Effect of the Head Reference Angle on the Mean Cerebral Blood Flow during Radionuclide-Angiography with the Patlak Plot Method with Technetium-99m Ethyl Cysteinate Dimer
Article Information
Hitoshi Hiraki1,3, Toshimune Ito2, Masahisa Onoguchi3*, Hirotatsu Tsuchikame4, Masaaki Shishido3,4, Takafumi Maeno4, Hiroki Sanada1, Masao Tago1,5
1Department of Central Radiology, Teikyo University Mizonokuchi Hospital, Kawasaki, Japan
2Department of Radiological Technology, Faculty of Medical Technology, Teikyo University, Tokyo, Japan
3Department of Quantum Medical Technology, Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Japan
4Department of Radiology, Saiseikai Yokohamashi Tobu Hospital, Yokohama, Japan
5Department of Radiology, Teikyo University Mizonokuchi Hospital, Kawasaki, Japan
*Corresponding Author: Masahisa Onoguchi, Department of Quantum Medical Technology, Graduate School of Medical Sciences, Kanazawa University, Kanazawa, Japan.
Received: 11 July 2022; Accepted: 20 July 2022; Published: 28 July 2022
Citation:
Hitoshi Hiraki, Toshimune Ito, Masahisa Onoguchi, Hirotatsu Tsuchikame, Masaaki Shishido, Takafumi Maeno, Hiroki Sanada, Masao Tago. Effect of the Head Reference Angle on the Mean Cerebral Blood Flow during Radionuclide-Angiography with the Patlak Plot Method with Technetium-99m Ethyl Cysteinate Dimer. Journal of Psychiatry and Psychiatric Disorders 6 (2022): 231-238.
View / Download Pdf Share at FacebookAbstract
Background: The purpose of this study was to clarify the effect of the head reference angle (jaw pull angle) in the cephalocaudal direction on mean cerebral blood flow (mCBF) during radionuclide (RI)-angiography using the Patlak Plot method in CBF Single Photon Emission Computed Tomography (SPECT) testing with 99mTc-ECD.
Methods: From January 2012 to December 2013, 290 normal patients were retrospectively evaluated with CBF SPECT testing with technetium-99m ethyl cysteinate dimer (99mTc-ECD). The brain Region of Interest (ROI) and mCBF were evaluated during RI-angiography.
Results: mCBF of all males and females aged 60–89 years was 40.4 ± 4.2 and 42.2 ± 3.6 (ml/100 g/min), respectively, showing a weak negative correlation with aging. The mCBF was lower in males than in females (p<0.05). The brain ROI size was significantly greater in males with a head reference angle below −10 degrees (p<0.05) and significantly smaller in males with an angle above 5.1degrees (p<0.05). Brain ROI size was significantly greater in females with a head reference angle below −10 degrees (p<0.05) and significantly smaller for females with an angle above 10 degrees (p<0.05). There was no significant difference in brain ROI sizes between males and females. The mCBF was significantly lower in both genders with head reference angle ±10 degrees (p<0.05).
Conclusion: The mCBF was found to be significantly lower when the head reference angle during RI-angiography using the Patlak Plot method and 99mTc-ECD was greater than ±10 degrees.
Keywords
Technetium-99m ethyl cysteinate dimer, Patlak plot, Region of interest, Mean cerebral blood flow, Head reference angle
Technetium-99m ethyl cysteinate dimer articles, Patlak plot articles, Region of interest articles, Mean cerebral blood flow articles, Head reference angle articles
Technetium-99m ethyl cysteinate dimer articles Technetium-99m ethyl cysteinate dimer Research articles Technetium-99m ethyl cysteinate dimer review articles Technetium-99m ethyl cysteinate dimer PubMed articles Technetium-99m ethyl cysteinate dimer PubMed Central articles Technetium-99m ethyl cysteinate dimer 2023 articles Technetium-99m ethyl cysteinate dimer 2024 articles Technetium-99m ethyl cysteinate dimer Scopus articles Technetium-99m ethyl cysteinate dimer impact factor journals Technetium-99m ethyl cysteinate dimer Scopus journals Technetium-99m ethyl cysteinate dimer PubMed journals Technetium-99m ethyl cysteinate dimer medical journals Technetium-99m ethyl cysteinate dimer free journals Technetium-99m ethyl cysteinate dimer best journals Technetium-99m ethyl cysteinate dimer top journals Technetium-99m ethyl cysteinate dimer free medical journals Technetium-99m ethyl cysteinate dimer famous journals Technetium-99m ethyl cysteinate dimer Google Scholar indexed journals Patlak plot articles Patlak plot Research articles Patlak plot review articles Patlak plot PubMed articles Patlak plot PubMed Central articles Patlak plot 2023 articles Patlak plot 2024 articles Patlak plot Scopus articles Patlak plot impact factor journals Patlak plot Scopus journals Patlak plot PubMed journals Patlak plot medical journals Patlak plot free journals Patlak plot best journals Patlak plot top journals Patlak plot free medical journals Patlak plot famous journals Patlak plot Google Scholar indexed journals Region of interest articles Region of interest Research articles Region of interest review articles Region of interest PubMed articles Region of interest PubMed Central articles Region of interest 2023 articles Region of interest 2024 articles Region of interest Scopus articles Region of interest impact factor journals Region of interest Scopus journals Region of interest PubMed journals Region of interest medical journals Region of interest free journals Region of interest best journals Region of interest top journals Region of interest free medical journals Region of interest famous journals Region of interest Google Scholar indexed journals Mean cerebral blood flow articles Mean cerebral blood flow Research articles Mean cerebral blood flow review articles Mean cerebral blood flow PubMed articles Mean cerebral blood flow PubMed Central articles Mean cerebral blood flow 2023 articles Mean cerebral blood flow 2024 articles Mean cerebral blood flow Scopus articles Mean cerebral blood flow impact factor journals Mean cerebral blood flow Scopus journals Mean cerebral blood flow PubMed journals Mean cerebral blood flow medical