Effect of Transcutaneous Cervical Spinal Cord Stimulation on Trunk Function in Subjects with Cervical Spinal Cord Injury
Article Information
Hatice Kumru1,2,3,*, Yolanda Castillo-Escario4,5,6, Raimon Jane4,5,6, Joan Vidal1,2,3, Loreto García Alén1,2,3
1Fundación Institut Guttmann, Institut Universitari de Neurorrehabilitació Adscrit a la UAB, Badalona, 08916, Spain
2Universitat Autónoma de Barcelona, Barcelona, 08193, Spain
3Fundació Institut d’Investigació en Ciéncies de la Salut Germans Trias i Pujol, Badalona, 08916, Spain
4Department of Automatic Control, Universitat Politècnica de Catalunya-Barcelona Tech (UPC), Barcelona, 08028, Spain
5Institute for Bioengineering of Catalonia (IBEC), Barcelona Institute of Science and Technology, Barcelona, 08028, Spain
6Centro de Investigación Biomédica en Red de Bioingeniería, Biomateriales y Nanomedicina (CIBER-BBN), Madrid, 28029, Spain
*Corresponding Author: Hatice Kumru, MD, PhD, Hospital de Neurorehabilitació Institut Guttmann
Camí Can Ruti s/n. Barcelona, 08916 Barcelona, Spain.
Received: 28 November 2023; Accepted: 06 December 2023; Published: 16 December 2023
Citation: Hatice Kumru, Yolanda Castillo-Escario, Raimon Jane, Joan Vidal, Loreto García Alén. Effect of Transcutaneous Cervical Spinal Cord Stimulation on Trunk Function in Subjects with Cervical Spinal Cord Injury. Journal of Spine Research and Surgery. 5 (2023): 117-126
View / Download Pdf Share at FacebookAbstract
This study aimed to examine how cervical transcutaneous spinal cord stimulation (tSCS) influences trunk muscle activity and movement patterns while individuals with SCI perform a reaching task. Trunk stability is crucial for daily activities, and spinal cord injury (SCI) often impairs this stability. By evaluating muscle activity and movement patterns during a specific task, the researchers seek to understand whether tSCS has an impact on enhancing trunk muscle control and movement.
Methods:
Nineteen subjects with cervical or high thoracic SCI participated in the randomized mix of parallel group and crossover clinical trial, consisting of an intervention group (n=13; tSCS) and control group (n=10), and four of them participated in both groups. We used the American Spinal Injury Association Impairment Scale (AIS) scale to evaluate clinical motor and sensory deficits, and the clinical trunk impairment control test (static and dynamic equilibrium and static equilibrium with upper limbs). In addition, data from electromyography (EMG) and smartphone accelerometers were recorded during a reaching task that required trunk tilting. Outcome measures included response time (RespT) until pressing a target button, EMG onset latencies and amplitudes, and trunk tilt, lateral deviation, and other movement features from accelerometry. Patients were evaluated before and after eight sessions of tSCS applied at C3-4 and C6-7 at 30 Hz during upper extremity rehabilitation.
Result:
The results showed in tSCS group significant improvement in total motor strength, while trunk control evaluated by clinical scales did not show significant changes in any group. Additionally, there were no significant changes in any EMG or smartphone variables, except response time (RespT), which was faster in the controls after last session. Changes as absolute or percentage values were similar in both groups in all parameters.
Significance:
tSCS applied at two cervical segments showed significant improvement in total motor score but not in trunk control in SCI individuals. This is a crucial finding, suggesting that while tSCS at cervical segments positively affected certain aspects of motor function, this did not translate into improvements in trunk stability.
