Efficacy and Safety of Intravenous Ferric Carboxymaltose In Patients with Chronic Heart Failure and Associated Iron Deficiency: Insights from a Tertiary Care Research Institute In India
Article Information
Rajendra Kumar Jain1, Mohammed Sadiq Azam2, Onkar C Swami3*, Neha Jain4
1Head of Department of Cardiology, KIMS Group of Hospitals, Secunderabad, India
2Consultant, Department of Cardiology, KIMS Group of Hospitals, Secunderabad, India
3Director, Medical Affairs, Emcure Pharmaceuticals Limited, India
4Senior Manager, Medical Affairs, Emcure Pharmaceuticals Limited, India
*Corresponding author: Onkar C Swami. Director, Medical Affairs, Emcure Pharmaceuticals Limited, Pune – 411057, India.
Received: 20 April 2023; Accepted: 27 April 2023; Published: 19 June 2023
Citation: Rajendra Kumar Jain, Mohammed Sadiq Azam, Onkar C Swami, Neha Jain. Efficacy and Safety of Intravenous Ferric Carboxymaltose In Patients with Chronic Heart Failure and Associated Iron Deficiency: Insights from a Tertiary Care Research Institute In India. Cardiology and Cardiovascular Medicine. 7 (2023): 158-161.
View / Download Pdf Share at FacebookAbstract
Objectives: To assess the efficiency and safety on Intravenous Ferric Carboxymaltose (IV FCM) in correcting Iron Deficiency (ID) in patients with chronic heart failure (CHF).
Methods: A Prospective, Observational single center study was performed in a tertiary care teaching institute in India for 12 weeks. Inclusion criteria was patients of both genders, age > 18 years, stable and ambulatory with CHF and associated ID (Absolute & Functional) and belonging to New York Heart Association (NYHA) Class II- III. Patients who didn’t meet the inclusion criteria were excluded from the study. Assessment of NYHA Class, Ejection Fraction (EF) and Iron indices [S. Haemoglobin (Hb), S. Iron, S. Ferritin, Total Iron Binding Capacity (TIBC) and Transferrin Saturation (TSAT)] was done at baseline and 12 weeks after giving IV FCM. A p value of < 0.05 was considered significant.
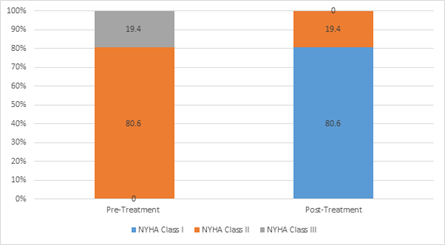
Results: Total 31 patients completed the study (14 males and 17 females). The mean age of patients was 60.81 years and mean Serum Ferritin level was 13.08 μg/l. Majority had HFpEF (61.3%) followed by HFrEF (32.3 %). Upon administering IV FCM, 100% improvement was seen in NYHA class while mean EF increased by 2.8% (p=0.001). A statistically significant improvement was also seen in Hb and Iron indices. No adverse event was reported.
Conclusion: Patients treated with IV FCM showed improvement in EF, NYHA class and Iron indices over 12-weeks and had no adverse reactions. IV FCM effectively and safely corrects ID in patients with CHF.
Keywords
Heart Failure; Iron Deficiency; Ferric Carboxymaltose
Heart Failure articles; Iron Deficiency articles; Ferric Carboxymaltose articles
Article Details
1. Introduction
Iron Deficiency (ID) is a commonly observed co-morbidity seen in patients with chronic conditions such as Heart Failure (HF), renal failure, autoimmune diseases such as rheumatoid arthritis, SLE and various other conditions [1]. The prevalence of ID in HF is nearly 70% in acute heart failure (AHF) and 50% in chronic heart failure (CHF) patients [2]. Given such high prevalence of ID in HF and its clinical implications on disease prognosis, it is important to screen every patient of HF for ID. If detected, patient should be appropriately treated for . Additionally, ID can be present in patients with HF regardless of the presence of anemia i.e. low Hb levels [3]. Current evidence highlights the need to recognize ID as an independent predictor of mortality in patients with HF [4]. It has been reported that ID has a greater negative impact on exercise capacity than Iron Deficiency Anaemia (IDA) in patients with heart failure [5]. S. ferritin and Transferrin Saturation (TSAT) are two serum biomarkers to identify ID. While Absolute ID is defined as S. Ferritin <100 µg/l, functional ID is defined as S. Ferritin between 100 and 300 µg/l when TSAT is <20% [1].
