Effectiveness of Tongue cleaning as a component of Oral care on the fasting Plasma Desacyl Ghrelin concentration in very elderly individuals: Six-Week Randomized controlled trial
Article Information
Kazuo Sonoki1*, Maya Izumi1, Yuko Ohta2, Masayo Fukuhara2, Masaharu Nagata3, Sumio Akifusa1
1Unit of Interdisciplinary Education, School of Oral Health Sciences, Kyushu Dental University, Kitakyushu, Japan
2Division of General Internal Medicine, Department of Health Promotion, Kyushu Dental University, Kitakyushu, Japan
3Shin-eikai Hospital, Kitakyushu, Japan
*Corresponding author: Dr. Kazuo Sonoki, MD PhD, Unit of Interdisciplinary Education, School of Oral Health Sciences, Kyushu Dental University, Manazuru 2-6-1, Kokurakita-ku, Kitakyushu, Fukuoka 803-8580, Japan
Received: 29 May 2021; Accepted: 08 June 2021; Published: 30 June 2021
Citation:
Kazuo Sonoki, Maya Izumi, Yuko Ohta, Masayo Fukuhara, Masaharu Nagata, Sumio Akifusa. Effectiveness of Tongue cleaning as a component of Oral care on the fasting Plasma Desacyl Ghrelin concentration in very elderly individuals: Six-Week Randomized controlled trial. Dental Research and Oral Health 4 (2021): 039-048
View / Download Pdf Share at FacebookAbstract
To investigate whether tongue cleaning as a component of oral care affects the fasting plasma desacyl ghrelin concentrations in elderly people with the peak expiratory flow (PEF) as the measure of coughing ability. This randomized controlled trial was conducted in Kitakyushu, Japan from July 2017 to November 2018. We randomly assigned 52 enrolled Japanese subjects from three nursing care facilities to a routine oral care group (control group) and an oral care with twice-daily tongue cleaning group (intervention group). Before and after 6 weeks of each version of oral care, the PEF of each subject was measured and blood was collected. A final total of 48 subjects (39 females, 9 males, mean ± SD age 88.4 ± 7.5 years) was analyzed. The PEF increased significantly in the intervention group (1.62 ± 0.89 L/s to 2.07 ± 1.09 L/s) but not in the control group (1.76 ± 1.05 L/s to 1.53 ± 0.67 L/s). The fasting plasma desacyl ghrelin concentrations in the intervention group did not change significantly (181 ± 102 fmol/mL to 175 ± 98 fmol/mL) whereas those in the control group decreased significantly (168 ± 114 fmol/mL to 142 ± 72 fmol/mL). The change of the plasma desacyl ghrelin concentration was significantly correlated with the change in albumin (correlation coefficient = 0.292, p<0.05), but not with that in PEF. Tongue cleaning accompanied by improved coughing ability seemed to help prevent the decrease in the fasting plasma desacyl ghrelin concentration in very elderly individuals.
Keywords
Dental care, Ghrelin, Appetite regulation, Elderly 80 and over
Dental care articles; Ghrelin articles; Appetite regulation articles; Elderly 80 and over articles
Article Details
1. Introduction
With the increasing interest in oral care, the prevalence of tongue cleaning is expanding [1]. Tongue cleaning is usually performed to remove tongue coating, and it is thus thought to contribute to oral bacterial control and halitosis management. It was recently reported that tongue cleaning exerts several other positive effects on an individual's oral status and even body condition, i.e., increasing the perceived intensity of salty taste [2], combating gingival inflammation [3], and improving digestive power [4]. In 2016, Izumi et al. reported that tongue cleaning added to routine oral care improved the peak expiratory flow (PEF), an index of coughing ability that is known to be involved in the onset of aspiration pneumonia [5]. Regarding the mechanism underlying the increase in the PEF by tongue cleaning, they noted that coughing and the tongue are both controlled by the glossopharyngeal and vagus nerves. Their study was the first to demonstrate that stimulation of the tongue may affect the body's respiratory function through the nerve system. Ghrelin is an endogenous ligand for the growth hormone secretagogue receptor, and it stimulates the release of growth hormone [6]. It is a 28-amino-acid peptide hormone secreted mainly from the stomach, and the secretion of ghrelin increases the body's appetite for food; circulating ghrelin levels rise before meals and rapidly decline after eating or a gastrointestinal infusion of nutrients. In rats, an intracerebroventricular injection of ghrelin was confirmed to strongly stimulate feeding and