Keywords
Transcutaneous spinal cord stimulation; cervical spinal cord injury; trunk stability; EMG; Smartphone accelerometer
Transcutaneous spinal cord stimulation articles Transcutaneous spinal cord stimulation Research articles Transcutaneous spinal cord stimulation review articles Transcutaneous spinal cord stimulation PubMed articles Transcutaneous spinal cord stimulation PubMed Central articles Transcutaneous spinal cord stimulation 2023 articles Transcutaneous spinal cord stimulation 2024 articles Transcutaneous spinal cord stimulation Scopus articles Transcutaneous spinal cord stimulation impact factor journals Transcutaneous spinal cord stimulation Scopus journals Transcutaneous spinal cord stimulation PubMed journals Transcutaneous spinal cord stimulation medical journals Transcutaneous spinal cord stimulation free journals Transcutaneous spinal cord stimulation best journals Transcutaneous spinal cord stimulation top journals Transcutaneous spinal cord stimulation free medical journals Transcutaneous spinal cord stimulation famous journals Transcutaneous spinal cord stimulation Google Scholar indexed journals cervical spinal cord injury articles cervical spinal cord injury Research articles cervical spinal cord injury review articles cervical spinal cord injury PubMed articles cervical spinal cord injury PubMed Central articles cervical spinal cord injury 2023 articles cervical spinal cord injury 2024 articles cervical spinal cord injury Scopus articles cervical spinal cord injury impact factor journals cervical spinal cord injury Scopus journals cervical spinal cord injury PubMed journals cervical spinal cord injury medical journals cervical spinal cord injury free journals cervical spinal cord injury best journals cervical spinal cord injury top journals cervical spinal cord injury free medical journals cervical spinal cord injury famous journals cervical spinal cord injury Google Scholar indexed journals trunk stability articles trunk stability Research articles trunk stability review articles trunk stability PubMed articles trunk stability PubMed Central articles trunk stability 2023 articles trunk stability 2024 articles trunk stability Scopus articles trunk stability impact factor journals trunk stability Scopus journals trunk stability PubMed journals trunk stability medical journals trunk stability free journals trunk stability best journals trunk stability top journals trunk stability free medical journals trunk stability famous journals trunk stability Google Scholar indexed journals EMG articles EMG Research articles EMG review articles EMG PubMed articles EMG PubMed Central articles EMG 2023 articles EMG 2024 articles EMG Scopus articles EMG impact factor journals EMG Scopus journals EMG PubMed journals EMG medical journals EMG free journals EMG best journals EMG top journals EMG free medical journals EMG famous journals EMG Google Scholar indexed journals Smartphone accelerometer articles Smartphone accelerometer Research articles Smartphone accelerometer review articles Smartphone accelerometer PubMed articles Smartphone accelerometer PubMed Central articles Smartphone accelerometer 2023 articles Smartphone accelerometer 2024 articles Smartphone accelerometer Scopus articles Smartphone accelerometer impact factor journals Smartphone accelerometer Scopus journals Smartphone accelerometer PubMed journals Smartphone accelerometer medical journals Smartphone accelerometer free journals Smartphone accelerometer best journals Smartphone accelerometer top journals Smartphone accelerometer free medical journals Smartphone accelerometer famous journals Smartphone accelerometer Google Scholar indexed journals cervical articles cervical Research articles cervical review articles cervical PubMed articles cervical PubMed Central articles cervical 2023 articles cervical 2024 articles cervical Scopus articles cervical impact factor journals cervical Scopus journals cervical PubMed journals cervical medical journals cervical free journals cervical best journals cervical top journals cervical free medical journals cervical famous journals cervical Google Scholar indexed journals thoracic articles thoracic Research articles thoracic review articles thoracic PubMed articles thoracic PubMed Central articles thoracic 2023 articles thoracic 2024 articles thoracic Scopus articles thoracic impact factor journals thoracic Scopus journals thoracic PubMed journals thoracic medical journals thoracic free journals thoracic best journals thoracic top journals thoracic free medical journals thoracic famous journals thoracic Google Scholar indexed journals postural rehabilitation articles postural rehabilitation Research articles postural rehabilitation review articles postural rehabilitation PubMed articles postural rehabilitation PubMed Central articles postural rehabilitation 2023 articles postural rehabilitation 2024 articles postural rehabilitation Scopus articles postural rehabilitation impact factor journals postural rehabilitation Scopus journals postural rehabilitation PubMed journals postural rehabilitation medical journals postural rehabilitation free journals postural rehabilitation best journals postural rehabilitation top journals postural rehabilitation free medical journals postural rehabilitation famous journals postural rehabilitation Google Scholar indexed journals L1-L2 articles L1-L2 Research articles L1-L2 review articles L1-L2 PubMed articles L1-L2 PubMed Central articles L1-L2 2023 articles L1-L2 2024 articles L1-L2 Scopus articles L1-L2 impact factor journals L1-L2 Scopus journals L1-L2 PubMed journals L1-L2 medical journals L1-L2 free journals L1-L2 best journals L1-L2 top journals L1-L2 free medical journals L1-L2 famous journals L1-L2 Google Scholar indexed journals cerebral cortex articles cerebral cortex Research articles cerebral cortex review articles cerebral cortex PubMed articles cerebral cortex PubMed Central articles cerebral cortex 2023 articles cerebral cortex 2024 articles cerebral cortex Scopus articles cerebral cortex impact factor journals cerebral cortex Scopus journals cerebral cortex PubMed journals cerebral cortex medical journals cerebral cortex free journals cerebral cortex best journals cerebral cortex top journals cerebral cortex free medical journals cerebral cortex famous journals cerebral cortex Google Scholar indexed journals
Article Details
Introduction
Spinal cord injury (SCI) is a catastrophic event that culminates in a deficiency of sensorimotor and/or autonomic functions. Trunk instability is a major concern for people with SCI [13]. Particularly, lesions in the cervical to the thoracic region can paralyze trunk muscles, leading to a partial or complete loss of trunk stability [5]. The trunk plays a critical role in maintaining balance and stability during activities of daily living (ADLs). Therefore such impairments adversely affect an individual’s ability to carry out everyday activities including bed movements, unsupported sitting, and self-care duties [16] and their capacity to perform transfers, and reach for objects [20].