journals Mean cerebral blood flow free journals Mean cerebral blood flow best journals Mean cerebral blood flow top journals Mean cerebral blood flow free medical journals Mean cerebral blood flow famous journals Mean cerebral blood flow Google Scholar indexed journals Head reference angle articles Head reference angle Research articles Head reference angle review articles Head reference angle PubMed articles Head reference angle PubMed Central articles Head reference angle 2023 articles Head reference angle 2024 articles Head reference angle Scopus articles Head reference angle impact factor journals Head reference angle Scopus journals Head reference angle PubMed journals Head reference angle medical journals Head reference angle free journals Head reference angle best journals Head reference angle top journals Head reference angle free medical journals Head reference angle famous journals Head reference angle Google Scholar indexed journals Brain Perfusion Indexes articles Brain Perfusion Indexes Research articles Brain Perfusion Indexes review articles Brain Perfusion Indexes PubMed articles Brain Perfusion Indexes PubMed Central articles Brain Perfusion Indexes 2023 articles Brain Perfusion Indexes 2024 articles Brain Perfusion Indexes Scopus articles Brain Perfusion Indexes impact factor journals Brain Perfusion Indexes Scopus journals Brain Perfusion Indexes PubMed journals Brain Perfusion Indexes medical journals Brain Perfusion Indexes free journals Brain Perfusion Indexes best journals Brain Perfusion Indexes top journals Brain Perfusion Indexes free medical journals Brain Perfusion Indexes famous journals Brain Perfusion Indexes Google Scholar indexed journals Cerebral Blood Flow articles Cerebral Blood Flow Research articles Cerebral Blood Flow review articles Cerebral Blood Flow PubMed articles Cerebral Blood Flow PubMed Central articles Cerebral Blood Flow 2023 articles Cerebral Blood Flow 2024 articles Cerebral Blood Flow Scopus articles Cerebral Blood Flow impact factor journals Cerebral Blood Flow Scopus journals Cerebral Blood Flow PubMed journals Cerebral Blood Flow medical journals Cerebral Blood Flow free journals Cerebral Blood Flow best journals Cerebral Blood Flow top journals Cerebral Blood Flow free medical journals Cerebral Blood Flow famous journals Cerebral Blood Flow Google Scholar indexed journals Computed Tomography articles Computed Tomography Research articles Computed Tomography review articles Computed Tomography PubMed articles Computed Tomography PubMed Central articles Computed Tomography 2023 articles Computed Tomography 2024 articles Computed Tomography Scopus articles Computed Tomography impact factor journals Computed Tomography Scopus journals Computed Tomography PubMed journals Computed Tomography medical journals Computed Tomography free journals Computed Tomography best journals Computed Tomography top journals Computed Tomography free medical journals Computed Tomography famous journals Computed Tomography Google Scholar indexed journals Mean Cerebral Blood Flow articles Mean Cerebral Blood Flow Research articles Mean Cerebral Blood Flow review articles Mean Cerebral Blood Flow PubMed articles Mean Cerebral Blood Flow PubMed Central articles Mean Cerebral Blood Flow 2023 articles Mean Cerebral Blood Flow 2024 articles Mean Cerebral Blood Flow Scopus articles Mean Cerebral Blood Flow impact factor journals Mean Cerebral Blood Flow Scopus journals Mean Cerebral Blood Flow PubMed journals Mean Cerebral Blood Flow medical journals Mean Cerebral Blood Flow free journals Mean Cerebral Blood Flow best journals Mean Cerebral Blood Flow top journals Mean Cerebral Blood Flow free medical journals Mean Cerebral Blood Flow famous journals Mean Cerebral Blood Flow Google Scholar indexed journals Single Photon Emission Computed Tomography articles Single Photon Emission Computed Tomography Research articles Single Photon Emission Computed Tomography review articles Single Photon Emission Computed Tomography PubMed articles Single Photon Emission Computed Tomography PubMed Central articles Single Photon Emission Computed Tomography 2023 articles Single Photon Emission Computed Tomography 2024 articles Single Photon Emission Computed Tomography Scopus articles Single Photon Emission Computed Tomography impact factor journals Single Photon Emission Computed Tomography Scopus journals Single Photon Emission Computed Tomography PubMed journals Single Photon Emission Computed Tomography medical journals Single Photon Emission Computed Tomography free journals Single Photon Emission Computed Tomography best journals Single Photon Emission Computed Tomography top journals Single Photon Emission Computed Tomography free medical journals Single Photon Emission Computed Tomography famous journals Single Photon Emission Computed Tomography Google Scholar indexed journals
Article Details
Abbreviations:
BPI: Brain Perfusion Indexes; CBF: Cerebral Blood Flow; CT: Computed Tomography; mCBF: Mean Cerebral Blood Flow; MRI: Magnetic Resonance Imaging; RI: Radionuclide; ROI: Region of Interest; SPECT: Single Photon Emission Computed Tomography
1. Introduction
In Japan, the application of Single Photon Emission Computed Tomography (SPECT) for cerebral blood flow changed from mainly inpatients to mainly outpatients due to the spread of the diagnosis procedure combination system. In addition, the indications for SPECT changed from cerebrovascular diseases to degenerative diseases. Furthermore, the population changed due to aging factors such as an increase in the number of people aged 65 years and over, which was the result of the decline in age group-specific mortality rates. Against this background, the number of patients undergoing cerebral perfusion SPECT examinations was elderly and an increasing number of patients had difficulty with the imaging head position due to spinal flexion and other factors.
