Effect of The Gluten-Free Diet on Quality of Life, Gastrointestinal Symptoms and Immune System in Patients with Fibromyalgia and Non-Celiac Wheat Sensitivity. Fibromyalgia and Non-Celiac Wheat Sensitivity
Article Information
Claudia Schinocca1#, Diana Di Liberto2#, Pasquale Mansueto1, Marianna Lo Pizzo2, Giulia Grasso1, Emilia Messana1, Francesco Dieli2, Francesco Ciccia3, Antonio Carroccio*1 and Giuliana Guggino1*
1Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy
2Central Laboratory of Advanced Diagnosis and Biomedical Research (CLADIBIOR), University of Palermo, Palermo, Italy
3Department of Precision Medicine - University of Campania "Luigi Vanvitelli", Naples, Italy
# Claudia Schinocca, Diana Di Liberto contribute equally to this work.
* The last two authors share the senior authorship.
*Corresponding author: Giuliana Guggino, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy.
Received: 01 September 2021; Accepted: 08 September 2021; Published: 30 December 2021
Citation:
Claudia Schinocca, Diana Di Liberto, Pasquale Mansueto, Marianna Lo Pizzo, Giulia Grasso, Emilia Messana, Francesco Dieli, Francesco Ciccia, Giuliana Guggino and Antonio Carroccio. Effect of The Gluten-Free Diet on Quality of Life, Gastrointestinal Symptoms and Immune System in Patients with Fibromyalgia and Non-Celiac Wheat Sensitivity. Fibromyalgia and Non-Celiac Wheat Sensitivity. Journal of Biotechnology and Biomedicine 4 (2021): 211-227.
View / Download Pdf Share at FacebookAbstract
Fibromyalgia (FM) is a clinical syndrome characterized by chronic pain. FM patients complain hyperalgesia and allodynia and they are frequently affected by Non Celiac Wheat Sensitivity (NCWS), a condition where gastrointestinal and extraintestinal symptoms are triggered by gluten and/or wheat ingestion. The gluten-free diet (GFD) impact was evaluated on fibromyalgia-related and gastrointestinal symptoms, health-related quality of life and immune response of patients with both FM and NCWS in order to detect a possible pathogenetic role of wheat/gluten in the triggering of the inflammatory process. Peripheral blood from 8 FM patients, 10 FM and NCWS patients (FM+NCWS patients), 13 NCWS patients and 14 healthy subjects (HS) was taken before and after 8 weeks of GFD and used for cytofluorimetric analysis. In all FM+NCWS patients after GFD almost all the clinical parameters used to evaluate musculoskeletal and systemic symptoms related to FM were reduced as well as intestinal symptoms present before GFD. Moreover, pro-inflammatory cytokines production (IFN-γ, TNF- α, IL-17 and IL-22) by T helper (Th) cells of FM+NCWS patients was reduced after GFD. Our findings suggest that gluten/wheat could represent one of the triggering factors of the inflammatory condition playing an important role in the etiopathogenesis of both FM and NCWS.
Keywords
Adaptative immunity; Fibromyalgia (FM); Gluten Free Diet (GFD); Non Celiac Wheat Sensitivity (NCWS); Pro-inflammatory Cytokines
Adaptative immunity articles Adaptative immunity Research articles Adaptative immunity review articles Adaptative immunity PubMed articles Adaptative immunity PubMed Central articles Adaptative immunity 2023 articles Adaptative immunity 2024 articles Adaptative immunity Scopus articles Adaptative immunity impact factor journals Adaptative immunity Scopus journals Adaptative immunity PubMed journals Adaptative immunity medical journals Adaptative immunity free journals Adaptative immunity best journals Adaptative immunity top journals Adaptative immunity free medical journals Adaptative immunity famous journals Adaptative immunity Google Scholar indexed journals Fibromyalgia (FM) articles Fibromyalgia (FM) Research articles Fibromyalgia (FM) review articles Fibromyalgia (FM) PubMed articles Fibromyalgia (FM) PubMed Central articles Fibromyalgia (FM) 2023 articles Fibromyalgia (FM) 2024 articles Fibromyalgia (FM) Scopus articles Fibromyalgia (FM) impact factor journals Fibromyalgia (FM) Scopus journals Fibromyalgia (FM) PubMed journals Fibromyalgia (FM) medical journals Fibromyalgia (FM) free journals Fibromyalgia (FM) best journals Fibromyalgia (FM) top journals Fibromyalgia (FM) free medical journals Fibromyalgia (FM) famous journals Fibromyalgia (FM) Google Scholar indexed journals Gluten Free Diet (GFD) articles Gluten Free Diet (GFD) Research articles Gluten Free Diet (GFD) review articles Gluten Free Diet (GFD) PubMed articles Gluten Free Diet (GFD) PubMed Central articles Gluten Free Diet (GFD) 2023 articles Gluten Free Diet (GFD) 2024 articles Gluten Free Diet (GFD) Scopus articles Gluten Free Diet (GFD) impact factor journals Gluten Free Diet (GFD) Scopus journals Gluten Free Diet (GFD) PubMed journals Gluten Free Diet (GFD) medical journals Gluten Free Diet (GFD) free journals Gluten Free Diet (GFD) best journals Gluten Free Diet (GFD) top