Actually, postural rehabilitation to improve trunk function has been identified as one of the highest priorities for optimizing the recovery of SCI individuals. While transcutaneous spinal cord stimulation (tSCS) shows promise as a treatment for trunk stability in subjects with cervical spinal cord injuries, it is important to note that this technique is still in the early stages of development [19,17,8]. When tSCS was applied at Th11-12 and/or L1-L2, there was an improvement in trunk and sitting functions with increased static and dynamic balance [19]. The application of tSCS also increased trunk extension, enabled upright sitting posture and improved ability to perform transfers [8], and increased unilateral reaching [17].
Previous reports emphasize the potential of tSCS at cervical level to affect not only the adjacent spinal cord, but also provide opportunities for remote neuromodulation [1,2,10,11]. It was previously reported that tSCS applied to cervical segments could increase cortical excitability, affecting the responsiveness of the cerebral cortex. This effect has been observed both in healthy individuals [10,11] and in individuals with spinal cord injuries [2]. In addition, modulation of the H-reflex in the lower limb has been reported when tSCS is applied to the cervical segments [1]. The H-reflex is a measure of spinal cord excitability, and its modulation can affect muscle and nerve responses in the lower extremities. This highlights the versatility of tSCS as a neuromodulation technique with applications, offering opportunities for remote therapeutic interventions.
Here, our hypothesis was that tSCS applied at the cervical level could positively impact trunk-related parameters in individuals with cervical or high thoracic SCI indicating a comprehensive understanding of the potential effects of neuromodulation beyond the targeted area. Therefore, the application of tSCS during upper extremity therapy with a robotic exoskeleton in SCI individuals with cervical or high thoracic SCI [6], our focus was to explore a potential improvements in trunk stability and the impact on trunk muscle activity, movement patterns, and trunk response to disrupting effect of startling acoustic stimuli (SAS).
Materials and Methods
Participants
The inclusion criteria were: i) clinical diagnosis of cervical or high thoracic SCI, either traumatic or non-traumatic in origin; ii) including complete and incomplete injuries; iii) classified as AIS A, B, C, or D according to the American Spinal Injury Association Impairment Scale (AIS) [9].
Exclusion criteria were: (i) unstable medical condition (cancer, acute infections, or other health issues that could potentially impact their participation); (ii) dependent on mechanical ventilation; (iii) severe spasticity (≥ 3 score on the Modified Ashworth scale – MAS); (iv) peripheral nerve injury; (v) intolerance of tSCS; peacemakers electronic implants, episodes of epilepsy; (vi) participating in another investigation.
The protocol was approved by the Ethics Committee of the Institute Guttmann and was carried out in accordance with the standards of the Declaration of Helsinki. All subjects were informed of all experimental procedures, after which each subject completed a signed informed consent. The study was conducted between December 2019 and January 2023.
Experimental Design
The experimental design at the beginning was a randomized, controlled clinical trial, which consisted of two groups: (i) intervention group: tSCS which realized during robotic exoskeleton for upper extremity and (ii) control group: the subjects realized their routine rehabilitation program including robotic exoskeleton for upper extremity. We used a computer-generated list as randomization strategy. Assignment of the subjects to the treatment interventions was random. If the subjects wanted to participate in tSCS following control condition, we gave them this possibility. Finally we realizaed a randomized mix of parallel group and crossover clinical trial. The duration of both interventions was 4 sessions per week during 2 weeks. All patients from each group were evaluated at baseline condition and after the last session. Total duration of clinical and neurophysiological assessments was around 3-4 hours. The study was carried out in the installations of the Guttmann Institute from January 2020 until December 2022.