SPECT provides information on brain function and blood circulation that is difficult to obtain from anatomical images obtained with Computed Tomography (CT) and Magnetic Resonance Imaging (MRI). SPECT has been shown to be useful in various central nervous system diseases [1-3]. Positron emission tomography using 18F-fluorodeoxyglucose (18F-FDG) is more sensitive than cerebral blood flow SPECT for detecting decreased glucose metabolism, especially in imaging studies carried out to assess dementia, but is not covered by insurance in Japan [4]. The number of cases in which it can be performed is limited in Japan. In addition, compared to 123I-based cerebral blood flow SPECT agents, 99mTc-based cerebral blood flow SPECT agents are more suitable for screening degenerative diseases and require shorter acquisition times. Therefore, SPECT of cerebral blood flow using radiopharmaceuticals such as technetium-99m ethyl cysteinate dimer (99mTc-ECD) [5-8] is used in many facilities because it is known to be useful for differential diagnoses of dementia by assessing areas of reduced blood flow [9,10]. It is important to understand cerebral blood flow dynamics in central nervous system diseases in the elderly using cerebral blood flow SPECT [11,12]. It is especially useful when the entire brain is diffusely hypoperfused [13]. In cerebral blood flow SPECT using 99mTc-ECD, the Patlak Plot method, which was developed by Matsuda et al. [14] is widely used because it is noninvasive and simple to use [14-19]. The Patlak Plot method requires radionuclide-angiography (RI-angiography) of the patient's frontal view of the tracer to pass from the heart to the brain immediately after the tracer is administered intravenously for quantitative analysis [14]. From the obtained RI-angiography images, the Regions of Interest (ROIs) were hand-drawn over the bilateral brain hemispheres (ROIbrain) and aortic arch (ROIaorta) [14]. The ROIbrain should be set in the parenchyma of the brain, avoiding the scalp, nasal cavity, and cerebellum [20-21]. In particular, it has been shown that the setting of the ROIbrain has the greatest impact on quantitative analysis [21]. In order to set the ROIbrain only in the parenchyma of the brain, the angle of jaw pull to the cephalocaudal direction of the head during RI-angiography is important, and there is no report that has verified the influence from that angle. The purpose of this study was to clarify the effect of the reference angle of the head during RI-angiography using the Patlak Plot method on mean cerebral blood flow (mCBF) in cerebral blood flow SPECT using 99mTc-ECD.
2. Materials and Methods
2.1. Patients
Between January 2012 and December 2013, 290 patients who visited an outpatient memory loss clinic and underwent cerebral blood flow SPECT scans and were judged to be normal were included in the study. One cerebral blood flow SPECT study per patient was included. The patients ranged in age from 60 to 89 years, the mean age and standard deviation were 77.8 ± 7.0 years for males and 78.8 ± 6.0 years for females (Table 1). The patients with no apparent abnormality on cerebral blood flow SPECT, no abnormal signal areas on head MRI or head CT performed on the same day as cerebral blood flow SPECT, and the patients who walked independently were included in the study. This study was approved by the ethical review committee of the institution to which the researcher belongs.
|
Age-group |
Male |
Female |
||||
|
Number |
Mean |
SD |
Number |
Mean |
SD |
|
|
60–69 years |
15 |
65.3 |
2.9 |
13 |
65.5 |
2.3 |
|
70–79 years |
45 |
75.3 |
2.8 |
74 |
75.3 |
2.6 |
|
80–89 years |
49 |
84 |
2.7 |
94 |
83.5 |
2.7 |
|
All |
109 |
77.8 |
7 |
181 |
78.8 |
6 |
Table 1: Age stratified participants.
2.2. Equipment and acquisition conditions
A two-detector gamma camera Symbia-E equipped with a low energy high resolution collimator and a workstation GMS-7700R (Cannon Medical Systems Inc.) were used for processing. To prevent increased blood flow in the visual area due to light stimulation, the patient was placed in a supine position on a bed and kept in a resting position with closed eyes [22]. Dynamic acquisition was started 10 minutes later simultaneously with radiopharmaceutical administration. Dynamic acquisition was performed with a detector placed in front of the patient, covering the area from the heart to the brain.
The detector was set so that the detector in front of the patient and the patient’s body surface (head to chest) were parallel and at the shortest distance apart. 99mTc-ECD was administered into the ulnar cutaneous vein of the right arm using 20 mL saline at a constant flush for 10 seconds and under the constant conditions of at least 15 mL of saline and an infusion rate of 1.5–2.0 mL/sec. This ensured the conditions for a constant rate of infusion of 1.0–1.5 mL/sec or more23). The angle of the right arm during administration was raised to a right angle (approximately 90 degrees) between the arm and the body to improve bol usability (Figure 1) [20,23]. One frame per second was acquired for 120 seconds with a 128 × 128 matrix immediately after administration, and the magnification ratio was set to 1.0 [24]. If it was difficult to secure a vessel in the ulnar cutaneous vein of the right arm, the elbow median vein and radial cutaneous vein of the right arm were selected, in that order, and only three vessels were included. After the dynamic acquisition was completed, the indwelling needle was removed and planar images of both sides of the head were acquired using a 512 × 512 matrix, 2.67× magnification, and one minute acquisition time to measure the angle of the head during dynamic acquisition (Figure 2). SPECT acquisition was started 5 minutes after administration. SPECT acquisition was performed using a 128 × 128 matrix, 2.29× magnification (pixel size 2.1 mm), 90 projections (4°/step, continuous), six sets of 3 minutes per frame, 18 minutes of acquisition time, and a 13.5 cm rotation radius [25]. The filtered back projection method was used for image reconstruction, and a Butterworth filter (cutoff frequency 0.60 cycles/cm, order 8) was used as a preprocessing filter. The attenuation was corrected using the Chang method (attenuation coefficient of 0.09 cm−1, and a uniform attenuation map was created with a background threshold of 7%. The photopeak window for 99mTc was set as a 15% energy window centered at 141 keV. The mCBF was calculated using the Patlak Plot method, which was similar to the method reported by Matsuda et al. [15,16]. The ROIbrain and ROIaorta were hand-drawn [14] for Patlak Plot measurements and the ratio of the ROIbrain size to the ROIaorta size was 10:1 (Figure 3) [14]. A ROIbrain was set on each side to avoid the scalp and nasal cavity and did not extend beyond the cerebral hemispheres. The ROIaorta was set so that it did not extend beyond the aortic arch and did not overlap with the venous or pulmonary circulatory phases. The Brain Perfusion Indexes (BPIs) of the left and right cerebral hemispheres were calculated from the time activity curves obtained from the ROIbrain and ROIaorta, respectively, using the Patlak Plot method and the obtained BPI was converted to the mCBF using the relationship formula between BPI and mCBF reported in a previous study by Matsuda et al. [16]. This equation used the early picture method as the conversion equation to 133Xe. The equation is shown below.