to increase the rats' body weight gain. Ghrelin was also shown to have numerous central and peripheral actions, including the stimulation of gut motility and gastric acid secretion; the modulation of sleep, taste sensations and reward-seeking behavior; the regulation of glucose metabolism, the suppression of brown fat thermogenesis, the modulation of stress and anxiety, protection against muscle atrophy, and the improvement of cardiovascular functions such as vasodilatation and cardiac contractility [6]. Of ghrelin's many effects, protection against muscle atrophy would be beneficial for elderly people, in whom frailty and sarcopenia are serious concerns. In a study using mice, ghrelin impaired muscle atrophy [7] and in humans, treatment with an oral ghrelin mimetic, MK-677 (ibutamoren) resulted in a significantly higher increase in the fat free mass compared to a placebo [8]. Two major forms of ghrelin exist in blood: n-octanoyl-modified ghrelin (acyl ghrelin) and desacyl ghrelin. The acylated form is essential for the above-mentioned biological activities of ghrelin. Desacyl ghrelin has been considered the degradation product of acyl ghrelin without biological activities [9], but it was recently reported to prevent muscle atrophy in mice, as did acyl ghrelin [7]. Desacyl ghrelin circulates in the bloodstream at much higher concentrations than acyl ghrelin, due to the shorter half-life of acyl ghrelin and plasma ghrelin diacylation [10]. The desacyl ghrelin concentration is thus speculated to be an indicator of acyl ghrelin secretory function [9,11]. Appropriate oral care is known to contribute to elderly people's health as it helps prevent aspiration pneumonia and maintain the nutritional status [12,13]. It was reported that functional oral care improved the plasma active ghrelin dynamics in elderly people [14]. The finding that tongue cleaning increased the PEF (possibly through the glossopharyngeal and vagus nerves; [5]) suggests that tongue cleaning may influence the secretion of ghrelin from the stomach. In addition, an increase in digestive power after tongue cleaning revealed by a questionnaire suggested the possible involvement of ghrelin's effects [4]. In light of these findings, we hypothesized that compared to routine oral care alone, tongue cleaning as a component of oral care could have more favorable effects on the acyl ghrelin secretion in elderly people. We conducted the present study to compare the change in plasma desacyl ghrelin concentrations (as an indicator of the acyl ghrelin secretion in very elderly individuals) after 6 weeks of oral care that included tongue cleaning with the change provided by 6 weeks of routine oral care alone.
2. Materials and Methods
2.1 Study design and population
This randomized controlled trial (parallel design) was conducted in accordance with the CONSORT statement for randomized trials of non-pharmacologic treatments at two nursing homes and one long-term-care hospital in Kitakyushu City, Fukuoka prefecture, Japan [15]. The primary outcome was the difference in the plasma desacyl ghrelin concentration in the intervention and control groups between the baseline and the end of the intervention period. The secondary outcome was the differences in the values of variables including PEF in both groups between the baseline and the end of the intervention period. The sample size, n=54, was calculated using the G*Power 3.1 software program, based on an α error of 0.05, 1-β of 0.8, and the effect size of 0.8. As the eligibility criteria, individuals who were bedridden or unable to maintain a sitting position, those with difficulty communicating, and those in poor health were excluded from the study in consideration of the measurement of PEF and blood collection. An independent researcher who was not involved in the outcome assessments assigned the subjects randomly to each group. Both the examiner conducting the outcome assessments and the subjects were blinded to the group allocation. Prior to the study, all of the subjects were given a full explanation of the study (both verbal and written), and all subjects provided informed consent to participate. If a subject was considered to have difficulty communicating, a legally acceptable representative such as a family member was given a full explanation of the study, and he/she provided informed consent. The study was conducted with the approval of the Ethics Committee at Kyushu Dental University (No. 17-1).