Neurological assessment
AIS scale was used to evaluate the clinical motor and sensory deficit according to American Spinal Injury Association (ASIA) Impairment Scale [9]. AIS-A is sensory and motor complete SCI; B: sensory incomplete and motor complete SCI; C and D: sensory and motor incomplete SCI and AIS-E: normal sensory and motor function. The sensory and motor score assessment was carried out with the participant in a supine position.
To evaluate trunk stability, we used the clinical trunk impairment control test (static and dynamic equilibrium and static equilibrium with upper limbs) [15].
Neurophysiological assessment during simple reaction time of body displacement to evaluate trunk function
Participants were instructed to perform a simple reaction time task to reach a switch on the wall [3,4]. In the initial position, subjects were sitting in a wheelchair, with the hip flexed at 90°, the knees flexed at 90°, the arms resting on their legs or the armrests. The target switch button was placed in front of them, aligned with the subject’s midline, at a distance of 15 cm of the index fingertip with the arm extended.
The participants had to raise their arm and flex the trunk forward to reach the button as fast as possible but trying not to lose balance to the imperative signal (IS). The IS was a low-intensity electrical signal (between 3.6-8.1 mA, 0.2 ms duration) applied to the right little finger. If participant did not feel IS, it was delivered on the right shoulder. Participants performed 20 trials, in 5 (25%) of which a startle auditory stimulus (SAS) was presented simultaneously with the IS. The SAS was obtained by discharging a magnetic coil on top of a metallic platform, reaching a sound intensity of 125 dB for 250 ms [3,4].
Electromyographic (EMG) and smartphone recordings during body displacement
EMG data were collected from 8 muscles on the left side of the body: sternocleidomastoid (SCM), middle deltoid (DEL), trapezius (TRA), pectoralis major (PECT), upper abdominal Th6 (ABD), and paraspinal muscles at cervical C3 (PC), thoracic T6 (PT), and lumbar L2 (PL) levels. We asked the patients to use their left hand to press the button. If the left hand was more affected, the patient reached with the right hand. EMG signals were recorded at a sampling rate of 10 kHz by means of a ten-channel EMG system (Synergy, VIASYS Healthcare UK Ltd., 2005), using disposable adhesive surface electrodes (outer diameter 20 mm; Technomed) that were attached over the muscle belly. The remaining two channels of the EMG system were used to record 1) the activity of the orbicularis oculi muscle (OOc) and thus measure the blink reflex, and 2) the electrical artefact generated when pressing the wired switch button. Each trial was recorded for 3 seconds, starting 500 ms prior to the IS to evaluate the basal activity [3,4].
In addition, a smartphone (Samsung Galaxy S5) was placed on the subjects’ chest, over the sternum, using an elastic band, to collect triaxial accelerometer data for motion analysis with the smartphone built-in sensor (MPU-6500 three-axis MEMS accelerometer with 16-bits ADCs, TDK InvenSense, San Jose, CA, United States). The smartphone accelerometer x-axis was in the transverse (left-to-right) direction, the y-axis in the longitudinal (superior-to-inferior) direction, and the z-axis in the anteroposterior direction. Accelerometer data were sampled at 200 Hz and stored as a text file.
From the tilt angle signal, we calculated the maximum trunk inclination angle PeakAng., the duration and angular velocity of the forward movement (from the beginning of the movement to the time of the maximum tilt angle), and the maximum peak-to-peak distance in the first 200 ms, [3,4]. Data processing and analysis was performed with custom written Matlab code (r2018a, Mathworks Inc.).
Interventions
Transcutaneous electrical spinal cord stimulation
Electric stimulation was delivered using the transcutaneous electrical stimulator BioStim-5 (Cosyma Inc., Moscow, Russia). tSCS was delivered simultaneously at two sites of cervical spinal cord along the midline between spinous processes C3-C4 and C6-C7, through 2 cm diameter hydrogel adhesive electrodes (axion GmbH, Hamburg, Germany) as cathodes and two 5×12 cm rectangular electrodes placed symmetrically over the iliac crests as anodes [6]. The intensity of stimulation at each spinal level was set at 90% of rest motor threshold induced by single-pulse tSCS at Abductor Pollicis Brevis (APB) muscle [4] of the less affected hand or of the right hand if both hand were similarly affected. tSCS consisted of biphasic rectangular 1-ms pulses, each one filled with a carrier frequency of 10 kHz (i.e., each 1-ms pulse was composed of ten 0.1-ms biphasic rectangular pulses), at a frequency of 30 Hz. tSCS was delivered with time patterns of 30s of stimulation followed by 60 s resting for 1 h. during robotic exoskeleton for upper extremity.