mCBF = 2.60 × BPI + 19.8. (1)
The ROIbrain and ROIaorta sizes were recorded when the mCBF was calculated. Lassen's correction (α value: 2.59) [26,27] was applied to the quantitative images using the report by Kawahata et al. [28]. A board-certified nuclear medicine technologist with more than 20 years of experience in nuclear medicine performed the entire process, and the entire series of actions from image processing to quantitative analysis was performed by one person.
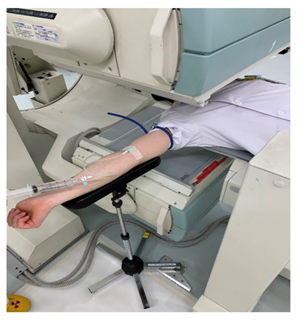
Figure 1: Right upper extremity positioning during radiopharmaceutical administration.
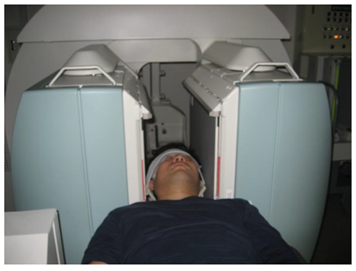
Figure 2: Positioning during head bilateral image acquisition.
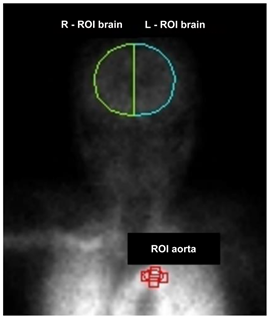
Figure 3: Regions of Interest (ROIs) drawn over the aortic arch and the bilateral brain hemispheres in sequential images of radionuclide angiography using Technetium-99m Ethyl Cysteinate Dimer (99mTc-ECD).
2.3. Head reference angle measurements
The bilateral planar images of the head taken after the end of the dynamic acquisition period were used to measure the jaw pull angle in the head-caudal direction during the dynamic acquisition (Figure 4). The jaw pull angle of the head in the cephalocaudal direction was measured using a straight line connecting the frontal and occipital poles of the cerebrum as the reference line, and the average of the two angles was used. The reference angle range was defined as ±5.0 degrees around the reference angle. Angles were classified as −10.0 degrees or less, −9.9 to −5.1 degrees, and −5.0 to 5.0 degrees (reference angle), 5.1 to 9.9 degrees, and 10.0 degrees or more.
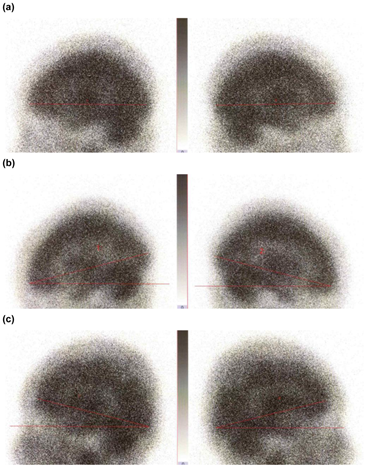
Figure 4: Bilateral images of the head for measurement of the head-jaw pull angle (a) ±0 degrees (reference angle); (b) Jaw outward (- direction); (c) Jaw backward (+direction).
2.4. Radiopharmaceutical
The 99mTc-ECD syringe formulation 600 MBq (FUJIFILM Toyama Chemical Co., Ltd.) was administered as a full dose. Doses ranged from 600 to 900 MBq.
2.5. Statistical analysis
Statistical processing was performed using Graphpad Prism 9. The mean between the two groups was tested for significant differences using an unpaired t test. The Kruskal–Wallis and Dunnet-type multiple testing methods were used to test for significant differences between groups at each angle. A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Relationship between age and gender of subjects and mCBF
Table 2 and Figure 5 show mCBF (ml/100g/min) according to the sex of the subjects. The overall mean for males was 40.4 ± 4.2 and 43.5 ± 4.6 for those aged 60–69 years, 41.1 ± 4.0 for those aged 70–79 years, and 38.8 ± 3.4 for those aged 80–89 years. The correlation coefficient between age and mCBF was −0.40. The overall mean for females was 42.2 ± 3.6, and it was 43.0 ± 3.5 for those aged 60–69 years, 42.9 ± 3.9 for those aged 70–79 years, and 41.5 ± 3.2 for those aged 80–89 years. The correlation coefficient between age and mCBF was −0.22. The overall mean mCBF for males was significantly lower than the overall mean mCBF for females (p < 0.05) (Figure 6).