journals Gluten Free Diet (GFD) free medical journals Gluten Free Diet (GFD) famous journals Gluten Free Diet (GFD) Google Scholar indexed journals Non Celiac Wheat Sensitivity (NCWS) articles Non Celiac Wheat Sensitivity (NCWS) Research articles Non Celiac Wheat Sensitivity (NCWS) review articles Non Celiac Wheat Sensitivity (NCWS) PubMed articles Non Celiac Wheat Sensitivity (NCWS) PubMed Central articles Non Celiac Wheat Sensitivity (NCWS) 2023 articles Non Celiac Wheat Sensitivity (NCWS) 2024 articles Non Celiac Wheat Sensitivity (NCWS) Scopus articles Non Celiac Wheat Sensitivity (NCWS) impact factor journals Non Celiac Wheat Sensitivity (NCWS) Scopus journals Non Celiac Wheat Sensitivity (NCWS) PubMed journals Non Celiac Wheat Sensitivity (NCWS) medical journals Non Celiac Wheat Sensitivity (NCWS) free journals Non Celiac Wheat Sensitivity (NCWS) best journals Non Celiac Wheat Sensitivity (NCWS) top journals Non Celiac Wheat Sensitivity (NCWS) free medical journals Non Celiac Wheat Sensitivity (NCWS) famous journals Non Celiac Wheat Sensitivity (NCWS) Google Scholar indexed journals Pro-inflammatory Cytokines articles Pro-inflammatory Cytokines Research articles Pro-inflammatory Cytokines review articles Pro-inflammatory Cytokines PubMed articles Pro-inflammatory Cytokines PubMed Central articles Pro-inflammatory Cytokines 2023 articles Pro-inflammatory Cytokines 2024 articles Pro-inflammatory Cytokines Scopus articles Pro-inflammatory Cytokines impact factor journals Pro-inflammatory Cytokines Scopus journals Pro-inflammatory Cytokines PubMed journals Pro-inflammatory Cytokines medical journals Pro-inflammatory Cytokines free journals Pro-inflammatory Cytokines best journals Pro-inflammatory Cytokines top journals Pro-inflammatory Cytokines free medical journals Pro-inflammatory Cytokines famous journals Pro-inflammatory Cytokines Google Scholar indexed journals chronic disease articles chronic disease Research articles chronic disease review articles chronic disease PubMed articles chronic disease PubMed Central articles chronic disease 2023 articles chronic disease 2024 articles chronic disease Scopus articles chronic disease impact factor journals chronic disease Scopus journals chronic disease PubMed journals chronic disease medical journals chronic disease free journals chronic disease best journals chronic disease top journals chronic disease free medical journals chronic disease famous journals chronic disease Google Scholar indexed journals NCWS diagnosis articles NCWS diagnosis Research articles NCWS diagnosis review articles NCWS diagnosis PubMed articles NCWS diagnosis PubMed Central articles NCWS diagnosis 2023 articles NCWS diagnosis 2024 articles NCWS diagnosis Scopus articles NCWS diagnosis impact factor journals NCWS diagnosis Scopus journals NCWS diagnosis PubMed journals NCWS diagnosis medical journals NCWS diagnosis free journals NCWS diagnosis best journals NCWS diagnosis top journals NCWS diagnosis free medical journals NCWS diagnosis famous journals NCWS diagnosis Google Scholar indexed journals gastrointestinal articles gastrointestinal Research articles gastrointestinal review articles gastrointestinal PubMed articles gastrointestinal PubMed Central articles gastrointestinal 2023 articles gastrointestinal 2024 articles gastrointestinal Scopus articles gastrointestinal impact factor journals gastrointestinal Scopus journals gastrointestinal PubMed journals gastrointestinal medical journals gastrointestinal free journals gastrointestinal best journals gastrointestinal top journals gastrointestinal free medical journals gastrointestinal famous journals gastrointestinal Google Scholar indexed journals fatigue articles fatigue Research articles fatigue review articles fatigue PubMed articles fatigue PubMed Central articles fatigue 2023 articles fatigue 2024 articles fatigue Scopus articles fatigue impact factor journals fatigue Scopus journals fatigue PubMed journals fatigue medical journals fatigue free journals fatigue best journals fatigue top journals fatigue free medical journals fatigue famous journals fatigue Google Scholar indexed journals Pharmacia Biotech articles Pharmacia Biotech Research articles Pharmacia Biotech review articles Pharmacia Biotech PubMed articles Pharmacia Biotech PubMed Central articles Pharmacia Biotech 2023 articles Pharmacia Biotech 2024 articles Pharmacia Biotech Scopus articles Pharmacia Biotech impact factor journals Pharmacia Biotech Scopus journals Pharmacia Biotech PubMed journals Pharmacia Biotech medical journals Pharmacia Biotech free journals Pharmacia Biotech best journals Pharmacia Biotech top journals Pharmacia Biotech free medical journals Pharmacia Biotech famous journals Pharmacia Biotech Google Scholar indexed journals
Article Details
3. Introduction