All assessments were carried out before (pre) and after last session (post) except the clinical trunk impairment control test (static and dynamic equilibrium and static equilibrium with upper limbs) was done after 2 weeks of last session also for follow-up assessment.
Data Processing and Statistical Analysis
EMG traces contaminated by artefacts were removed by visual inspection. The response time (RespT) was calculated as the time from the IS to the button press.
EMG onset latencies from IS were calculated for each muscle and trial. To calculate the EMG envelopes, EMG signals were full-wave rectified and low-pass filtered at 20 Hz. The baseline activity was calculated over the 600 ms prior to the IS and subtracted from all signals. Then, for each muscle and trial, the mean absolute value (MAV) of the EMG envelope from the onset of the muscle to the RespT was calculated, as a measure of the EMG amplitude.
The smartphone accelerometer data was used to monitor the trunk tilt and lateral angle signals, which are the angles calculated in the ZY plane, and the XY plane, respectively. These angles were estimated based on the projection of the gravity acceleration on the axes of the accelerometer [3,4].
For each feature, clinical, neurophysiological and smartphone data were averaged across trials to obtain a single measure for each subject and condition (non-SAS vs. SAS pre and post tSCS vs. control). Then, descriptive statistics, i.e., means and standard deviations (SD), were calculated for each group (tSCS and control).
Normality was assessed using the Shapiro-Wilk test. Since the assumptions for parametric analyses were not met, Wilcoxon signed-rank tests were conducted to compare pre and post non-SAS and after than pre and post SAS trials in each group except the total motor score for so we used the parametric test: Student's t test. While Mann-Whitney U tests were performed to determine differences in absolute value or percentage (%) changes following intervention or during follow-up between tSCS vs. control group. The alpha level was set at 0.05 for all comparisons.
Results
Twenty-five people were selected for this study, but six of them were excluded due to not meeting inclusion criteria (n=3); declined to participate (n=1); COVID-19 (n=1); infection (n=1) (Figure 1).
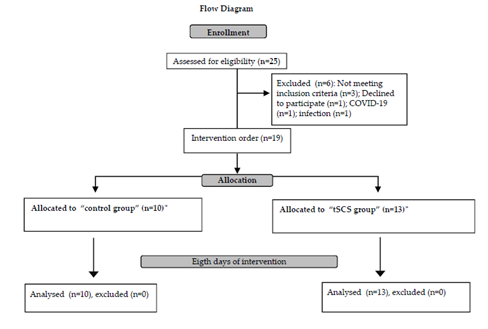
Figure 1: Flow Diagram
Flow Diagram. tSCS: transcutaneous spinal cord stimulation; *:4 individuals first were included in control group and at least one week later in tSCS group
Nineteen individuals with cervical (n=18) or high thoracic(n=1) SCI participated in this study: 13 in tSCS and 10 in control group. Four of them participated in both groups at least one week after the follow-up period (Figure 1).
All subjects were able to complete the experiment. Two patients could not realize following assessment because of living in the other city. All subjects had cervical SCI except one with high thoracic lesion. The clinical and demographical characteristics of all the subjects with SCI were given in Table 1.
|
Intervention |
Age |
Sex |
SCI level |
AIS |
Etiology |
Time since SCI (months) |
total motor score |
total motor score |
tSCS intensities at C3-C4/C6-C7 (mA) |
|
pre |
post |
||||||||
|
tSCS* |
25 |
M |
C4 |
A |
Trauma |
4 |
11 |
15 |
67/86 |
|
tSCS |
46 |
M |
C4 |
C |
Trauma |
4 |
21 |
23 |
80/80 |
|
tSCS* |
36 |
M |
C5 |
B |
Trauma |
14 |
14 |
15 |
74/86 |
|
tSCS |
36 |
M |
C7 |
D |
Trauma |
6 |
74 |
74 |
63/85 |
|
tSCS |
28 |
M |
C4 |
C |
Trauma |
6 |
52 |
56 |
63/85 |
|
tSCS |
28 |
M |
C5 |
A |
Trauma |
4 |
28 |
28 |
86/86 |
|
tSCS |
38 |
M |
C4 |
B |
Trauma |
5 |
48 |
48 |
54/77 |
|
tSCS* |
56 |
M |
C4 |
C |
Trauma |
9 |
47 |
50 |
86/86 |
|
tSCS |
60 |
M |
C5 |
D |
Trauma |
3 |
88 |
90 |
86/86 |
|
tSCS* |
22 |
F |
C7 |
C |
Trauma |
5 |
44 |
51 |
85/86 |
|
tSCS |
55 |
M |
C3 |
D |
Trauma |
4 |
87 |
91 |
86/86 |
|
tSCS |
47 |