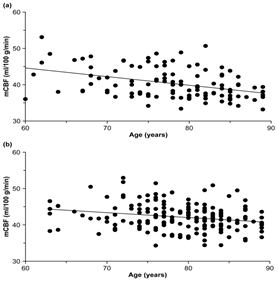
Figure 5: Relationship between age and mean cerebral blood flow (mCBF) by sex (a) Male; (b) Female.
|
Age-group |
Male |
Female |
||||
|
mCBF |
SD |
Correlation |
mCBF |
SD |
Correlation |
|
|
60–69 years |
43.5 |
4.6 |
−0.40 |
43 |
3.5 |
−0.22 |
|
70–79 years |
41.1 |
4 |
42.9 |
3.9 |
||
|
80–89 years |
38.8 |
3.4 |
41.5 |
3.2 |
||
|
All |
40.4 |
4.2 |
42.2 |
3.6 |
||
|
mCBF (ml/100 g/min) |
||||||
Table 2: Relationship between age and mean cerebral blood flow (mCBF) by gender and age group.
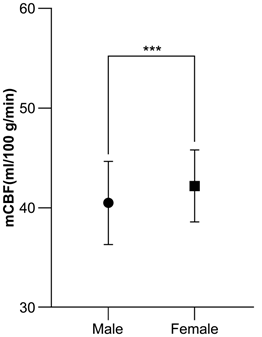
Figure 6: Comparison of male and female mCBF
3.2. The ROIbrain relationship for different reference angles
Table 3 and Figure 7 show the ROIbrain number of pixles by gender and reference angle classification of the subjects. The overall mean for males was 177.0 ± 17.9 and it was 197.3 ± 10.9 for −10.0 degrees and below, 184.9 ± 15.3 for −9.9 to −5.1 degrees, 178.3 ± 17.3 for −5.0 to 5.0 degrees, 165.6 ± 13.3 for 5.1 to 9.9 degrees, and 162.6 ± 11.1 for 10.0 degrees and above. Compared to the reference angle of −5.0 to 5.0 degrees, the values below −10.0 degrees were predominantly higher (p < 0.05), while the values between 5.1 and 9.9 and above 10.0 were significantly lower (p<0.05). There was no significant difference between −9.9 and −5.1. The overall mean for females was 172.8 ± 20.1 and it was 193.0 ± 17.5 for −10.0 degrees and below, 171.7 ± 12.8 for −9.9 to −5.1 degrees, 169.8 ± 15.0 for −5.0 to 5.0 degrees, 158.5 ± 11.2 for 5.1 to 9.9 degrees, and 142.7 ± 10.3 for 10.0 degrees or above. Compared to the reference angle of −5.0 to 5.0 degrees, the values below −10.0 degrees were predominantly higher (p < 0.05) and those above 10.0 degrees were predominantly lower (p < 0.05). There was no significant difference between −9.9 to −5.1 and 5.1 to 9.9 degrees. There was no significant difference in the overall mean ROIbrain between males and females (Figure 8).
|
Angle |
Male |
Female |
||
|
ROI brain |
SD |
ROI brain |
SD |
|
|
<−10° |
197.3 |
11 |
193 |
18 |
|
−9.9° to −5.1° |
184.9 |
15 |
171.7 |
13 |
|
–5.0° to 5.0° |
178.3 |
17 |
169.8 |
15 |
|
5.1° to 9.9° |
165.6 |
13 |
158.5 |
11 |
|
10°< |
162.6 |
11 |
142.7 |
10 |
|
All |
177 |
18 |
172.8 |
20 |
Table 3: Relationship between head reference angle and brain Region of Interest (ROI) by gender.
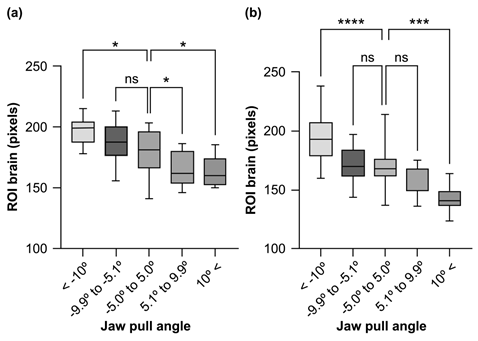
Figure 7: Relationship between brain ROI and head reference angle by gender (a) Male; (b) Female.
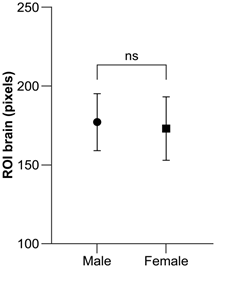
Figure 8: Comparison of male and female brain ROI.
3.3. Relationship between the mCBF at different reference angles
Table 4 and Figure 9 show mCBF (ml/100g/min) by gender and reference angle classification, respectively. The overall mean for males was 40.4 ± 4.2 and it was 37.3 ± 1.4 for −10.1 degrees and below, 40.5 ± 3.4 for −10.0 to −5.1 degrees, 41.5 ± 4.3 for −5.0 to 5.0 degrees, 40.0 ± 4.8 for 5.1 to 10.0 degrees, and 37.9 ± 2.0 for 10.1 degrees and above. Compared to the reference angle of −5.0 to 5.0 degrees, the values below −10.1 degrees and above 10.1 degree were significantly lower (p < 0.05). There was no significant difference between −10.0 to −5.1 degrees and 5.1 to 10.0 degrees. The overall mean for females was 42.2 ± 3.6 and it was 41.2 ± 3.1 for −10.1 degrees and below, 42.8 ± 3.5 for −10.0 to −5.1 degrees, 42.9 ± 3.8 for −5.0 to 5.0 degrees, 42.5 ± 3.6 for 5.1 to 10.0 degrees, and 39.5 ± 2.5 for 10.1 degrees and above. Compared to the reference angle of −5.0 to 5.0 degrees, the values below −10.1 degrees were predominantly low (p < 0.05) and above 10.1 degrees were significantly low (p < 0.05). There was no significant difference between −10.0 to −5.1 degrees and 5.1 to 10.0 degrees.