Fibromyalgia (FM) is a chronic widespread pain syndrome characterized by systemic symptoms, as headache, cognitive dysfunction, mood disorders, sleep disturbance, musculoskeletal pain, paresthesia, fatigue, insomnia and irritable bowel syndrome (IBS)-like symptoms without a well-defined organ’s disease [1-4]. The etiology of FM is still not completely understood [5-7]. A big debate on the possibility to consider FM as an inflammatory chronic disease is still ongoing. In fact, proinflammatory cytokines (i.e. interleukin IL-1RA, IL-6, and IL-8, but not IL-4, IL-5, and IL-13) have been already described as involved in the pathogenesis of FM, but so far only few data are present in literature regarding their role [8,9]. Additionally, FM is often associated with other inflammatory disease, such as rheumatic diseases (i.e. spondyloarthritis, connective tissue disease, etc.), with a prevalence of 2 to 4% [10,11], or with Non-Celiac Gluten Sensitivity (NCGS), even there are no data about its prevalence [12-16]. In fact, FM patients experience gastrointestinal symptoms as IBS-like symptoms and dyspepsia [17] and gluten-free diet (GFD) would seem to lead to a reduction of both gastrointestinal and extraintestinal symptoms [14]. NCGS, or better defined Non-Celiac Wheat Sensitivity (NCWS) [18], is a condition where gastrointestinal and extraintestinal symptoms are triggered by wheat, in the absence of Celiac Disease (CD) and Wheat Allergy (WA) [19,20]. The term NCWS is more accurate, because wheat components, other than gluten, seem to contribute to disease symptoms [21]. Many of the extraintestinal symptoms are comparable to those present in FM patient, most common are headache or “foggy mind”, anxiety, joint and muscle pain, leg or arm numbness and fatigue [15,22-25]. NCWS pathogenesis has also been attributed to very different mechanisms, however, very little is known about the immunological response that is activated in the intestinal mucosa as a consequence of wheat ingestion. Both innate and adaptive immunity have been suggested as being involved in the immunological response of NCWS patients and can share some trigger with FM [26-30]. From this background, the aim of this study was to evaluate the effect of GFD on fibromyalgia-related symptoms, health-related quality of life, gastrointestinal symptoms and immune system of patients with both FM and NCWS diagnosis to detect a possible pathogenetic role of wheat/gluten in the triggering of the inflammatory process in FM patients.
4. Patients and Methods
4.1 Patients
63 patients, all females, mean age ± standard deviation (SD) 52 ± 6 years, with primary FM, with and without gastrointestinal symptoms, were recruited for this study. All the patients fulfilled the 1990 American College of Rheumatology classification criteria [31] and the 2010 American College of Rheumatology preliminary diagnostic criteria [32], consecutively referred at the Policlinico “Paolo Giaccone” University Hospital, Palermo, Italy, Rheumatology Department, and Internal Medicine Department. All FM patients were evaluated at the Internal Medicine Unit and 45 patients who self-reported symptoms improvement on wheat-free diet were evaluated to underwent to diagnostic procedure for NCWS diagnosis. Exclusions criteria for enrolling in the present study were: medications that interfere with the immune system (steroid and/or immunosuppressive therapy), autoimmune disorders, secondary FM, inflammatory bowel diseases. Therefore, only 41 were identified as FM+self-reported NCWS patients and were found eligible for the research, thus enrolled in the study. All selected FM+self-reported NCWS patients accepted to undergo a 4-weeks period of wheat-containing diet, containing 100 grams daily of wheat-based foods, on the patient’s choice (bread, pasta, etc); at the end of this period (T0) they were clinically evaluated. Then, they underwent an 8-weeks period of GFD and were newly clinically evaluated at the end of this period (T1) and 30 of them were classified as FM+NCWS patients. Informed consent was obtained from all participants. Finally, 18 FM patients unrelated to NCWS, 13 NCWS patients unrelated to FM on wheat-containing diet, and 14 age- and sex-matched healthy subjects (HS) were also recruited as control groups (controls). Additionally, peripheral blood mononuclear cells (PBMC) were studied in subgroups of the above patients: 8 FM, 10 FM+NCWS, 13 NCWS patients and 14 HS as disease control groups was analyzed at baseline (T0) and after 8 weeks of GFD (T1). The study was recorded at the Clinicaltrials.gov (registration number NCT03022513, “Fibromyalgia-like Joint/Muscle Pain and Synovitis in Non-celiac Wheat Sensitivity Patients”) and was approved by the Ethical Committee of the University of Palermo after ascertaining its compliance with the dictates of the Declaration of Helsinki (registration number 02/2018).