M |
C6 |
C |
Trauma |
10 |
57 |
59 |
86/86 |
|
tSCS |
42 |
M |
C4 |
D |
Trauma |
3 |
69 |
86 |
80/76 |
|
control* |
25 |
M |
C4 |
A |
Trauma |
8 |
15 |
15 |
- |
|
control |
46 |
M |
C4 |
C |
Trauma |
6 |
23 |
23 |
- |
|
control* |
36 |
M |
C4 |
B |
Trauma |
5 |
12 |
14 |
- |
|
control |
38 |
M |
C7 |
C |
Trauma |
3 |
48 |
48 |
- |
|
control* |
56 |
M |
C4 |
C |
Medical |
6 |
46 |
46 |
- |
|
control |
58 |
M |
C6 |
A |
Trauma |
6 |
44 |
46 |
- |
|
control |
21 |
M |
C6 |
B |
Trauma |
9 |
22 |
22 |
- |
|
control |
53 |
M |
T1 |
D |
Medical |
3 |
51 |
65 |
- |
|
control |
32 |
M |
C5 |
D |
Trauma |
3 |
90 |
94 |
- |
|
control* |
22 |
F |
C7 |
C |
Trauma |
4 |
42 |
44 |
- |
*Individual with SCI participated first in control group and than at least one week of wash-out, in tSCS group.
Table 1: The clinical and demographical characteristics of all the subjects with SCI
The age was similar between both groups (39.9±12.4 years in tSCS and 38.7±14.0 years in control groups; p. 0.83). Duration of SCI was 5.9±3.2 months in tSCS and 5.3±2.1 months in control group (p. 0.58). Total motor score were similar between control vs. tSCS group at baseline condition (pre intervention) (39.3±21.8 vs. 49.2±24.8 respectively; p. 0.34).
Neurological assessments
After the last session of tSCS, total motor score improved significantly (from 49.2±24.8 to 52.8±26.1; p=0.015), but in control group it did not reach a significant level (from 39.3±21.8 to 41.7±23.6; p=0.11). There were not significant changes in the absolute value of the total motor score in tSCS and in control group (p. 0.23; Table 1).
Clinical trunk impairment control test was similar between tSCS (17.2±9.0) and control group (13.7±6.5) at baseline condition (p=0.20). Table 2 shows the pre, post and follow-up values of clinical trunk impairment control test, static and dynamic equilibrium, and static equilibrium with upper limbs for each SCI subject.
There were no significant changes in static or dynamic equilibrium, static equilibrium with upper limbs, or in total clinical trunk impairment control test in tSCS, neither in control group, after last session (p>0.05), neither during follow-up period (p>0.05; Table 2).
SD: standard deviation; NA: not available; pvalue according to Wilcoxon-test {comparison between pre vs. post, and pre vs. follow-up)
Table 2: Clinical trunk impairment control test (static and dynamic equilibrium and static equilibrium with upper limbs)
Electromyographic (EMG) and smartphone accelerometer recordings during body displacement
Outcome measures from EMG and smartphone accelerometer in the tSCS and control groups, are described in Table 3, both in SAS and non-SAS trials.
The columns show the Mean for each group and condition. RespT: response time; ActDur: Action duration; LateralDev: lateral deviation, AngVel: angle velocity, PeakAccAng: peak acceleration angle. SCM: sternocleidomastoid muscle; DEL: deltoid; TRA: trapezius; PECT:pectoralis, ABD: abdominal muscle; paraspinal muscles at cervical C3 (PC), thoracic T6 (PT), and lumbar L2 (PL) levels .
Table 3: Outcome measures from EMG and smartphone accelerometer in the tSCS and control groups, both in SAS and non-SAS trials.
Table 4 shows the p-values according to Mann-Whitney U Test corresponding to the pre-intervention comparison between the two groups: tSCS vs. control group. Both groups were similar for all pre-intervention variables from EMG and smartphone accelerometry (Table 4; p>0.05 for all comparisons).
Table 4: The homogeneity of two groups pre-intervention (pre-tSCS vs. pre-control group) (p-value according to Mann-Whitney U Test)
Table 5A shows statistical analysis and p-values between pre and post comparison in each group according to Wilcoxon signed rank test, while Table 5B shows the comparison between tSCS vs. control group for absolute value and percentage changes following intervention (Mann-Whitney U Test).
