scopus journals Tetuximab PubMed journals Tetuximab medical journals Tetuximab free journals Tetuximab best journals Tetuximab top journals Tetuximab free medical journals Tetuximab famous journals Tetuximab Google Scholar indexed journals Histone deacetylase inhibitors articles Histone deacetylase inhibitors Research articles Histone deacetylase inhibitors review articles Histone deacetylase inhibitors PubMed articles Histone deacetylase inhibitors PubMed Central articles Histone deacetylase inhibitors 2023 articles Histone deacetylase inhibitors 2024 articles Histone deacetylase inhibitors Scopus articles Histone deacetylase inhibitors impact factor journals Histone deacetylase inhibitors Scopus journals Histone deacetylase inhibitors PubMed journals Histone deacetylase inhibitors medical journals Histone deacetylase inhibitors free journals Histone deacetylase inhibitors best journals Histone deacetylase inhibitors top journals Histone deacetylase inhibitors free medical journals Histone deacetylase inhibitors famous journals Histone deacetylase inhibitors Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Epigenetics articles Epigenetics Research articles Epigenetics review articles Epigenetics PubMed articles Epigenetics PubMed Central articles Epigenetics 2023 articles Epigenetics 2024 articles Epigenetics Scopus articles Epigenetics impact factor journals Epigenetics Scopus journals Epigenetics PubMed journals Epigenetics medical journals Epigenetics free journals Epigenetics best journals Epigenetics top journals Epigenetics free medical journals Epigenetics famous journals Epigenetics Google Scholar indexed journals Acetoxychavicol acetate-28 articles Acetoxychavicol acetate-28 Research articles Acetoxychavicol acetate-28 review articles Acetoxychavicol acetate-28 PubMed articles Acetoxychavicol acetate-28 PubMed Central articles Acetoxychavicol acetate-28 2023 articles Acetoxychavicol acetate-28 2024 articles Acetoxychavicol acetate-28 Scopus articles Acetoxychavicol acetate-28 impact factor journals Acetoxychavicol acetate-28 Scopus journals Acetoxychavicol acetate-28 PubMed journals Acetoxychavicol acetate-28 medical journals Acetoxychavicol acetate-28 free journals Acetoxychavicol acetate-28 best journals Acetoxychavicol acetate-28 top journals Acetoxychavicol acetate-28 free medical journals Acetoxychavicol acetate-28 famous journals Acetoxychavicol acetate-28 Google Scholar indexed journals polymerase chain reaction articles polymerase chain reaction Research articles polymerase chain reaction review articles polymerase chain reaction PubMed articles polymerase chain reaction PubMed Central articles polymerase chain reaction 2023 articles polymerase chain reaction 2024 articles polymerase chain reaction Scopus articles polymerase chain reaction impact factor journals polymerase chain reaction Scopus journals polymerase chain reaction PubMed journals polymerase chain reaction medical journals polymerase chain reaction free journals polymerase chain reaction best journals polymerase chain reaction top journals polymerase chain reaction free medical journals polymerase chain reaction famous journals polymerase chain reaction Google Scholar indexed journals Mitogen-activated protein kinase articles Mitogen-activated protein kinase Research articles Mitogen-activated protein kinase review articles Mitogen-activated protein kinase PubMed articles Mitogen-activated protein kinase PubMed Central articles Mitogen-activated protein kinase 2023 articles Mitogen-activated protein kinase 2024 articles Mitogen-activated protein kinase Scopus articles Mitogen-activated protein kinase impact factor journals Mitogen-activated protein kinase Scopus journals Mitogen-activated protein kinase PubMed journals Mitogen-activated protein kinase medical journals Mitogen-activated protein kinase free journals Mitogen-activated protein kinase best journals Mitogen-activated protein kinase top journals Mitogen-activated protein kinase free medical journals Mitogen-activated protein kinase famous journals Mitogen-activated protein kinase Google Scholar indexed journals 4-phenyl butyric acid articles 4-phenyl butyric acid Research articles 4-phenyl butyric acid review articles 4-phenyl butyric acid PubMed articles 4-phenyl butyric acid PubMed Central articles 4-phenyl butyric acid 2023 articles 4-phenyl butyric acid 2024 articles 4-phenyl butyric acid Scopus articles 4-phenyl butyric acid impact factor journals 4-phenyl butyric acid Scopus journals 4-phenyl butyric acid PubMed journals 4-phenyl butyric acid medical journals 4-phenyl butyric acid free journals 4-phenyl butyric acid best journals 4-phenyl butyric acid top journals 4-phenyl butyric acid free medical journals 4-phenyl butyric acid famous journals 4-phenyl butyric acid Google Scholar indexed journals