|
Angle |
Male |
Female |
||
|
mCBF |
SD |
mCBF |
SD |
|
|
<−10° |
37.3 |
1.4 |
41.2 |
3.1 |
|
−9.9° to −5.1° |
40.5 |
3.4 |
42.8 |
3.5 |
|
−5.0° to 5.0° |
41.5 |
4.3 |
42.9 |
3.8 |
|
5.1° to 9.9° |
40 |
4.8 |
42.5 |
3.6 |
|
10°< |
37.9 |
2 |
39.5 |
2.5 |
|
All |
40.4 |
4.2 |
42.2 |
3.6 |
Table 4: Relationship between head reference angle and mCBF by gender.
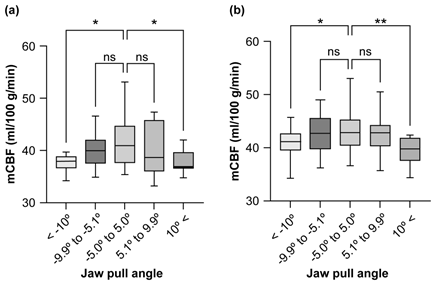
Figure 9: Relationship between head reference angle and mCBF by gender (a) Male; (b) Female.
4. Discussion
In this study, we evaluated the effect of the reference angle of the head in the cephalocaudal direction (jaw pull angle) during RI-angiography to measure the mCBF in a quantitative analysis of cerebral blood flow using the Patlak Plot method with 99mTc-ECD (Figure 4). Otake et al. [21] reported that the inclusion or exclusion of the cerebellum and nasal cavity in the ROIbrain is a major factor and that this has the greatest impact on quantitative analyses [21]. The ROIbrain size was verified based on the reference angle of the head in the cephalocaudal direction during RI-angiography and its effect on mCBF was verified. First, the relationship between age and gender of the subjects and mCBF was verified and compared with previous studies (Table 2; Figures 5 and 6). The results showed that there was a significant difference in the mCBF between males and females. We therefore evaluated the mCBF of males and females separately. Next, we examined the relationship between the reference angle and ROIbrain size by gender and found that when the reference angle was greater than ±10 degrees, the ROIbrain size was likely to be over- or under-set for both genders (Table 3; Figures 7 and 8). Next, the relationship between the reference angle and the mCBF by gender was examined and it was shown that the mCBF may be underestimated when the ROIbrain size was overestimated or underestimated (Table 4; Figure 9).
The relationship between age and sex of the patients and mCBF was negative and weak, with a mCBF of −0.40 for males aged 60 to 89 years. Similarly, females showed a negative and weak correlation of −0.22 (Table 2). This was in agreement a report by Tanaka et al. [29] that suggested mCBF had a significant negative correlation with aging [29-30]. Next, a comparison of mCBF between males and females showed that males predominantly had a lower mCBF than females. This is consistent with the results of the present study, in which Takeda et al. [31] reported that cerebral blood flow in males was lower than that in females [31]. In a study by Matsuda et al. [15] in which the Patlak Plot method with 99mTc-HMPAO was used, mCBF was reported to be 44.9 ± 2.2 ml/100g/min on average in 60- to 76-year-olds, and the present results were similar to these values. Therefore, we believe that the subject of our study is appropriate.
In terms of the relationship between the ROIbrain sizes for different reference angles, first, the mean ROIbrain size for males was larger than the mean ROIbrain size for females with respect to the size of the ROIbrain for each gender (Table 3). Allen et al. reported that males had larger brain volumes than females [32], and their results were similar to those of Allen et al. However, the images were obtained from a system with a resolution of approximately 10%, and no significant difference was found due to the blurring of nuclear medicine images caused by pixel size and low counts (Figure 7). Next, for the male ROIbrain size, values below −10.0 degrees were significantly higher than the reference ROIbrain, while values between 5.1 and 9.9 degrees and above 10.0 were significantly lower than the reference ROIbrain (Figure 6) The ROIbrain size of the male subject was significantly higher than −10.0 degrees because the ROIbrain size was set widely because the ROIbrain was set using image acquisition from the front of the head, with the cerebral hemispheres collapsed toward the feet, as shown in Figure 4(b). In addition, as can be seen from Figure 4(c), the ROIbrain size from the frontal image was influenced by the ROIbrain from the front view, and the occipital region could not be included in the ROIbrain image, resulting in an underestimation of the ROIbrain and a significantly low value for the ROIbrain size. In a report by Yano et al. [24] the definition was based on the assumption that the jaw was not extremely raised or lowered, and an extremely strong reference angle was shown to affect the ROIbrain, which was consistent with their results. Next, for the female ROIbrain size, the reason was considered to be the same as that for the male ROIbrain size, but because the female ROIbrain size was smaller than the male ROIbrain size, there was a significant difference only between the values below −10.0 degrees and above 10.0 degrees.
The relationship between the reference angle and mCBF showed that mCBF was significantly lower below −10.0 degrees and above 10.0 degrees in both males and females (Figure 8). The ROIbrain size was significantly larger when the reference angle value was less than −10.0 degrees, suggesting that the ROIbrain size included regions other than the cerebral hemispheres and included the cerebellum. This reduced the counts/pixels included in the ROIbrain measurements, resulting in a significantly lower mCBF value. Similarly, the ROIbrain size was significantly smaller at values of 10.0 degrees or greater. This is because one of the characteristics of 99mTc-ECD brain distribution is overestimation of occipital lobe blood flow [33], and when the ROIbrain exceeds 10.0 degrees, there is a high possibility that the occipital lobe is not included. Therefore, the number of counts/pixels included in the ROIbrain was reduced and mCBF was considered to be significantly lower.