2.2 Tongue cleaning procedure
The tongue cleaning was performed by a staff member of the nursing home or hospital, with a mucosal brush (DENT. ERAC 510; Lion Dental Products, Tokyo) along with routine oral care as described by Izumi et al. [5]. These staff members were instructed by a trained dental hygienist to gently brush the subject's tongue from the base to the apex 10 times with sufficient pressure to bend the bristles of the mucosal brush, once in the morning and once in the evening during the subject's regular oral care.
2.3 Measures
We obtained the subjects' demographic characteristics and physical health status including activities of daily living (ADLs; Barthel index), cognitive function (MMSE; Mini-Mental State Examination), nutritional status (MNA-SF; Mini-Nutritional Assessment-Short Form) and comorbidity condition (the Charlson comorbidity index) from a standard questionnaire and the medical records of the two nursing homes and the long-term-care hospital. Each subject's oral health status, including the number of teeth, posterior teeth occlusion (total number of functional tooth units), and plaque index was examined by a trained dentist before (as the baseline) the oral care [16]. Each subject's swallowing function (MWST; modified water swallow test), tongue pressure (TPM-01; JMS Co., Tokyo), and PEF (CHESTGRAPH HI-105; CHEST M.I., Tokyo) were measured by a single dental hygienist before (as the baseline) and after the 6 weeks of either version of the oral care. Blood samples for analyses including the plasma desacyl ghrelin measurement were collected before breakfast at approx. 7:00 a.m. before and after the 6-week intervention period. For the measurement of the subjects' plasma desacyl ghrelin concentrations, total blood collected with aprotinin in an EDTA-containing tube that was centrifuged immediately, and the separated plasma was immediately added with 1N HCl (10% volume of plasma volume). The obtained plasma was stored at −80°C until measurement. The plasma desacyl ghrelin concentrations were measured by a Desacyl-Ghrelin ELISA kit (Mitsubishi Kagaku Iatron, Tokyo).
2.4 Statistical analyses
For the subjects' clinical characteristics, we evaluated the significance of the difference by χ2-test for categorical valuables. The Mann-Whitney U-test was used for the comparison of continuous variables between the two groups. The changes in the measured values between before and after the 6-week oral care period were compared using Wilcoxon's signed rank sum test. The linear correlation between the change in the plasma desacyl ghrelin concentration (ΔGhrelin = desacyl ghrelin after oral care − desacyl ghrelin before oral care) and the change in other measured variables such as the PEF values (i.e., ΔPEF = PEF after oral care − PEF before oral care) was tested by Pearson's correlation coefficient. Levene's test was applied for a homogeneous analysis of variance in the two groups. We performed a multivariate linear regression analysis to determine which of the variables measured after the 6 weeks of oral care (tongue pressure, body mass index [BMI], PEF, white blood cells [WBC], red blood cells [RBC], platelets, albumin, aspartate transaminase [AST], creatinine, total cholesterol, and HbA1c) and the subjects' clinical characteristics at the baseline (sex, age, Barthel index, number of teeth) influenced the post-oral-care plasma desacyl ghrelin concentration as the dependent variable by the backward selection method. All data are presented as the mean ± SD. Results were considered significant when the p-value was <0.05 (SPSS 17.0, SAS, Cary, NC).
3. Results
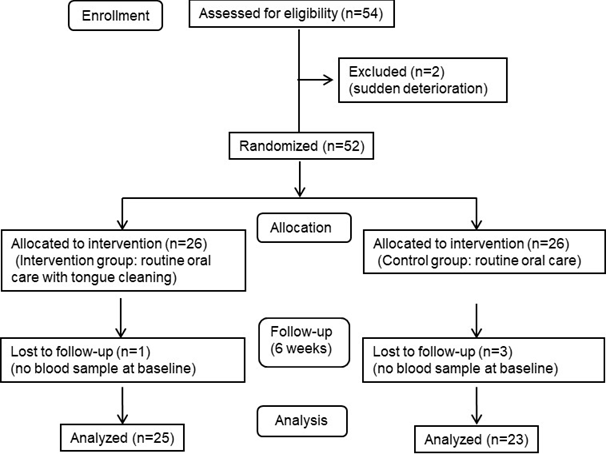
Figure 1 illustrates the flow of this parallel design study and the number of participants at each stage. Fifty-two subjects (40 females, 12 males, mean ± SD age 88.6 ± 7.3 years) were randomly assigned to the routine oral care-alone group (the control group, n=26) and the oral care including twice-daily tongue cleaning group (the intervention group, n=26) during the period from July 2017 to May 2018. Four of the 52 subjects were excluded from the follow-up and statistical analysis because blood samples had not been collected at the baseline survey. A final total of 48 subjects (39 females, 9 males, mean ± SD age 88.4 ± 7.5 years) had completed each intervention as of November 2018, and their resultant data was analyzed.