Article Details
Abbreviations:
CHOP - CCAAT/enhancer-binding protein homologous protein; EGFR - Epidermal growth factor receptor; MAPK - Mitogen-activated protein kinase; OSCC - Oral squamous cell carcinoma; HDAC - Histone deacetylase; HDACi - Histone deacetylase inhibitors; 4-PBA - 4-phenyl butyric acid; TUNEL - TdT-mediated dUTP-biotin nick end labeling; ACA-28 - Acetoxychavicol acetate-28; PCR - polymerase chain reaction
1. Introduction
Oral squamous cell carcinoma (OSCC) is a cancer origin-ating from the oral mucosal epithelium that accounts for more than 90% of malignant tumors of the oral cavity and considerably influences the quality of life. Smoking is the predominant cause of OSCC [1] although, viruses such as human papillomavirus, particularly type 16, are thought to play a role in OSCC development [2]. At the cellular level, tumor suppressors (p53 and p16) are often lost whereas oncogenes, including epidermal growth factor receptor (EGFR) are amplified or mutated. Increased EGFR gene copy numbers and protein overexpression result from regulatory pathway changes, gene structure changes, or gene amplification, and these play an important role in carcinogenesis [3]. EGFR is an important therapeutic target and prognostic factor for OSCC treatment. Treatment with the monoclonal antibody cetuximab exerts beneficial clini-cal effects. Cetuximab specifically binds to and degrades EGFR. In addition, cetuximab inhibits the binding of EGFR to its ligands and blocks the downstream signal trans-duction pathways, leading to inhibition of proliferation and apoptosis induction in tumor cells [4]. RAS somatic mutations are negative predictors of the clinical efficacy of anti-EGFR antibodies. Therefore, routine screening for mutations in K-RAS is used in colorectal cancer for patient selection prior to treatment [5, 6]. However, in OSCC, primary RAS mutations are rare and the importance of the mutations remain unclear [7, 8].
Epigenetic DNA and histone modifications alter chromatin conformation and play a predominant role in the control of transcription and regulation of genes. Histone deacetylases (HDACs) are powerful epigenetic regulators that act as transcriptional repressors by removing acetyl groups from histones. HDACs are dysregulated in many cancers, including OSCC, making them a therapeutic target for the treatment of cancer [9]. However, experimental and clinical studies have demonstrated that the currently available histone deacetylase inhibitors (HDACi) are not adequate as single-agent therapies against OSCC [9, 10], despite the fact that HDACi exert significant antitumor effects in combination therapies. The combination of HDACi with other standard chemotherapeutic agents could achieve higher anticancer effects in OSCC [11, 12]. Based on these reports, we hypothesized that cetuximab sensitivity is caused by the presence of epigenetic alterations and the approaches employed to screen candidate materials for rep-urposing and/or repositioning cetuximab in commercially available HDACi. Therefore, we investigated whether HDACi have a beneficial effect when combined with cetux-
imab in OSCC cells expressing high levels of EGFR.
2. Methods
2.1 Cell culture
B88 and KOSC-2 cells are human squamous cell carcinoma (SCC) cell lines established from SCCs of the tongue [13]. B88 was a generous gift from the Kanagawa Cancer Center Research Institute (Kanagawa, Japan). KOSC-2 was pur-chased from the JCRB Cell Bank (Osaka, Japan). In this study, both cells were routinely cultured in RPMI-1640 (Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, penicillin (100 U/ml), and streptomycin (100 μg/ml). Cultures were maintained in a humidified incubator at 37°C in 5% CO2.
2.2 HDACi screening
Drug screening was performed using the DiscoveryPak HDAC Inhibitor Set (splitomicin, apicidin, valproic acid, trichostatin A, 4-phenyl butyric acid [4-PBA]) obtained from BioVision (Milpitas, CA, USA). Cetuximab was purchased from Merck Serono (Burlington, MA, USA). B88 and KOSC-2 cells were plated at 10,000 cells/well in a 96-well plate. After incubation for 24 h, HDACi, cetuximab (500 mg/ml), or both were added. After 24 h of incubation, the cell toxicity was evaluated using a Cell Counting Kit-8 assay (Dojindo Laboratories, Kumamoto, Japan). Absor-bance at 450 nm was measured using a microplate reader (Thermo Scientific Multiskan FC, Thermo Fisher Scientific Life Sciences, Waltham, MA, USA).