Miyazaki et al. [34] reported that the relationship between ROI and mCBF showed that as the counts/pixel in the ROIbrain increased, mCBF also increased proportionally, which is consistent with their results.
These results are consistent with the report by Yano et al. [24] which showed that an extremely strong jaw pull angle, such as an extremely raised or lowered jaw during RI-angiography, affects the ROIbrain size when mCBF is obtained using the Patlak Plot method and 99mTc-ECD [33]. In the present validation, it was inferred that mCBF is significantly lower when the jaw is extremely lowered below −10.0 degrees and when the jaw is extremely raised above 10.0 degrees, due to the effect of the size of the ROIbrain.
Based on the results of this verification, the effect on mCBF at reference angles greater than ±10 degrees is shown in Table 5 as a relative error. It was clear that mCBF was significantly lower when the reference angle was ±10 degrees. Therefore, when dynamic acquisition was performed at a reference angle of ±10 degrees or higher, the mCBF can be converted to the mCBF at the reference angle by using the correction coefficient shown in Table 6.
|
Angle |
Male |
Female |
|
mCBF |
mCBF |
|
|
<−10° |
−10.0% |
−4.0% |
|
10°< |
−8.6% |
−7.9% |
Table 5: Relative error of mCBF by reference angle.
|
Angle |
Male |
Female |
|
mCBF |
mCBF |
|
|
<−10° |
×1.11 |
×1.04 |
|
10°< |
×1.09 |
×1.09 |
Table 6: Correction coefficient of mCBF by reference angle.
This study has several limitations. First, the Patlak Plot method requires many manual operations, and since the data differs from patient to patient, it has been reported that the results may vary depending on the surgeon's experience level and processing standards [21], and it is necessary to verify this series of processes with many surgeons. If interoperator variability is minimized, the accuracy may be further improved. Second, mCBF has been reported to decrease with age [17]. The present study was conducted with elderly subjects aged 60 to 89 years (mean age: approximately 80 years). Continuous follow-up of the subjects may improve the accuracy of the normality judgment and the accuracy of determining that the cause of the decrease in mCBF was due to the size of the ROIbrain. Further validation with a larger number of subjects may improve the accuracy, especially with respect to the collection of reference angles at ±10.0 or higher. Finally, because RI-angiography records data from the anterior aspect, it has been reported that the BPI is significantly lower in cases where the ischemic area is deep or occipital [34], and another method should be selected if these are known in advance.
5. Conclusions
In order to obtain mCBF using the Patlak Plot method with 99mTc-ECD, we measured the reference angle of the head during RI-angiography and verified the effect on ROIbrain sizes and the effect on mCBF. Our results suggested that the reference angle of the head ±10 degrees or less during RI-angiography may reduce the effect on mCBF.
Acknowledgement
The authors thank the staff of the Department of Radiology, Saiseikai Yokohamashi Tobu Hospital.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors have no relevant conflicts of interest to disclose.
References
- Ell PJ, Jarritt PH, Costa DC, et al. Functional Imaging of the Brain. Seminars in Nuclear Medicine 17 (1987): 214-229.
- Juni JE, Waxman AD, Devous MD, et al. Society of Nuclear Medicine Procedure Guideline for Brain Perfusion Single Photon Emission Computed Tomography (SPECT) Using Tc-99m Radiopharmaceuticals, Version 2.0. Society of Nuclear Medicine Procedure Guidelines Manual 39 (2002): 113-118.
- Kapucu OL, Nobili F, Varrone A, et al. EANM Procedure Guideline for Brain Perfusion SPECT Using 99mTc-Labelled Radiopharmaceuticals, Version 2. European Journal of Nuclear Medicine and Molecular Imaging 36 (2009): 2093-2102.
- Shivamurthy VK, Tahari AK, Marcus C, et al. Brain FDG PET and the Diagnosis of Dementia American Journal of Roentgenology 204 (2015): W76-W85.
- Vallabhajosula S, Zimmerman RE, Picard M, et al. Technetium-99m ECD: A New Brain Imaging Agent: In Vivo Kinetics and Biodistribution Studies in Normal Human Subjects. Journal of Nuclear Medicine 30 (1989): 599-604.
- Walovitch RC, Hill TC, Garrity ST, et al. Characterization of Technetium-99m-L,L-ECD for Brain Perfusion Imaging, Part 1: Pharmacology of Technetium-99m ECD in Nonhuman Primates. Journal of Nuclear Medicine 30 (1989): 1892-1901.
- Léveillé J, Demonceau G, De Roo M, et al. Characterization of Technetium-99m-L,L-ECD for Brain Perfusion Imaging, Part 2: Biodistribution and Brain Imaging in Humans. Journal of Nuclear Medicine 30 (1989): 1902-1910.
- Léveillé J, Demonceau G, Walovitch RC. Intrasubject Comparison Between Technetium-99m-Ecd and Technetium-99m-Hmpao in Healthy Human Subjects. Journal of Nuclear Medicine 33 (1992): 480-484.
- Nakajima K, et al. Guidelines for the Treatment of Dementia Diseases 2017. The Journal of the Japanese Society of Internal Medicine 107 (2018): 544-549.
- Ishii K. Dementia: Advances in Diagnosis and Treatment. Nihon Naika Gakkai Zasshi. The Journal of the Japanese Society of Internal Medicine 100 (2011): 2116-2124.