Table 1 summarizes the characteristics of the subjects in the control group and intervention group at the baseline before the start of the 6-week oral care protocol and the changes in the values of variables from before to after 6 weeks of each version of oral care. The mean age of the subjects in both groups was >85; i.e., the subjects were considered 'oldest-old,' and the ages were not significantly different between the two groups. The MMSE scores of the intervention group were slightly higher than those of the control group, but the difference was not significant. There was no significant between-group difference in the male-to-female ratio, Barthel index, MNA-SF score, number of teeth, tongue pressure, BMI, or PEF values. Of the blood test data, the fasting plasma desacyl ghrelin concentrations were equal in the two groups, but the values of RBC, hemoglobin, and AST in the intervention group were slightly lower than those in the control group.
Over the 6 weeks of each version of oral care, the mean value of tongue pressure in both groups declined, but not significantly. In the control group, the PEF did not change significantly (1.76 ± 1.05 L/s to 1.53 ± 0.67 L/s, n.s.), but in the intervention group, the PEF increased significantly from the baseline to the end of the 6-week intervention (1.62 ± 0.89 L/s to 2.07 ± 1.09 L/s, p<0.05) as Izumi et al. reported in 2016. The control group's fasting plasma desacyl ghrelin concentrations decreased significantly (168 ± 114 fmol/mL to 142 ± 72 fmol/mL, p<0.05), but in the intervention group, these concentrations did not change significantly (181 ± 102 fmol/mL to 175 ± 98 fmol/mL, n.s.). The values of WBC, hemoglobin, and albumin in the control group and those of RBC and hemoglobin in the intervention group all declined slightly but significantly.
Table 2 provides the correlation relationship between the change in the fasting plasma desacyl ghrelin concentrations (ΔGhrelin) and the change in other measured variables. ΔGhrelin and ΔAlbumin were significantly positively correlated (Pearson correlation coefficient, R= 0.292, p=0.047), but there was no significant correlation between ΔGhrelin and the change in other measured variables. We next compared the variance of ΔGhrelin in both groups by performing Levene's test for a homogeneous analysis. The variance of ΔGhrelin in the intervention group was smaller than that in the control group (506.8 vs. 2804.8), and the variance of ΔGhrelin in the two groups was significantly unequal (F value = 7.366, p<0.05).
|
Intervention group |
Control group |
|||
|
Before |
After |
Before |
After |
|
|
Men/women |
5/20 |
- |
4/19 |
- |
|
Age, yrs |
88.4±6.7 |
- |
88.4±8.4 |
- |
|
Barthel index |
39.2±21.2 |
- |
38.7±17.9 |
- |
|
MMSE score |
17.5±5.9 |
- |
13.7±8.9 |
- |
|
MNA-SF score |
10.1±1.9 |
- |
10.2±1.8 |
- |
|
Teeth number |
7.8±9.7 |
- |
4.9±6.4 |
- |
|
MWST score |
4.2±0.9 |
4.2±1.0 |
3.9±1.1 |
4.1±0.9 |
|
Tongue pressure, kPa |
17.5±10.2 |
15.7±12.5 |
16.8±10.9 |
12.0±8.8 |
|
BMI, kg/m2 |
20.1±3.9 |
21.0±4.3 |
20.7±4.9 |
23.3±4.6 |
|
PEF, L/s |
1.62±0.89 |
2.07±1.09 † |
1.76±1.05 |
1.53±0.67 |
|
Desacyl ghrelin, fmol/mL |
181±102 |
175±98 |
168±114 |
142±72 † |
|
WBC, /μL |
5640±1979 |
5014±1031 |
5687±1432 |
5058±1189 † |
|
RBC, ×104/μL |
371±60* |
353±53 † |
412±57 |
392±55 |
|
Hemoglobin, g/dL |
11.1±1.6* |
10.5±1.6 † |
12.3±1.6 |
11.7±1.6 † |
|
Platelet, ×104/μL |
22.3±6.4 |
21.9±9.5 |
22.8±5.4 |
22.9±4.1 |
|
Albumin, g/dL |
3.6±0.4 |
3.5±0.3 |
3.8±0.4 |
3.6±0.3 † |
|
AST, U/L |
18.4±5.4* |
18.5±6.0 |
22.7±11.6 |
19.9±3.6 |
|
Creatinine, mg/dL |
0.91±0.47 |
0.94±0.52 |
0.78±0.40 |
0.79±0.38 |
|
Total cholesterol, mg/dL |
173±39 |
171±39 |
189±46 |
182±43 |
|
HbA1c, % (NGSP) |
5.7±0.8 |
5.7±0.8 |
5.8±0.6 |
5.8±0.6 |
Table 1: Characteristics of the participants and the changes in parameters from before to after 6 weeks of oral care.