2.3 RNA extraction and quantitative RT-PCR
The EGFR mRNA levels were determined at 24 h and 48 h post-treatment with 4-PBA (5 mM), cetuximab (500 mg/ml), or both by RT-qPCR. Total RNA was extracted from B88 cells using TRIzol reagent (Invitrogen, La Jolla, CA, USA) according to the manufacturer’s protocol. Two micrograms of cellular RNA were converted to cDNA following the manufacturer’s instructions for the specific reverse transcription kit (Invitrogen). Quantitative RT-PCR was performed using primers specific for EGFR (Forward: 5'-AAGTGTAAGAAGTGCGAAGG-3'. Reverse: 5'-GGAGGAGTATGTGTGAAGGA-3'.). The degree of exp-ression was quantified using a relative standard curve and normalized to GAPDH that was amplified using specific primers (Forward: 5'-GTCAACGGATTTGGTCGTATT-3'. Reverse: 5'-GATCTCGCTCCTGGAAGATGG-3'.).
2.4 Cell viability, cytotoxicity, and caspase 3/7 activities
Cell viability and caspase 3/7 activities were evaluated in B88 cells treated with 4-PBA (5 mM), cetuximab (500 mg/ml), or both using the ApoToxGlo Triplex Assay kit (Promega, Madison, WI, USA) following the manufactu-rer’s instructions. The ApoToxGlo Triplex Assay combines three assay chemistries to assess viability, cytotoxicity, and caspase activation events in the same cell-based assay. Briefly, 10 µl of viability/cytotoxicity reagent containing both GF-AFC substrate and bis-AAF-R110 substrates were added to all wells. After incubation for 1 h at 37°C, the Relative Fluorescence Units (RFU) was determined at 370 Ex/535 Em for cell viability and at 485 Ex/535 Em for cytotoxicity. To determine the effect on caspase-3/7 activity of B88 cells treated with 4-PBA and cetuximab, 10 µl of luminogenic caspase-3/7 substrate, which contains the tetrapeptide sequence DEVD were added to all wells. The Relative Luminescence Units (RLU) from the caspase-3/7 activated was measured after 30 min of incubation. The fluorescence and luminescence were detected using a FilterMax-F5 (Molecular Devices LLC, Sunnyvale, CA, USA).
2.5 TdT-mediated dUTP-biotin nick end labeling assay
For the in situ analysis of DNA fragmentation, TdT-mediated dUTP-biotin nick end labeling (TUNEL) staining was performed using an ApopTag peroxidase in situ apoptosis detection kit (Merck Millipore, MA, USA) according to the manufacturer's instructions. The apoptotic index (average percentage of apoptotic cells labeled by the TUNEL method) was determined as the number of DAB-stained cells versus the total number of cells using light microscopy. A minimum of 1,000 cells were counted in random fields.
2.6 Western blot
Cells were lysed in a radioimmunoprecipitation assay buffer 24 h after drug treatment. The protein concentration was measured using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Rockford, IL, USA). Samples were run on sodium dodecyl sulfate-polyacrylamide gel and transferred onto polyvinylidene difluoride membranes. After blocking with 5% skim milk, the membranes were incubated with primary antibodies. Blots were then incu-bated with peroxidase-conjugated secondary antibodies and detected by ECL chemiluminescence, and images were captured with ImageQuant LAS-500 analyzer (GE Healthcare Life Sciences, Pittsburgh, PA, USA).
Primary antibodies against the following proteins were used: EGFR, p-EGFR, ERK, Akt, p-AKT, cleaved-PARP, and GRP78 (Santa Cruz Biotechnologies, Santa Cruz, CA, USA); B-cell lymphoma 2 (bcl-2), CCAAT/enhancer-bind-ing protein homologous protein (CHOP), and p-ERK (Cell Signaling Technology, Beverly, MA, USA); H3K9,14ac, H3K27ac, and H4K16ac (MAB Institute, Nagano, Japan); β-actin (Sigma-Aldrich, St. Louis, MO, USA).