Table 5A: Statistical analysis and p-values between pre and post comparison in each group according to Wilcoxon signed rank test
After the last session in the tSCS group, there were not any significant changes in EMG or smartphone variables with or without SAS condition:RespT, action duration, lateral deviation, angle velocity, peak acceleration angel, onset latencies and MAV in EMG for all muscles did not significantly change with the intervention. The same happened in the control group: none of the parameters changed after two weeks of upper limb rehabilitation (p>0.05 for each comparison between pre and post-intervention, Table 5A), except RespT, which was accelerated in the non-SAS trials after the last session (p=0.05).
There were not any significant changes in absolute value or in the % changes between tSCS vs. control group (p>0.05; Table 5B).
Table 5B: Groups comparison (tSCS vs control group) for absolute value and percentage (%) changes following each intervention (Mann-Whitney U Test)
Discussion
Despite the observed total motor score improvement following tSCS, we did not find any significant changes in trunk control in these individuals. This is a crucial finding, suggesting that while tSCS at two cervical segments positively affected certain aspects of motor function as published previously [6], but this did not translate into improvements in trunk stability and its EMG and smartphone accelerometer measurements.
We have methodological differences with the previous studies: (i) Our study was randomized mix of parallel group and crossover clinical trial and we applied tSCS during upper limb therapy in 13 cervical SCI individuals during 8 days; (ii) we used the clinical trunk impairment control test (static and dynamic equilibrium and static equilibrium with upper limbs; (iii) EMG and smartphone assessments to evaluate the trunk stability [3,4]. However, Rath et al. (2018) [17] studied 8 chronic cervical or thoracic SCI subjects following one session of tSCS. They used monophasic rectangular 1ms pulses, frequency at 30 Hz at T11 level, and 15 Hz at L1, carrier frequency 10 kHz, and intensity 10 to 150mA. Reported an elevated activity of the trunk muscles contributing to improved trunk control, and increased multi-directional seated stability. Keller et al (2021) [8] applied tSCS at intensity of 20-200 mA at T11 at 30 Hz, L1 at 15 Hz, and C5 at 30 Hz during 22 experiments in 8 children with cervical or thoracic SCI ages 3–14 years old. It was non-randomized, non-blinded pilot clinical trial. They reported increased trunk extension, enabled upright sitting posture. Tharu et al. (2022) [19] included just five subjects with chronic complete cervical SCI participated in 24-week therapy that combined tSCS and conventional task-specific rehabilitation (TSR) in the first 12 weeks, followed by TSR alone for another 12 weeks. tSCS was delivered simultaneously at T11 and L1 spinal levels, at a frequency ranging from 20–30 Hz with 0.1–1.0 ms biphasic pulse width. They reported improved trunk and sitting functions with increased static and dynamic balance.
The principal mechanism of tSCS is a non-invasive activation of neuronal networks of the spinal cord likely including the recruitment of afferent fibers in the posterior root in order to elevate spinal network excitability [18,14]. The excitability of spinal interneuronal networks without directly producing action potentials can be readily modulated by changing the networks’ physiological state [7]. For that reason, the placement of tSCS electrodes is an important consideration in order to target specific segments of the spinal cord and promote desired functional outcomes. The previous studies, tSCS applied at thoracic and lumber segments, [17,19] in addition to one cervical segment [8]. In our study, tSCS was applied to two cervical segments (C3-4 and C6-7).
Other important differences in the application duration of tSCS between our study and previous three studies highlight an important aspect of study design. We applied tSCS over eight days in adult SCI individuals, which contrast with studies that utilized a single session [17,8] and involved children [8]. Tharu et al. (2022) [19] applied tSCS and conventional task-specific rehabilitation during 12 weeks. Al those three studies were done in chronic SCI individuals [17,8,19]. Additionally, our study exclusively focused on individuals with cervical or one with upper thoracic SCI, emphasizing the severity of the condition compared to thoracic SCI.
Our study acknowledges the limitations: (i) using only two cervical segments for stimulation. Consideration of thoracic and lumbar segments might offer a broader perspective on the effects of tSCS on trunk stability; ii) the study notes the absence of a specific trunk exercise combined with tSCS. Incorporating such exercises, as demonstrated in previous research [19], might potentially enhance neuromodulation.
In conclusion, while our study demonstrates robustness through its control group and diverse assessment methods, the limitations suggest areas for improvement and avenues for future research. Consideration of tSCS at thoracic and lumbar segments and exploration of combined trunk interventions may provide a more comprehensive understanding of the potential benefits of tSCS on trunk stability following SCI.