*p<0.05 vs. Control group, †p<0.05 vs. Before. AST: aspartate transaminase, BMI: body mass index, MMSE: Mini-Mental State Examination, MNA-SF: Mini-Nutritional Assessment-Short Form, PEF: peak expiratory flow, RBC: red blood cells, WBC: white blood cells.
|
Correlation coefficient |
p-value |
|
|
ΔTongue pressure, N |
0.029 |
0.846 |
|
ΔBMI, kg/m2 |
−0.002 |
0.992 |
|
ΔPEF, L/s |
−0.006 |
0.966 |
|
ΔWBC, /μL |
0.092 |
0.537 |
|
ΔRBC, ×104/μL |
−0.075 |
0.671 |
|
ΔHemoglobin, g/dL |
−0.040 |
0.791 |
|
ΔPlatelet, ×104/μL |
0.149 |
0.319 |
|
ΔAlbumin, g/dL |
0.292 |
0.047 |
|
ΔAST, U/L |
0.126 |
0.399 |
|
ΔCreatinine, mg/dL |
0.152 |
0.307 |
|
ΔTotal cholesterol, mg/dL |
0.195 |
0.190 |
|
ΔHbA1c, % (NGSP) |
−0.226 |
0.126 |
WBC: white blood cells, RBC: red blood cells.
Table 2: Correlation relationship between ΔGhrelin (fmol/mL) and the change in other measured variables.
Table 3 lists the variables from among those in Table 1 that were revealed to significantly affect the post-oral-care plasma desacyl ghrelin concentration as a dependent variable calculated by the multivariate linear regression analysis. According to the unstandardized coefficients, male gender, the number of teeth, BMI, and the AST positively influenced the plasma desacyl ghrelin concentration, but the subjects’ age, WBC count, and total cholesterol negatively influenced the concentration.
|
Variable |
Unstandardized coefficients |
p |
95%CI for B |
||
|
B |
SE |
Lower |
Upper |
||
|
Men/women |
151.559 |
27.835 |
0 |
90.294 |
212.823 |
|
Age, yrs |
−3.743 |
1.447 |
0.025 |
−6.927 |
−0.559 |
|
Teeth no. |
4.78 |
0.97 |
0 |
2.646 |
6.915 |
|
BMI, kg/m2 |
9.48 |
2.463 |
0.003 |
4.058 |
14.902 |
|
WBC, /μL |
−0.024 |
0.008 |
0.009 |
−0.042 |
−0.007 |
|
AST, U/L |
10.807 |
2.388 |
0.001 |
5.551 |
16.063 |
|
Total cholesterol, mg/dL |
−1.292 |
0.282 |
0.001 |
−1.913 |
−0.671 |
The multivariate linear regression was performed by the backward selection method. AST: aspartate transaminase, BMI: body mass index, WBC: white blood cells.
Table 3: Multivariate linear regression model for the plasma desacyl ghrelin concentration after 6 weeks of oral care.