The latest ESC 2021 guidelines recommend screening of all CHF patients for ID by S. Ferritin and TSAT work-up (Class IC ) [6]. There is no evidence that oral iron therapy is effective for correcting ID win patients with HF [7]. There is, however, substantial evidence supporting use of IV Ferric Carboxymaltose (FCM) for correction of ID in patients with HF [8]. FAIR-HF, CONFIRM-HF, EFFECT-HF and AFFIRM-HF studies reported improvement in exercise capacity, quality of life, iron indices and hospitalization rate in patients with HF and associated ID with IV FCM administration. Recently published IRON-CRT trial highlighted additional benefits of FCM in patients HFrEF by improving myocardial contractility and cardiac reverse remodelling. Also treatment with FCM resulted in improved EF and end systolic volume, improved cardiac performance (negative FFR to positive FFR), improved functional status and improved peak exercise capacity (Peak VO2) over 3 months [9]. Despite substantial clinical evidence favoring IV FCM for treatment of ID in patients with HF, the evidence of use of FCM for treating ID in patients with HF in India remains limited. Therefore, the present study aims to assess the efficacy and safety of IV FCM on the improvement of symptoms and iron indices in Indian patients with HF with coexisting ID.
2. Materials and Methods
This was a single-center, prospective, observational study at a tertiary care teaching institute in India. Patients who met the inclusion criteria of age > 18 years, both genders and stable chronic heart failure with NYHA class II- III and with associated ID were recruited in the study. Baseline parameters of New York Heart Association (NYHA) class, Ejection Fraction (EF) and Iron indices (S. Haemoglobin, Ferritin, Iron, Total Iron Binding Capacity and Transferrin Saturation) were measured. If found to have either absolute or functional ID, then iron requirement was then calculated using patients Hb and weight. Based on its results, IV FCM was given as single bolus of 1000mg to all the patients. Patients were then followed up after 12 weeks of IV FCM administration. The primary outcome measures of the study were- improvement in NYHA class, EF and Iron indices of the patients’ pre-administration statuses. The secondary outcomes measure was occurrence of any adverse event during the 12-week study period. The values of respective indices were recorded and analyzed using paired T-test and McNemar chi square test. A p value < 0.05 was considered significant. Study was approved by institutional ethics committee and study participants provided written informed consent prior to enrolment.
3. Results
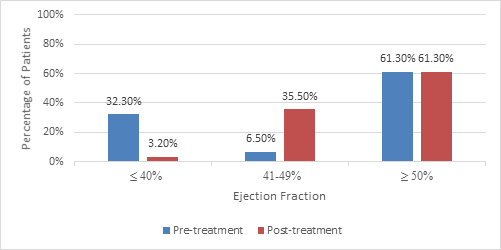
Total 31 patients with CHF and coexisting ID, matching inclusion and exclusion criteria received IV FCM were analyzed (Table 1& 2). Majority of patients had HFpEF (61.3%) followed by HFrEF (32.3%), and HFmrEF (6.5%). Post treatment with IV FCM 1000mg, there were significant improvement in EF. Mean EF post treatment increased by 2.48 % (p=0.001). Also, significant improvement in NYHA class was noted. All NYHA class III and II improved to class II and I respectively (Figure 1& 2). No major adverse events noted during the study.
Table 1: Demographic Details of Patients Enrolled in the Study
|
Patient Characteristics |
Observed Value |
|
Number of Patients (N) |
31 |
|
Age, years; (mean ± SD) |
60.81 ± 11.59 |
|
Male: Female (n=14:17) |
1.01.21 |
Table 2: Ejection Fraction and Iron Indices: Pre and Post FCM Treatment
|
Patient Characteristics |
Observed Value |
P Value |
|
|
Baseline |
12 Weeks |
||
|
Ejection Fraction, %; (mean ± SD) |
52.32 ± 10.64 |
54.81 ± 9.06 |
0.001 † |
|
Haemoglobin, gm/dl; (mean ± SD) |
10.75 ± 1.37 |
12.21 ± 1.69 |
<0.001 ‡ |
|
Serum Ferritin, ng/mL; (mean ± SD) |
13.08 ± 14.29 |
70.48 ± 103.93 |
0.001‡ |
|
Serum Iron, g/dl; (mean ± SD) |
33.12 ± 12.25 |
202.52 ± 120.1 |
<0.001‡ |
|
Total Iron Binging Capacity, mcg/dl; (mean ± SD) |
390.03 ± 73.89 |
241.19 ± 154.71 |
<0.001‡ |
|
Transferrin saturation, %; (mean ± SD) |
34.12 ± 68.13 |
45.71 ± 27.55 |
0.006‡ |
HFpEF: Heart failure with preserved ejection fraction; HFmrEF: Heart failure with mid-range ejection fraction; HFrEF: Heart failure with reduced ejection fraction.