4.2 NCWS diagnosis
NCWS was diagnosed after CD or WA had been ruled out in all the studied patients according to the following criteria: (1) negative serum assays for CD-anti-tissue transglutaminase (anti-tTG) IgA and anti-deamidated gliadin peptide (anti-DGP) IgG antibodies; (2) absence of intestinal villous atrophy, documented in all the patients carrying the DQ2 and/or the DQ8 HLA haplotypes (thus irrespective of CD-specific serum antibody negativity); (3) negative IgE-mediated immune-allergy tests to wheat (skin prick tests and/or specific serum IgE detection). In all cases, both serum antibodies and the intestinal histology were evaluated when patients had a minimum intake of 100 grams of wheat daily since at least 2 weeks. Furthermore, CD diagnosis was considered likely if patients were positive for anti-endomysial antibodies (EmA) in the medium of cultured duodenal biopsies, even if the villus/crypt ratio in the duodenal mucosa was normal. Consequently, these patients were not included in the NCWS group. Subsequently, we adopted similar criteria for NCWS diagnosis used in previous studies: resolution of symptoms on a standard elimination diet without wheat, cow’s milk, yeast, and other food(s) causing self-reported symptoms, followed by symptom reappearance on a wheat challenge. In the present study, however, we did not perform double-blind placebo-controlled (DBPC) challenge but a simpler open challenge. Additional inclusion criteria were: age above 18 years, follow-up duration longer than 12 months after the initial diagnosis, and at least two outpatient visits during the follow-up period [1,33,34].
4.3 Elimination diet open challenge for NCWS diagnosis
Briefly, before final NCWS diagnosis, all patients were placed on a standard elimination diet, which excluded wheat, cow’s milk and yeast, and in patients with self-reported food hypersensitivity also other food(s) causing symptoms. Food diaries were kept during the elimination diet to assess dietary intake and adherence to the diet. Patients who became asymptomatic after 4 weeks of the elimination diet underwent open challenges. During all phases of the evaluation, the severity of symptoms was recorded: a 10-point visual analog scale (VAS, with 0 representing no and 10 intolerable symptoms), was used by patients to assess the following overall and specific symptoms: diarrhea (increased passing of and/or urgent need to pass loose stools), constipation (decreased passing of stools or feeling of incomplete evacuation), abdominal pain or discomfort, abdominal distension, bloating, increased flatus, heartburn, acid regurgitation, nausea and vomiting. Extraintestinal symptoms were also recorded: rash/dermatitis, headache, brain fog, fatigue, fainting, numbness of the limbs, joint/muscle pains, oral/tongue lesions, or other specific symptoms reported by individual patients. The challenges were stopped when severe clinical reactions occurred for at least 2 consecutive days (increase in VAS score >30% over the basal value) for gastrointestinal and/or extraintestinal symptoms. Challenges were considered positive and NCWS confirmed when the same symptoms that had been initially present reappeared after their disappearance on the elimination diet, on the wheat flour challenge, and when a VAS score was >30% over the basal values. Challenges for other foods in patients with suspected multiple food hypersensitivity were performed in an open fashion also, with an identical method.
4.4 Physical, emotional and social assessment
Disease severity, widespread pain, fatigue, quality of life and gastrointestinal symptoms of FM+NCWS patients were assessed at T0 and T1, by using the Tender Points Count, the Visual Analogue Scale (VAS) for pain and fatigue, the Widespread Pain Index (WPI), the Symptom Severity Scale (SS), the Revised Fibromyalgia Impact Questionnaire (FIQR), and the Gastrointestinal Symptom Rating Scale (GSRS).
4.4.1 Tender Points Count and intensity pain score: Manual tender points (TP) examination was assessed for number and severity as described in the 1990-ACR guidelines [35]. Digital pressure of approximately 4 Kg was applied at each of the 18 predefined TP sites, and the patient’s pain response at each site was scored between 0=no pain to 10=severe pain.
4.4.2 Visual Analogue Scale (VAS) for pain and fatigue: The VAS is a straight, 100-mm line (10 cm) that represents continuous pain or fatigue intensity, where the left end of the line indicates “no pain” and/or “no fatigue” while the other end denotes “pain or fatigue as bad as it could possibly be.” Patients indicated their level of pain (in mm), by marking a single point on a line 38.