Funding:
This research partially financiated by “REGAIT-PID2021-124111OB-C31 funded by MCIN/AEI/ 10.13039/501100011033 and by ERDF A way of making Europe” to HK. And it was also supported in part by the Government of Catalonia (CERCA Program), the Secretaria d'Universitats i Recerca de la Generalitat de Catalunya (GRC 2021 SGR 01390), and the Spanish Ministry of Science and Innovation (PID2021-126455OB-I00 MCIN/AEI/FEDER).
Conflicts of Interest
The authors declares no conflicts of interest.
References
- Barss TS, Parhizi B, Mushahwar VK. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J Neurophysiol 123 (2020): 158-166.
- Benavides FD, Jo HJ, Lundell H, et al. Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia. J Neurosci 40 (2020): 2633-2643.
- Castillo-Escario Y, Kumru H, Valls-Solé J, et al. Assessment of trunk flexion in arm reaching tasks with electromyography and smartphone accelerometry in healthy human subjects. Sci Rep 11 (2021a): 5363.
- Castillo-Escario Y, Kumru H, Valls-Solé J, et al. Quantitative evaluation of trunk function and the StartReact effect during reaching in patients with cervical and thoracic spinal cord injury. J Neural Eng (2021b): 18.
- Friederich AR, Audu ML, Triolo RJ. Characterization of the force production capabilities of paralyzed trunk muscles activated with functional neuromuscular stimulation in individuals with spinal cord injury. IEEE Trans Biomed Eng 68 (2020): 2389–2399.
- García-Alén, L, Kumru, H, Castillo-Escario, Y, et al. Transcutaneous Cervical Spinal Cord Stimulation Combined with Robotic Exoskeleton Rehabilitation for the Upper Limbs in Subjects with Cervical SCI: Clinical Trial. Biomedicines 11 (2023): 589.
- Gerasimenko, Y.P, Lu, D.C, Modaber, M, et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma 32 (2015): 1968-1980.
- Keller A, Singh G, Sommerfeld JH, et al. Noninvasive spinal stimulation safely enables upright posture in children with spinal cord injury. Nat Commun. 12 (2021): 5850.
- Kirshblum, S.C, Burns, S.P, Biering-Sorensen, F, et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 34 (2011): 535-546.
- Kumru, H, Flores, A, Rodríguez-Cañón, M, et al. Cervical Electrical Neuromodulation Effectively Enhances Hand Motor Output in Healthy Subjects by Engaging a Use-Dependent Intervention. J. ClinMed 10 (2021a): 195.
- Kumru H, Rodríguez-Cañón M, Edgerton VR, et al. Transcutaneous Electrical Neuromodulation of the Cervical Spinal Cord Depends Both on the Stimulation Intensity and the Degree of Voluntary Activity for Training. A Pilot Study. J Clin Med 10 (2021b): 3278.
- Kumru H, García-Alén L, Ros-Alsina A, et al. Transcutaneous Spinal Cord Stimulation Improves Respiratory Muscle Strength and Function in Subjects with Cervical Spinal Cord Injury: Original Research. Biomedicines 11 (2023): 2121.
- Milosevic M, Masani K, Kuipers MJ, et al. Trunk control impairment is responsible for postural instability during quiet sitting in individuals with cervical spinal cord injury. Clin Biomech Elsevier Ltd. 30 (2015): 507-512.
- Milosevic, M, Masugi, Y, Sasaki, A, et al. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 121 (2019): 1672-1679.
- Quinzaños J, Villa A R, Flores A A et al. Proposal and validation of a clinical trunk control test in individuals with spinal cord injury Spinal Cord 52 (2014): 449-454.
- Patel K, Milosevic M, Nakazawa K, et al. Wheelchair neuroprosthe¬sis for improving dynamic trunk stability. IEEE Trans Neural Syst Rehabil Eng. 25 (2017): 2472-2479.
- Rath M, Vette AH, Ramasubramaniam S, et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma 35 (2018): 2540-2553.
- Sayenko, D.G, Rath, M, Ferguson, A.R, et al. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 36 (2019): 1435-1450.
- Tharu NS, Alam M, Ling YT, et al. Combined Transcutaneous Electrical Spinal Cord Stimulation and Task-Specific Rehabilitation Improves Trunk and Sitting Functions in People with Chronic Tetraplegia. Biomedicines 11 (2022): 34.
- Triolo RJ, Boggs L, Miller ME, et al. Implanted electrical stimulation of the trunk for seated postural stability and function after cervical spinal cord injury: a single case study. Arch Phys Med Rehabil 90 (2009): 340-347.