2.7 Statistical analysis
All experiments were performed in triplicate and the values represent the average of at least three independent experiments. We expressed all data as mean ± standard deviation. One-way analysis of variance (ANOVA) with Bonferroni’s post-hoc test was used to analyze the differences between the combination of cetuximab and HDACi vs. individual treatments. p Values <0.05 were considered significant. We used SPSS software for Windows (V26.0; SPSS Inc., Chicago, IL, USA) for all statistical analyses.
3. Results
3.1 Effect of the combination of HDACi and Cetuximab in OSCC cell proliferation
To investigate the effect of HDACi in OSCC cells, B88 and KOSC-2 cells were treated with cetuximab, HDACi (200nM trichostatin A, 5mM 4-PBA, 60μM splitomicin, 50nM apicidin, 1mM valproic acid), or the combination of cetuximab and HDACi. Both cells showed similar findings. The cetuximab-treated group was slightly differentiated from the control group. When cells were treated with 4-PBA and trichostatin A, significant toxicity was observed (Figure 1A and B). Moreover, when treated with the combination, additive or synergistic action was observed. No difference was observed in splitomicin, apicidin, and valproic acid (Figure 1C-E). The results indicated 4-PBA as the most useful HDACi.
Figure 1: Cetuximab resistance cells were improved susceptibility of cetuximab by treatment with 4-PBA. KOSC-2 and B88 cells were treated with cetuximab and/or HDACi [trichostatin A (A), 4-PBA (B), splitomicin (C), apicidin (D), valproic acid (E)] for 24 h. The effect of cetuximab and/or HDACi on cell proliferation as measured by cell counting kit-8 assay. 4-PBA was most useful in HDACi. Values represent the mean ±SD of three independent experiments. *p<0.05.
3.2 Morphological changes in B88 cells after exposure to 4-PBA and/or cetuximab
B88 cells have an epithelial sheet-like structure under standard incubation conditions (Figure 2A). B88 cell shape did not change when treated with cetuximab (Figure 2B); when treated with 4-PBA, B88 changed from an epithelial sheet-like structure to a spindle shape (Figure 2C). B88 cells showed marked changes and exhibited signs of cellular damage when treated with 4-PBA and cetuximab, including disruption of the intercellular junction complexes, swollen or prominent nuclei, shrunken cytosol, ruptured nuclear and plasma membranes, or nuclear fragmentation (Figure 2D).
Figure 2: 4-PBA caused a significant effect in morphology. B88 cells were treated with cetuximab and/or 4-PBA for 24 h. When treated with cetuximab, B88 had not changed (A, B). When treated with 4-PBA, B88 had changed shape from epithelial sheet-like structure to spindle shape (C). When treated with 4-PBA and cetuximab, B88 had changed markedly (D). Scale bars, 100 µm.
3.3 The mRNA levels of EGFR were elevated after treatment with 4-PBA
Treatment of B88 cells with cetuximab for 24 h significantly reduced EGFR mRNA expression by ~50% (Figure 3). Treatment of B88 cells with 4-PBA slightly increased EGFR mRNA expression. Interestingly, treat-ment of B88 cells with a combination of 4-PBA and cetuximab increased EGFR mRNA expression. It is evident from these findings that the mRNA levels of EGFR are affected by 4-PBA treatment.
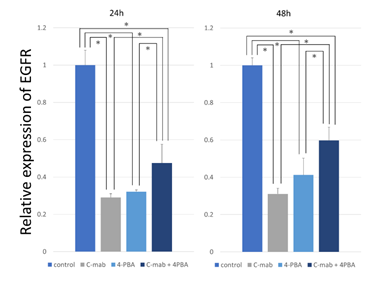
Figure 3: Expression analysis of EGFR. B-88 cells were treated with cetuximab and/or 4-PBA for 24 h and 48h. The amount of targeted EGFR gene was determined by quantitative real-time PCR with normalization to GAPDH. The relative amount of EGFR gene was determined using the 2−ΔΔCt method. When treated with cetuximab, EGFR expression was markedly decreased. When treated with 4-PBA and cetuximab, EGFR expression was increased. Values represent the mean ±SD of three independent experiments *p<0.05.