4.4.3 Widespread Pain Index (WPI) and Symptom Severity Scale (SS): WPI indicates the number of areas (accordingly to the new preliminary diagnostic ACR criteria of FM) in which the patient has had pain over the last week. The score could be between 0 and 19. SS is based on the patient’s experienced severity of fatigue, waking unrefreshed and cognitive symptoms. The patient indicated whether, during the past week, they have experienced these symptoms with a score scale (0=no problem, 1=slight or mild problem, 2=moderate problem, 3=severe problem) (2).
4.4.4 Revised Fibromyalgia Impact Questionnaire (FIQR): FIQR is an updated version (2009) of the Fibromyalgia Impact Questionnaire (FIQ). The FIQR has 21 items capturing 3 domains; the first domain (physical function) has 9 items, the second domain (overall impact) has 2 items, and the third domain (symptoms) has 10 items. All of the items are 0-10 numeric rating scales using 11 boxes, with higher numbers reflecting greater severity (1).
4.4.5 Gastrointestinal Symptom Rating Scale (GSRS): A modified version of the GSRS was found to be applicable in terms of symptoms evaluation. Briefly, a 10-point visual analog scale (VAS, with 0 representing no and 10 intolerable symptoms), was used by patients to assess the following overall and specific symptoms: diarrhea (increased passing of and/or urgent need to pass loose stools), constipation (decreased passing of stools or feeling of incomplete evacuation), abdominal pain or discomfort, abdominal distension, bloating, increased flatus, heartburn, acid regurgitation, nausea, and vomiting.
4.5 PBMCs isolation and staining
Peripheral blood from 8 patients with only FM, 10 patients that fulfilled both FM and NCWS diagnostic criteria (FM+NCWS), 13 patients with only NCWS and 14 healthy controls was taken before and after 8 weeks of GFD and used for intracellular cytokines and surface markers detection by fluorescence-activated cells sorting analysis. Peripheral blood mononuclear cells (PBMC) were obtained by density gradient centrifugation using Ficoll Hypaque (Pharmacia Biotech, Uppsala, Sweden) from FM+NCWS patients before (T0) and after GFD (T1), and controls. Cell viability (trypan blue dye exclusion) was always >95%. Cells, cultured in RPMI 1640 medium (Euroclone, MI, Italy) supplemented with 10% fetal cow serum (FCS), L-glutamine (Euroclone, MI, Italy) and antibiotics (Euroclone, MI, Italy), were activated with anti-CD3/CD28 beads (ratio 1:2), and/or ionomycin (Sigma, St. Louis, MO, US, 1μg/mL final concentration), and phorbolmyristate acetate (PMA, Sigma, St. Louis, MO, 150ng/mL final concentration) for 6h in the presence of 10 mcg/ml of monensin (Sigma, St. Louis, MO) to block protein secretion, and then stained with appropriate monoclonal antibodies (mAbs). Flow cytometry analysis was performed using a FACSAria (BD Biosciences, CA, USA). At least 100.000 cells (events), gated on lymphocytes region, were acquired for each sample. Data were analyzed with FlowJo software programs (BD Biosciences). The antibodies used were the following: FITC anti-human CD3 (clone UTCHT1, Biolegend, San Diego, CA, USA), PerCp anti-human CD4 (MEM241, Abcam, Cambridge, UK), Pe-Cyanine 7 anti-human CD8 (clone SK1, eBIoscience Affimetrix Inc), APC anti-human interferon (IFN)-γ (clone 4S.B3, Biolegend, San Diego, CA, USA), PE anti-human tumor necrosis factor (TNF)-α (clone MAb11, Biolegend, San Diego, CA, USA), PE anti-human interleukin (IL)-17 (clone eBio 64CAP17, eBIoscience Affimetrix Inc., San Diego, CA, USA), APC anti-human IL-22 (clone 2G12A41, Biolegend, San Diego, CA, USA), PE anti-human FoxP3 (Miltenyi Biotec, USA), Pe-Cyanine 7 anti-human CD25 (clone M-A251 Biolegend, San Diego, CA, USA), APC anti-human IL10 (clone JES3-9D7 Biolegend, San Diego, CA, USA) and APC anti-human IL-5 (clone TRFK5, Biolegend, San Diego, CA, USA).
4.6 Statistical analysis
All data were analyzed using GraphPad Prism version 8.0.1 (GraphPad). The data were analyzed, in order to highlight statistical significance, using the t-test. Only P value <0.05 were considered significant.
5. Results
Thirty of the sixty-three FM patients included in the study received a NCWS diagnosis (47.6%) by means of symptoms improvement on wheat-free diet and their reappearance on wheat-challenge as the flow-chart of the study shows (Figure 1).