- Nakano S, Asada T, Matsuda H, et al. Effects of Healthy Aging on the Regional Cerebral Blood Flow Measurements Using 99mTc-ECD SPECT Assessed with Statistical Parametric Mapping. Nihon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics 37 (2000): 49-55.
- Catafau AM, Parellada E, Lomeña F, et al. Baseline, Visual Deprivation and Visual Stimulation 99Tcm-HMPAO-Related Changes in Visual Cortex Can Be Detected with a Single-Head SPET System. Nuclear Medicine Communications 17 (1996): 480-484.
- Niida H. A Practice of Various Quantitative Methods for Cerebral Blood Flow Using Brain SPECT-Notes in Clinical Application (Clinical Technology Course). Nihon Hoshasen Gijutsu Gakkai Zasshi. Japanese Journal of Radiological Technology 58 (2002): 640-650.
- Matsuda H, Tsuji S, Shuke N, et al. A Quanttitative Approach to Technetium-99m Hexamethylpropylene Amine Oxime. European Journal of Nuclear Medicine 19 (1992): 195-200.
- Matsuda H, Tsuji S, Shuke N, et al. Noninvasive Measurements pf Regional Cerebral Blood Flow Using Technetium-99m Hexamethylpropylene Amine Oxime. European Journal of Nuclear Medicine 20 (1993): 391-401.
- Matsuda H, Yagishita A, Tsuji S, et al. A Quanttitative Approach to Technetium-99m Ethyl Cysteinate Dimer: A Comparison with Technetium-99m Hexamethylpropylene Amine Oxime. European Journal of Nuclear Medicine 22 (1995): 633-637.
- Matsuda H, Tsuji S, Shuke N, et al. Noninvasive Cerebral Blood Flow Quantification by 99mTc-HMPAO. Eizo Joho 25 (1993): 197-202.
- Fukuyama H. Patlak Method Concepts. Eizo Joho 25 (1993): 193-196.
- Tsuji S, Matsuda H, Shuke N, et al. Quantitative Analysis of Brain Perfusion Using Radionuclide Angiography with 99mTc-HMPAO. Kaku igaku. The Japanese Journal of Nuclear Medicine 30 (1993): 499-506.
- Miyazaki Y, Takimoto M, Shiozaki Z. Matsuda, Tsuji, and Shuke Method, For Calculating Stable mCBF. Eizo Joho 25 (1993): 203-213.
- Otake H, Ujita K, Matsubara K. Multicenter Trial for the Standardization of Mean Cerebral Blood Flow (mCBF) Measured by 99mTc-ECD Patlak Plot Method. Japanese Journal of Nuclear Medicine Technology 23 (2003): 473-476.
- Hasegawa T, Inao S, Senda K. Comparison of Cerebral Flow in Primary Visual Cortex by Administration of 99mTc-ECD While Having Eyes Open or Closed. Kaku Igaku. The Japanese Journal of Nuclear Medicine 33 (1996): 1239-1242.
- Hasegawa T, Yamashita K, Asano K, et al. A Study on the Methods of Tracer Administration in Patlak Plot Procedure. Eizo Joho Medical 28 (1996): 5-8.
- Yano K, Miyasaka T, Sato M. Development of the Automated Patlak Plot Method and Its Verification in Clinical Examples. Nihon Hoshasen Gijutsu Gakkai Zasshi. Japanese Journal of Radiological Technology 63 (2007): 247-256.
- Yamanaga T, Hasegawa S, Imoto A, et al. Guidelines for Standardization of Cerebral Blood Flow SPECT Imaging 1.0. Japanese Journal of Nuclear Medicine Technology. 37 (2017): 505-516.
- Friston KJ, Ashburner J, Frith CD, et al. Spatial Registration and Normalization of Images. Human Brain Mapping 2 (1995b): 189-210.
- Friberg L, Andersen AR, Lassen NA, et al. Retention of 99mTc-Bicisate in the Human Brain after Intracarotid Injection. Journal of Cerebral Blood Flow and Metabolism 14 (1994): S19-S27.
- Kawahata N, Daitoh N, Shirai F, et al. Reduction in Mean Cerebral Blood Flow Measurements Using 99mTc-ECD-SPECT During Normal Aging. Kaku Igaku. The Japanese Journal of Nuclear Medicine 34 (1997): 909-916.
- Tanaka S, Nakamura Y, Yagi Y, et al. Quantitative Measurement of Regional Cerebral Blood Flow Using 99mTc-HM-PAO SPECT.?The Japan Stroke Society 16 (1994): 334-340.
- Matsuda H, Maeda T, Yamada M, et al. Age-Matched Normal Values and Topographic Maps for Regional Cerebal Blood Flow Measurements by Xe-133 Inhalation. Stroke 15 (1984): 336-342.
- Takeda S, Kawai H, Matsuzawa T. Effects of Age on Cerebral Blood Flow—A Study with the Xe-133 Inhalation Method. Nihon Ronen Igakkai Zasshi. Japanese Journal of Geriatrics 23 (1986): 497-503.
- Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A Meta-Analysis of Sex Differences in Human Brain Structure. Neuroscience and Biobehavioral Reviews 39 (2014): 34-50.
- Nakagawara J, Nakamura J, Takeda R, et al. Assessment of Postischemic Reperfusion and Diamox Activation Test in Stroke Using 99mTc-ECD SPECT. Journal of Cerebral Blood Flow and Metabolism 14 (1994): S49-S57.
- Miyazaki Y, Kinuya S, Tonami N. A Simple Method to Obtain Semi-quantitative Index of Brain Perfusion Using 99mTc-HMPAO. Kaku Igaku. The Japanese Journal of Nuclear Medicine 31 (1994): 551-558.