3.4 Mechanism of cetuximab/4-PBA-induced cell death
B88 cell viability, cytotoxicity, and apoptosis were meas-ured using an ApoToxGlo Triplex Assay to further evaluate the effects of cetuximab/4-PBA. The 4-PBA treatment resulted in a dose-dependent reduction in the number of viable B88 cells (Figure 4A), and cell toxicity was increased (Figure 4B). In addition, treatment with different concentrations of 4-PBA (0.31, 0.62, 1.25, 2.5, 5.0, 10 mM) resulted in activation of caspase 3/7 (Figure 4C). These results indicate that 4-PBA increased cetuximab-induced cell toxicity and apoptosis in a dose-dependent manner.
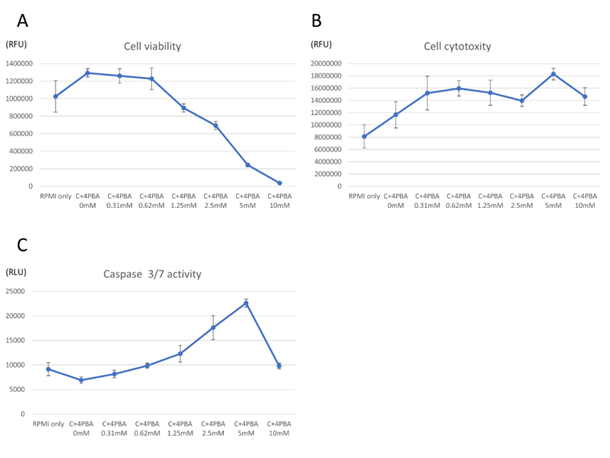
Figure 4: In vitro viability, cytotoxicity and caspase activity were dependent upon dosage of 4-PBA. B88 cells were cultured and treated with 4-PBA in 96-well format at the indicated doses for 24 h to measure the cellular viability, cytotoxicity and caspase activity using the ApoTox-Glo assay. B88 cells in suspension were treated with 4-PBA resulting in a dose-dependent decrease in cell viability (A) and concomitantly increase in cell cytotoxicity (B) and caspase-3/7 activation (C), which were consistent with apoptosis. Values represent the mean ±SD of three independent experiments.
3.5 Effects of the treatment with the combination of cetuximab and 4-PBAon apoptosis
We performed TUNEL staining to investigate the effects of the treatment with the combination of cetuximab and 4PBA on B88 cell apoptosis. We observed only a few apoptotic cells in the control group and cetuximab-treated group (Figure 5A, B and E); apoptosis was slightly increased following treatment of B88 with 4-PBA (Figure 5C and E). The most substantial increase in apoptosis was observed in the combination treatment group (Figure 5D and E). To further investigate the effects of the combination treatment with cetuximab and 4-PBA on apoptosis, the expression levels of CHOP, PERK Bcl-2, and PARP were examined by western blot analysis (Figure 6A). Our results indicated that the expression levels of CHOP, PERK, and Bcl-2 were decreased and that cleaved-PARP was increased in the combination treatment group.
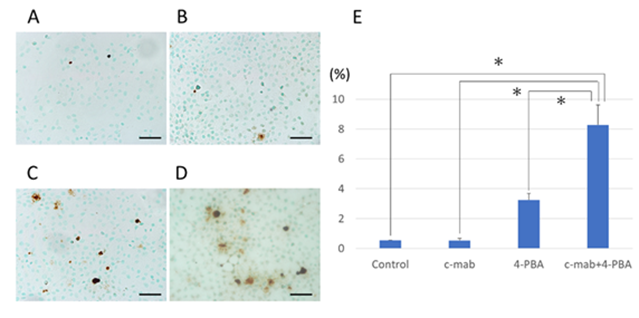
Figure 5: TUNEL assay to determine 4-PBA-induced apoptosis of B88 cells. TUNEL assay was used to confirm the induction of apoptosis in cetuximab and/or 4-PBA treated cells. (A) control, (B) c-mab, (C) 4-PBA, (D) c-mab + 4-PBA treated cells were confirmed by the appearance of TUNEL-positive cells. Scale bars, 50 µm. (E) Results were quantified as the percentage of TUNEL-positive cells in 4 high magnification fields per slide. Values represent the mean ±SD of three independent experiments. *p<0.05
3.6 Combination of cetuximab and 4-PBA activates mitogen-activated protein kinase /ERK pathway
EGFR signaling is transduced by two main pathways mediated by the PI3K-Akt and RAS-RAF-MEK-MAPK. To determine whether phosphorylation of EGFR induced by either 4-PBA or cetuximab is accompanied by activation of these pathways, we examined the levels of phos-phorylated Akt and ERK. Treatment with cetuximab alone did not change the levels of phosphorylated/total Akt and ERK (Figure 6B). Interestingly, treatment with 4-PBA upregulated the expression of phosphorylated ERK. In contrast, the levels of Akt did not change.