4. Discussion
We measured plasma desacyl ghrelin as an indicator of acyl ghrelin secretory function, and we thus interpret our findings as indicating that the acyl ghrelin secretory function in very elderly people decreases rapidly, but the tongue-cleaning intervention maintains this function [9,11]. As shown in Table 3, we observed that our subjects' ages affected the plasma desacyl ghrelin concentrations negatively. In the 362-subject subsample (mean age 45 years) of The Framingham Third Cohort, the fasting plasma ghrelin concentrations were reported to be inversely associated with age [17]. Nevertheless, in the present study it was surprising that the fasting plasma desacyl ghrelin concentrations decreased significantly within such a short period, i.e., 6 weeks. One possible reason for this significant decrease could be the advanced ages of the subjects in our control group (mean age 88.8 years); we also speculate that there might be age-related gastric mucosa atrophy, although our cohort did not include any subject with a gastric disease such as chronic gastritis or gastric cancer in their history.
We also observed a positive correlation between ΔGhrelin and ΔAlbumin (Table 2). The desacyl ghrelin value in the control group but not in the intervention group decreased, and the variance of ΔGhrelin in the intervention group was significantly smaller than that in the control group. Considering these results together, it appears that improving the desacyl ghrelin level is beneficial for an individual's nutritional status, and this improvement may have helped prevent muscle atrophy and eventually the enhancement of PEF in our intervention group. In humans, acyl ghrelin enhances eating and improves the nutritional status including the serum albumin level [18]. The plasma desacyl ghrelin concentrations observed in the present study thus seem to reflect the secretion of acyl ghrelin into the blood.
Regarding the mechanism underlying the increase in the subjects' PEF by tongue cleaning, the stimulation to the tongue may contribute to the respiratory function through the glossopharyngeal and vagus nerves as Izumi et al. suggested [5]. This mechanism also seems to be applicable to the secretion of acyl ghrelin from the stomach instead of the respiratory function. We thus expected to observe a positive correlation between the PEF and ghrelin values, but there was no significant correlation between ΔPEF and ΔGhrelin (Table 2). If stimulation of the tongue affects the PEF and the ghrelin secretion via the vagus efferent pathway simultaneously, an absence of a correlation between the PEF and ghrelin levels in the blood might be observed because the reactivity of the respiratory organs and the stomach caused by vagus efferent nerves might be different. Further studies are needed to clarify the correlation between respiratory function evaluated by the PEF and the ghrelin secretion from the stomach. Our study has some limitations that should be addressed. First, the subjects were very old (mean age ~88 years), and it is thus difficult to apply our findings to younger populations. With much younger subjects, it is likely that the fasting plasma desacyl ghrelin concentrations might increase with oral care that includes tongue cleaning as Kimura et al. reported [14]. A determination of the relationship between an individual's oral state and features of the systemic state such as the lung function and ghrelin level across the entire lifespan is desired. Another study limitation is that the number of subjects (n=52) was not sufficient for measuring the fasting plasma desacyl ghrelin concentrations. The coefficient of variation (CV) of the fasting plasma desacyl ghrelin concentrations in 48 subjects was 61.3% before oral care and 54.8% after oral care; the CV of ΔGhrelin was 301.3%. In light of these findings, the variability in the blood desacyl ghrelin levels seems to be very large and probably depends on the subjects' systemic state at the time of blood collection. Further studies of desacyl ghrelin levels should examine larger numbers of subjects.
Third, this study did not have a group without oral care for comparison with the two groups with oral care. Sumi et al. reported that oral care helped to maintain the serum albumin level in frail older people living in a nursing home [13]. This oral care intervention was professional oral care provided by a dentist three times a week, and in the control group, oral cleaning was done following the nursing home's oral care methods; the serum albumin level of the control group decreased significantly, as in our present control group. Regarding the serum albumin level, our intervention group including tongue cleaning corresponds to their oral care intervention group, and the presence or absence of tongue cleaning in oral care may be one of the points to consider when designing oral care to maintain the serum albumin level of elderly people. In future studies, it is necessary to explore a range of oral care interventions including tongue cleaning and to determine the optimal interventions for elderly people’s health.