*McNemar-Bowker Test (two-sided); † paired t test; ‡Wilcoxon sign rank test; p < 0.05: significant
EF- Ejection Faction; FCM- Ferric Carboxymaltose
NYHA: New York heart association (heart failure class); P value < 0.05
4. Discussion
The current worldwide prevalence of HF is 64.34 million (8.52 per 1,000 inhabitants). The estimated prevalence of HF in India is about 1% of the population. Overall, prevalence of HF has increased by ~36% since the year 1900. According to estimates, prevalence will probably grow to 9.81 per 1,000 inhabitants (+15.1%) by the year 2030, accounting for ~398.44 billion US $ worldwide expenditure. ID is one of the commonest co-morbidity seen in heart failure with significant clinical and prognostic implications on the disease and patient outcomes. There are various global studies that highlight the high prevalence of ID in patients with HF and the benefits achieved on correcting the same using IV Iron therapy. The latest guidelines on management of HF by the ESC 2021 recommend screening of every suspected HF case for ID. The guidelines also recommend using IV FCM in patients of HFrEF and HFmrEF and associated ID, to alleviate symptoms; improve exercise capacity and QOL; and to reduce the risk of HF hospitalization. There is lack of adequate and robust epidemiological data in India, that makes it difficult to recognize the true prevalence of anaemia and ID in HF. In addition to this, because of other factors such as malnutrition, worm infestation and infections, the prevalence of anaemia and ID in India might really differ than other countries. Hence, there is need for clinical data which may give clinical prognosis in HF with associated ID in Indian patients. The results of present study suggest that IV FCM therapy effectively corrects iron deficiency in patients with heart failure. It also helps improve the EF and NHYA functional class. With single bolus dose of 1000mg of IV FCM, a significant number of patients with HF with pre- treatment ejection < 40% (HFrEF) had improvement in their ventricular function and achieved higher EF (40-49%; HFmrEF) when followed up at 12 weeks. Similarly, patients showed improvement in their functional status as can be seen from their improved NYHA classes post treatment with IV FCM. This study also highlights the benefits of correcting ID in patients with HFpEF. Since no adverse events were reported during the study, it reaffirms the fact that IV FCM is a well tolerated for correcting ID in patients with HF. Our study provides Indian evidence regarding the efficacy of IV FCM in correction of ID in patients with HF. It also shows that ID, if present in patients with HF, is easily treatable with IV FCM which is given as a single bolus injection, as per the patient’s iron requirement. This study further reinforces the importance of screening every patient with heart failure for ID, irrespective of their signs and symptoms or NYHA functional class. IV FCM has shown to be the therapy of choice for correcting ID in patients with HF in multiple trials.[8] High dose of rapid iron administration with low chances of hypersensitivity reactions are important attributes of FCM for real-life situations. This helps in fast replenishment of iron stores and early improvement in clinical status of patients. In our study, a single bolus dose of 1000 mg IV FCM could replenish iron stores significantly as can be seen by the Iron indices levels at 12 weeks, which translated into functional benefits for the patient. Our study has certain limitations as well. Firstly, this was a single center non-comparative study with small sample size. Secondly, the study duration of 12 weeks is also short to understand the long term benefits of correction of ID in patients with HF using IV FCM. Lastly, the sample size of 31 is too small to extrapolate these findings to larger section and hence large multi-centric study may be needed to confirm these findings. Thus findings of this study need to be considered cautiously.
5. Conclusion
Iron Deficiency in patients with HF, including those with preserved Ejection Fraction (HFpEF) can be corrected effectively by IV FCM. IV FCM effectively replenishes iron stores in these patients when given as single 1000 mg dose. Apart from being efficacious, IV FCM is well tolerated for correction of Iron Deficiency in patients with HF as no adverse events were reported on its administration.
Conflicts of Interest:
The author reports no conflicts of interest with anyone with regards to this study. Dr. Neha Jain and Dr. Onkar Swami are full time employees of Emcure Pharmaceuticals Ltd. which actively markets Ferric Carboxymaltose.
References
- Van Aelst LN, Mazure D, Cohen-Solal A. Towards Holistic Heart Failure Management—How to Tackle the Iron Deficiency Epidemic?. Current Heart Failure Reports 14 (2017): 223-34.
- Rocha BM, Cunha GJ, Menezes Falcao LF. The burden of iron deficiency in heart failure: therapeutic approach. Journal of the American College of Cardiology 71 (2018): 782-93.
- Drozd M, Jankowska EA, Banasiak W, et al. Iron therapy in patients with heart failure and iron deficiency: review of iron preparations for practitioners. American Journal of Cardiovascular Drugs 17 (2017): 183-201.
- Cohen-Solal A, Leclercq C, Deray G, et al. Iron deficiency: an emerging therapeutic target in heart failure. Heart 100 (2014): 1414-20.
- Jankowska EA, Rozentryt P, Witkowska A, et al. Iron deficiency predicts impaired exercise capacity in patients with systolic chronic heart failure. Journal of cardiac failure 17 (2011): 899-906.
- McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European heart journal 42 (32021): 3599-726.
- Stewart CA. Intravenous ferric carboxymaltose for heart failure with iron deficiency: Editorial comment. European Journal of Heart Failure 20 (2018): 134.
- Anker SD, Kirwan BA, van Veldhuisen DJ, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. European journal of heart failure 20 (2018): 125-33.
- Martens P, Dupont M, Dauw J, P et al. Rationale and design of the IRON-CRT trial: effect of intravenous ferric carboxymaltose on reverse remodelling following cardiac resynchronization therapy. ESC Heart Failure 6 (2019): 1208-15.