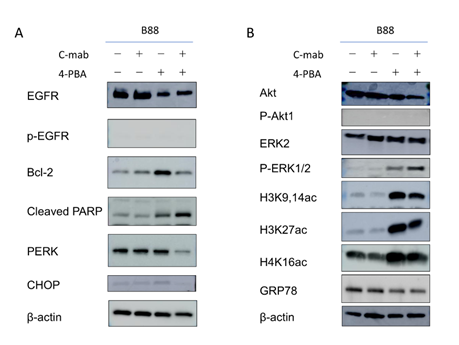
Figure 6: Western blotting after treatment with cetuximab and/or 4-PBA in B88 cells. Western blot analyses for apoptosis-related molecules and related pathways were performed. (A) Western blotting for apoptosis-related molecules after treatment. The expression levels of CHOP, PERK and Bcl-2 were decreased. The expression level of cleaved PARP was elevated by the treatment. (B) Western blotting for related pathways after treatment. The phosphorylation level of AKT was not changed. In contrast, the phosphorylation of ERK1/2 was increased by the 4-PBA treatment. The levels of H3K9/27ac, H3K14,27ac and H4K16ac were increased, GRP78 was decreased by the 4-PBA treatment. β-actin was used as an internal control.
3.7 Combination of cetuximab and 4-PBA modulates histone acetylation and ER stress in B88 cells
Histone acetylation level is a widely accepted marker for HDAC inhibition. For this reason, we examined the HDACi activity of 4-PBA in OSCC cells. Western blot analysis showed that 4-PBA treatment of B88 cells induced histone H3K9, H3K14, 27, and H4K16 acetylation. The levels of H3K9ac, H3K14ac, 27ac, and H4K16ac were increased (Figure 6B), indicating that the acetylation of H3 might play an important role in promoting EGFR gene transcription [14, 15]. Furthermore, we investigated whether 4-PBA could promote the EGFR signaling pathway. The results showed that 4-PBA significantly increased phosphorylation levels of phospho-ERK. The combination of cetuximab and 4-PBA further increased the expression. Interestingly, protein levels of the ER stress marker, GRP78, were significantly decreased in 4-PBA treated and combination of cetuximab and 4-PBA cells, but not in cetuximab only treated cells (Figure 6). These data also show that histone H3 hyperacetylation may correlate with ER stress.
4. Discussion
We screened the candidate materials for repurposing and/or repositioning of cetuximab in commercially available drugs and found that 4-PBA had the potential to induce apoptosis and upregulate the EGFR signaling pathway in OSCC cells. The role of 4-PBA has been established as an alleviator of ER stress. In addition, 4-PBA has antitumor activity as an HDACi. HDACi are divided into different classes based on their chemical properties, including hydroxamic acids, such as suberoylanilide hydroxamic acid (SAHA), trichostatin A; short chain fatty acids, such as 4-PBA and valproic acid; cyclic tetrapeptides, such as apicidin and depsipeptide (also known as FK228 or romidepsin); and benzamides [16, 17]. The varying action of 4-PBA with different HDACi is what improves ER stress. 4-PBA is a histone deacetylase inhibitor and a short-chain fatty acid chemical chaperon that stabilizes protein conformation, improves the capacity of ER folding, and facilitates proper trafficking of mutant proteins. Additionally, 4-PBA acts as an ammonia scaven-ger in patients with urea cycle disorders and hyperamm-onemia [18]. Subsequently, it has been shown that inhibition of the autophagy-mediated lipotoxic state causes increased cytosolic calcium levels [19]. It has also been demonstrated that 4-PBA inhibits tumor growth and epithelial–mesenchymal transition of human OSCC cells [20]. In this study, as expected from earlier research, 4-PBA prevented ER stress and histone deacetylation, and increased the expression of EGFR. It is also worth noting that cetuximab treatment with 4-PBA induced apoptosis and increased the expression of phospho-ERK in OSCC cells.