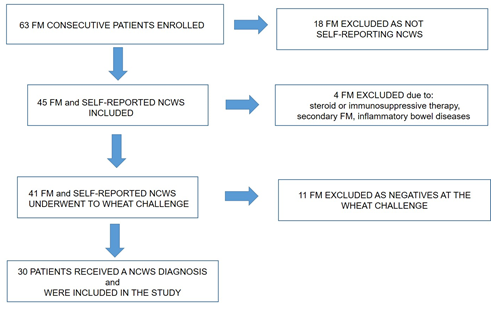
Figure 1: Flow chart of patients included in the study
5.1 Effect of GFD on disease severity, pain, fatigue, and quality of life of FM patients
In all FM+NCWS patients, adhering to the gluten-free diet, it was observed a reduction of almost all the clinical parameters used to evaluate musculoskeletal and systemic symptoms related to FM as shown in Table 1.
|
Parameters |
T0 |
T1 |
P value |
|
Tender Points Counts |
18.00 ± 0.00 |
12.00 ± 4.89 |
P<0.02 |
|
VAS for pain |
9.16 ± 1.32 |
8.33 ± 2.33 |
P<0.465 |
|
VAS for fatigue |
9.00 ± 1.09 |
9.16 ± 0.98 |
P<0.367 |
|
WPI |
16.33 ± 3.77 |
10.50 ± 3.50 |
P<0.0197 |
|
SS |
11.33 ± 0.81 |
7.16 ± 1.16 |
P<0.0001 |
|
FIQR |
88.65 ± 3.34 |
67.50 ± 6.40 |
P<0.0011 |
|
GSRS |
6 ± 2 |
3 ± 1 |
P<0.05 |
*VAS: Visual Analogue Scale; WPI:Widespread Pain Index; SS: Symptom Severity Scale; FIQR: Revised Fibromyalgia Impact Questionnaire, GSRS:Gastrointestinal Symptom Rating Scale.
Table 1: Effect of GFD on disease severity, pain, fatigue, and quality of life of FM patients. Data are expressed as media ± standard deviation (SD).
5.2 Effect of GFD on intestinal symptoms
Gastroenterological assessment demonstrated that all FM+NCWS patients after GFD had a significant reduction, but not their complete disappearance, of all intestinal symptoms present at T0 (see Table 1 GSRS).
5.3 CD4+ and CD8+ T cells percentage in FM+NCWS patients and controls
Figure 2 shows circulating CD4+ and CD8+ T cells percentage and ratio in FM+NCWS patients and controls by flow cytometric analysis. We didn’t find statistically significant differences between FM patients, NCWS patients and HS among circulating CD4+ cells (mean percentage ± standard deviation, SD, 50.7 ± 3.9%, 51.9 ± 7.8, and 49 ± 7.7%, respectively), and between FM patients and HS among CD8+ cells (26 ± 7.8%, and 24 ± 6.3%, respectively), while, on the contrary, NCWS patients showed statistically significant lower values of circulating CD8+ cells when compared to FM patients (18 ± 5.9 vs 26 ± 7.8, p=0.015) and HS (18 ± 5.9 vs 24 ± 6.3, p=0.013) (Figure 2A). No statistically significant differences we found, in circulating CD4+ and CD8+ cells, between FM+NCWS patients at T0 (46 ± 3.6 and 25.9 ± 9.79%), T1 (47.7 ± 10.3 and 24 ± 9.5%) and HS (49 ± 7.5 and 24 ± 6.3) (Figure 2B). However, we demonstrated an increase of the CD4/CD8 ratio, which is known to be physiologically approximately 2, as confirmed by HS (CD4+/CD8+=2.4), among FM+NCWS patients after GFD (CD4+/CD8+=2.4) when compared to the same patients before GFD (CD4 +/CD8+=1.92) (Figure 2C).
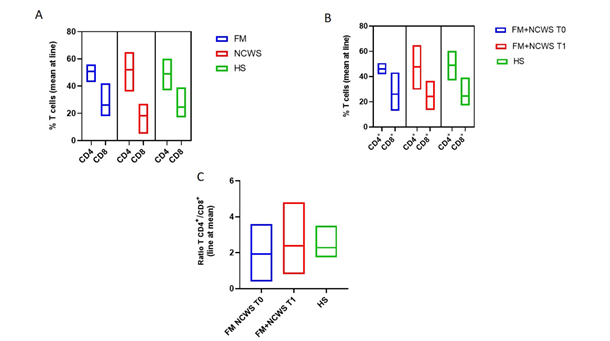
Figure 2: CD+ and CD8+ T cells percentage and ratio in patients and controls. (A) Cumulative data obtained by cytofluorimetric analyses of CD3+/CD4+ and CD3+/CD8+ T cells percentage in FM. NCWS patients and KS. (B) Cumulative data obtained by cytofluorimetric analyses of CD3+/CD4+ and CD3+/CD8+ T cells percentage in FM+NCWS patients at T0 and TI. and IN. (C) Cumulative data obtained by cytofluorimetric analyses of CD4+/CD8+ T cells ratio in FM+NCWS patients at T0 and T1, and HN.