In conclusion the fasting plasma desacyl ghrelin concentration, which we measured as an indicator of the acyl ghrelin secretory function, was observed to be intrinsically decreased in very elderly people, but tongue cleaning as a component of oral care resulting in an improvement of coughing ability had the effect of maintaining the fasting plasma desacyl ghrelin concentration (accompanied by the continued nutritional status), and this intervention may be beneficial for very elderly people who are at risk of malnutrition, sarcopenia, and/or frailty due to muscle atrophy. Tongue cleaning is meaningful in routine oral care, and caregivers of elderly individuals should be aware of its importance and instructed on how to properly provide tongue cleaning.
Acknowledgements
This work was supported by a Grant-in-Aid for Young Scientists (no. 18K17292) to Maya Izumi from Japan Society for the Promotion of Science (JSPS/KAKENHI). We are deeply grateful for the staff's cooperation at the two nursing homes (Yasuragi and Kiyomizu-no-Mori) and the long-term-care hospital, Nishino Hospital.
Conflicts of interest
None of the authors have any potential conflicts of interest associated with this research.
References
- Matsuda S, Saito T, Yoshida H, et al. Prevalence of tongue cleaning using a toothbrush: A questionnaire survey in Fukui Prefecture, Japan. BioMed Research International (2019).
- Seerangaiyan K, Jüch F, Atefeh F, et al. Tongue cleaning increases the perceived intensity of salty taste. Journal of Nutrition Health and Aging 22 (2018): 802-804.
- Acar B, Berker E, Tan Ç, et al. Effects of oral prophylaxis including tongue cleaning on halitosis and gingival inflammation in gingivitis patients - A randomized controlled clinical trial. Clinical Oral Investigations 23 (2019): 1829-1836.
- Tokinobu A, Yorifuji T, Sasai M, et al. Effects of tongue cleaning on Ayurvedic digestive power and oral health-related quality of life: A randomized cross-over study. Complementary Therapies in Medicine 36 (2018): 9-13.
- Izumi M, Takeuchi K, Ganaha S, et al. Effects of oral care with tongue cleaning on coughing ability in geriatric care facilities: A randomised controlled trial. Journal of Oral Rehabilitation 43 (2016): 953-959.
- Müller TD, Nogueiras R, Andermann ML, et al. Ghrelin. Molecular Metabolism 4 (2015): 437-460.
- Porporato PE, Filigheddu N, Reano S, et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. Journal of Clinical Investigation 123 (2013): 611-622.
- Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: A randomized trial. Annals of Internal Medicine 149 (2008): 601-611.
- Delhanty PJ, Neggers SJ, Van der Lely AJ. Mechanisms in endocrinology: Ghrelin: The differences between acyl- and des-acyl ghrelin. European Journal of Endocrinology 167 (2012): 601-608.
- Delporte C. Structure and physiological actions of ghrelin. Scientifica (2013).
- Hosoda H, Doi K, Nagaya N, et al. Optimum collection and storage conditions for ghrelin measurements: Octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clinical Chemistry 50 (2004): 1077-1080.
- Yoneyama T, Yoshida M, Ohrui T, et al. Oral care reduces pneumonia in older patients in nursing homes. Journal of the American Geriatrics Society 50 (2002): 430-433.
- Sumi Y, Ozawa N, Miura H, et al. Oral care help to maintain nutritional status in frail older people. Archives of Gerontology and Geriatrics 51 (2010): 125-128.
- Kimura T, Endoh M, Nagatomi E, et al. The relationship between functional oral care and plasma active ghrelin levels among dependent elderly people receiving enteral nutrition. Journal of the Kyushu Dental Society 66 (2012): 29-38 (in Japanese).
- Boutron I, Altman DG, Moher D, et al. Consort statement for randomized trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Annals of Internal Medicine 167 (2017): 40-47.
- Silness J, Loe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal condition. Acta Odontologica Scandinavica 22 (1964): 121-135.
- Ingelsson E, Larson MG, Yin X, et al. Circulating ghrelin, leptin, and soluble leptin receptor concentrations and cardiometabolic risk factors in a community-based sample. Journal of Clinical Endocrinology and Metabolism 93 (2008): 3149-3157.
- Kodama T, Ashitani J, Matsumoto N, et al. Ghrelin treatment suppresses neutrophil-dominant inflammation in airways of patients with chronic respiratory infection. Pulmonary Pharmacology and Therapeutics 21 (2008): 774-779.