The mitogen-activated protein kinase (MAPK) signaling pathway plays a central role in the regulation of gene expression, cellular growth, and survival [21]. Defects in the MAPK/ERK pathway lead to uncontrolled cell growth, a necessary step for the development of cancer [22]. Therefore, many researchers have suggested the role of ERK signaling activation in delivering a survival signal that counteracts proapoptotic signaling such as p38. ERK inhibitors have demonstrated preliminary antitumor activity and may be most effective against cancers with RAS, RAF, or MAPK pathway alterations. Several ERK inhibitors (BVD-523, GDC-0994, CC-90003, etc.) have been appro-ved by the Food and Drug Administration for advanced solid tumors [23]. In contrast, some studies in other organs have provided insights into the correlation between ERK activation and anticancer effects [24]. Depending on the cell type and stimulus, ERK activity will mediate different antiproliferative events, such as apoptosis. Satoh et al. [25] showed that acetoxychavicol acetate-28 (ACA-28) strongly stimulated ERK phosphorylation in melanoma cells. Consequently, ACA-28 specifically induced apoptosis that was abrogated when ERK activation was blocked with the specific MEK inhibitor U0126. There is a possibility that the induction of epigenetic alteration by 4-PBA affects the apoptosis linked to stimulation of oncogenic signaling, such as the MAPK/ERK pathway. Contrary to our findings, Shi et al. [26, 27] showed that 4-PBA promotes the migration of gastric cancer cells through upregulation of HER3/ HER4, subsequent to increased levels of acetyl-histone and activation of ERK signaling. The results of the present study do show that 4-PBA significantly increased phos-phorylation levels of phospho-ERK. However, these studies do not adequately account for the significant inhibition of gastric cancer cell proliferation in the presence of 4-PBA concentrations of 5 mM or above; therefore, the potential for antitumor effects seem to be lacking.
The present study clearly shows that the combination of 4-PBA and cetuximab upregulates phosphorylated ERK expression, although cell viability decreases and apoptosis increases. ER stress-induced apoptosis can be triggered via different pathways. CHOP plays an important role in ER stress-induced apoptosis [28]. CHOP is a transcription factor that positively promotes apoptosis following PERK activation. In this study, 4-PBA induced ER stress that was not evidenced by an increase in PERK and CHOP. Rather, the expression of PERK and CHOP in cells treated with 4-PBA and cetuximab was importantly attenuated. Taken together, these data suggest that 4-PBA induces apoptosis by triggering the EGFR signaling pathway. One of the limitations of this study is the discrepancy between mRNA and protein expression of EGFR in the combination treat-ment. The mRNA expression of EGFR increased in the combination treatment; however, the protein level of EGFR remained unchanged. Our results suggest a posttranscrip-tional modulation of EGFR expression. This may contribute to the activation of ERK. A further study of this pheno-menon should be conducted.
5. Conclusions
In summary, we demonstrated that cetuximab with 4-PBA induced histone acetylation and/or ER stress responses in OSCC cells, providing novel insights into the 4-PBA-mediated enhancement of antitumor activity by cetuximab. One interpretation of our results is that the EGFR signaling pathway is not affected by the accumulation of aberrant epigenetic alterations in oral cancer cells. It is clear that histone modification and/or ER stress prevents the action of cetuximab. Thus, therapeutic intervention using a chemical chaperon (4-PBA) that stabilizes misfolded proteins could provide a promising strategy for the enhancement of antitumor activity by cetuximab. Our findings provide experimental evidence for the application of combination therapy in the clinical treatment of OSCC. We hope the findings presented in this paper will contribute to a better understanding of the mechanisms of action of cetuximab.
Acknowledgments
The Authors would like to thank A. Shimomura for technical assistance with the experiments. The Authors would also like to thank Editage (www.editage.com) for English language editing.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang X, Wu J, Wang J, et al. Tobacco and oral squamous cell carcinoma: A review of carcinogenic pathways. Tob Induc Dis 17 (2019): 29.
- El-Naggar JKCC AK, Grandis JR, Takata T, et al. WHO Classification of Head and Neck Tumours. 4th ed. Lyon, France: IARC (2017).
- Ryott M, Wangsa D, Heselmeyer-Haddad K, et al. EGFR protein overexpression and gene copy number increases in oral tongue squamous cell carcinoma. Eur J Cancer 45 (2009): 1700-1708.
- Dutta PR, Maity A. Cellular responses to EGFR inhibitors and their relevance to cancer therapy. Can-cer Letters 254 (2007): 165-177.
- Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66 (2006): 3992-3995.
- Lievre A, Laurent-Puig P. Genetics: Predictive value of KRAS mutations in chemoresistant CRC. Nat Rev Clin Oncol 6 (2009): 306-307.
- Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol 7 (2010): 493-507.
- Lui VW, Hedberg ML, Li H, et al. Frequent mutation of the PI3K pathway in head and neck cancer defines predictive biomarkers. Cancer Discov 3 (2013): 761-769.
- Tasoulas J, Giaginis C, Patsouris E, et al. Histone deacetylase inhibitors in oral squamous cell carcin-oma treatment. Expert Opinion on Investigational Drugs 24 (2015): 69-78.
- Haigentz M, Kim M, Sarta C, et al. Phase II trial of the histone deacetylase inhibitor romidepsin in pati-ents with recurrent/metastatic head and neck cancer. Oral Oncology 48 (2012): 1281-1288.
- Shen J, Huang C, Jiang L, et al. Enhancement of cis-platin induced apoptosis by suberoylanilide hydroxa-mic acid in human oral squamous cell carcinoma cell lines. Biochem Pharmacol 73 (2007): 1901-1909.
- Erlich RB, Rickwood D, Coman WB, et al. Valproic acid as a therapeutic agent for head and neck squa-mous cell carcinomas. Cancer Chemotherapy and Pharmacology 63 (2009): 381-389.
- Uchida D, Begum N-M, Almofti A, et al. Possible role of stromal-cell-derived factor-1/CXCR4 signal-ing on lymph node metastasis of oral squamous cell carcinoma. Experimental Cell Research 290 (2003): 289-302.
- Cheung P, Tanner KG, Cheung WL, et al. Synergistic Coupling of Histone H3 Phosphorylation and Acetylation in Response to Epidermal Growth Factor Stimulation. Molecular Cell 5 (2000): 905-915.
- Yang W, Xia Y, Hawke D, et al. PKM2 Phospho-rylates Histone H3 and Promotes Gene Transcription and Tumorigenesis. Cell 150 (2012): 685-696.
- Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26 (2007): 5541-5552.
- Mai A, Altucci L. Epi-drugs to fight cancer: From chemistry to cancer treatment, the road ahead. The International Journal of Biochemistry & Cell Biology 41 (2009): 199-213.
- Brusilow SW. Phenylacetylglutamine may replace urea as a vehicle for waste nitrogen excretion. Pediatr Res 29 (1991): 147-150.
- Iannitti T, Palmieri B. Clinical and experimental applications of sodium phenylbutyrate. Drugs R D 11 (2011): 227-249.
- Qian K, Sun L, Zhou G, et al. Sodium Pheny-lbutyrate Inhibits Tumor Growth and the Epithelial-Mesenchymal Transition of Oral Squamous Cell Carcinoma In Vitro and In Vivo. Cancer Biother Radiopharm 33 (2018): 139-145.
- Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Research 12 (2002): 9-18.
- Downward J. Targeting RAS signalling pathways in cancer therapy. Nature Reviews Cancer 3 (2003): 11-22.
- Chin HM, Lai DK, Falchook GS. Extracellular Signal-Regulated Kinase (ERK) Inhibitors in Oncology Clinical Trials. Journal of Immunotherapy and Precision Oncology 2 (2020): 10-16.
- Cagnol S, Chambard J-C. ERK and cell death: Mechanisms of ERK-induced cell death – apoptosis, autophagy and senescence. The FEBS Journal 277 (2010): 2-21.
- Satoh R, Hagihara K, Matsuura K, et al. Identi-fication of ACA-28, a 1′-acetoxychavicol acetate analogue compound, as a novel modulator of ERK MAPK signaling, which preferentially kills human melanoma cells. Genes to Cells 22 (2017): 608-618.
- Shi X, Zheng C, Li C, et al. 4-Phenybutyric acid promotes gastric cancer cell migration via histone deacetylase inhibition-mediated HER3/HER4 up-regulation. Cell Biology International 42 (2018): 53-62.
- Shi X, Gong L, Liu Y, et al. 4-phenylbutyric acid promotes migration of gastric cancer cells by histone deacetylase inhibition-mediated IL-8 upregulation. Epigenetics 15 (2020): 632-645.
- Hu H, Tian M, Ding C, et al. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Frontiers in Immunology 9 (2019).