5.4 Cytokines production from T helper cells in FM+NCWS patients and controls
We analyzed the production of IFN-γ by T helper 1 (Th1) cells in FM+NCWS patients before and after GFD and in controls. As shown in Figure 3A, IFN-γ production by Th1 cells of FM+NCWS patients was dramatically reduced at T1 (mean percentage ± standard error of the mean, SEM, 1.60 ± 0.27%) when compared to T0 (12.90 ± 3.04%, P<0.01), becoming even lower than HS (3.27 ± 0.45%, p=0.013) and comparable to FM patients (1.64 ± 0.37%). IFN-γ production by Th1 cells was also higher in FM+NCWS patients at T0 and in NCWS patients than HS (12.9 ± 3.04% vs 3.27 ± 0.45%, P<0.05, and 16.8 ± 2.7% vs 3.27 ± 0.45%, P<0.01, respectively), and in NCWS patients compared to FM (16.8 ± 2.7% vs 1.64 ± 0.37%, p <0.0001) and FM+NCWS patients, both at T0 16.8 ± 2.7% vs 12.9 ± 3.04%, P=0.366) and T1 (16.8 ± 2.7% vs 1.60 ± 0.27%, P=0.0001). No others statistically significant differences were found.
Similarly, as shown in Figure 3B, TNF-α production by Th1 cells of FM+NCWS patients was reduced at T1 (4.6 ± 0.51%) when compared to T0 (11.37 ± 3.04%, P<0.05), becoming even lower than HS (6.84 ± 1.57%, p= 0.249). TNF-α production by Th1 cells was also higher in NCWS patients compared to FM (31.24 ± 3.79% vs 13.03 ± 2.79%, p=0.002), FM+NCWS patients, both at T0 (31.24 ± 3.79% vs 11.39 ± 3.04%, p=0.001) and T1 (31.24 ± 3.79% vs 4.616 ± 0.51%, p=0.0001) and HS (31.24 ± 3.79% vs 6.84 ± 1.57%, p=0.0015). No others statistically significant differences were found.
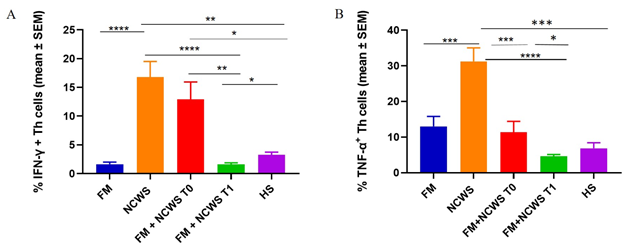
Figure 3: Cumulative data obtained by cytofluorimetric analyses of cytokines production from Th cells of patients, NCWS patients before GFD (FM+NCWS T0), FM+NCWS patients after GFD (FM+NCWS T1), and HS. (A) IFN-γ. Th cells from patients and controls; (B) TNF-α Th cells from patients and controls.
*P value <0.05
** P value <0.01
** P value <0.005
**** P value <0.0001
Figure 4 shows others Th1-cytokines production, in particular of IL-17 and IL-22 by Th cells. FM+NCWS patients before GFD showed a higher percentage of Th17 (1.59 ± 0.32%) when compared to FM patients (0.19 ± 0.049%, p=0.0004), NCWS patients (0.546 ± 0.119, P=0.0019) and HS (0.21 ± 0.02%, P<0.05). Moreover, IL-17 production by Th cells in FM+NCWS patients seems to be modulated by GFD since the production of IL-17 by T helper cells after GFD was reduced (0.27 ± 0.09%, P<0.05), becoming similar to FM and HS. Finally, NCWS patients showed higher production of IL-17 than FM patients (0.546 ± 0.119% vs 0.196 ± 0.049%, p=0.03) and HS (0.546 ± 0.119% vs 0.217 ± 0.028%, p<0.01). No others statistically significant differences were found (Figure 4A). We also observed that Th cells of FM+NCWS patients at T0 produced more IL-22 (1.01 ± 0.30%) than FM patients (0.640 ± 0.29%, p=0.40) and HS (0.67 ± 0.14%, p=0.206), and this production decreased after GFD (0.300 ± 0.07, p=0.044), becoming lower than HS (0.67 ± 0.14%, p=0.093). No others statistically significant differences were found (Figure 4B).
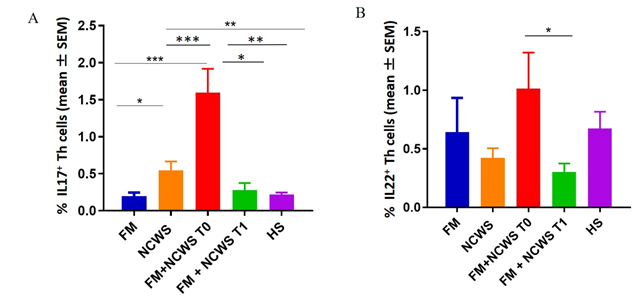
Figure 4: Cumulative data obtained by cytofluorimetric analyses of cytokines production from Th cells of FM patients, NCWS patients, FM+NCWS patients after GFD (FM+NCWS T0), FM+NCWS patients after GFD (FM+NCWS T1), and HS. (A) IL17. Th cells from patients and controls; (B) IL22 Th cells from patients and controls.
*P value <0.05
** P value <0.01
** P value <0.005
**** P value <0.0001
Therefore, NCWS patients without any sign or symptoms of FM remain the most important producers of IFN-γ and TNF-α confirming their inflammatory phenotype, while FM+NCWS patients before GFD produced higher levels of IL-17 and IL-22.
Figure 5A, showing representative dot plots from one FM+NCWS patient before and after GFD, highlights how GFD modifies the percentage of IFN-γ TNF-α IL-17, and IL-22 producing Th cells.
No statistically significant difference in the percentage of regulatory T cells (Treg) were found among FM+NCWS patients before and after GFD (0.25 ± 0.06% at T0 vs 0.45 ± 0.19% at T1, p=0.358 respectively) (data not shown). By contrast, after GFD a significant increase in IL-5 production by Th2 cells was observed (1.9 ± 0.728%), not only when compared to that found at T0 (0.59 ± 1.51%, p=0.0841), but also when compared to that found in HS (0.537 ± 0.081%, p=0.173) (data not shown).
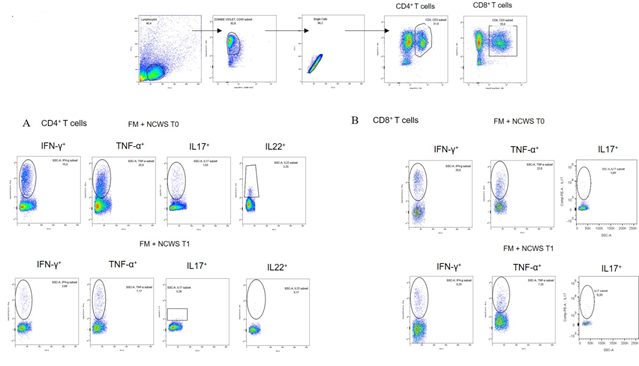
Figure 5: Representative dot plots showing the gating strategy for CD4+ T cells (A) and CD8+ T cells (B) as regards to IFN-γ, TNF-α, IL-17, and IL-22 production
5.5 Cytokines production from CD8+ T cells in FM+NCWS patients and controls
Flow cytometric analysis of peripheral blood CD3+/CD8+ T cells obtained from FM patients, NCWS patients, and FM+NCWS patients at T0 showed an increased production of proinflammatory cytokines, such as IFN-γ (13.79 ± 6.45%, 17.51 ± 5.17%, and 24.43 ± 5.54%, respectively), and TNF-α (8.55 ± 3.47%, 14.93 ± 4.03%, and 18.05 ± 3.66%, respectively), when compared to HS (10.12 ± 1.70%, and 9.59 ± 2.03%, respectively). Moreover, the production of IFN-γ and TNF-α was higher in FM+NCWS patients before GFD (24.43 ± 5.54%, and 18.05 ± 3.66%, respectively) with a reduction after GFD (9.96 ± 3.08%, and 8.39 ± 1.72%, respectively, P<0.05, for both), becoming comparable (IFN-γ) or lower (TNF-α, P<0.05) than HS. No others statistically significant differences were found (Figure 6A and 6B). We have further investigated the production of IL-17 from CD8+ T lymphocytes of FM+NCWS patients before (0.71± 0.35%) and after GFD (0.43 ± 0.22%). We didn’t find any significant difference in response to GFD and the percentages of IL-17+ cells from FM+NCWS patients, both before and after GFD, were comparable to those of FM patients (0.697 ± 0.505%) and HS (0.45 ± 0.08%) (Figure 6C). Finally, the production of IFN-γ, TNF-α, and IL17 by CD8+ T cells were also evaluated in patients affected by NCWS (17.51 ± 5.17%, 14.93 ± 4.03%, and 1.307 ± 0.46% respectively). NCWS patients showed an increase of these cytokines when compared to FM patients (p=0.674, p=0.417, p=0.453), FM+NCWS patients, both at T0 and T1 (p=0.385, p=0.619, p=0.386 and p=0.281, p=0.267, p=0.217 respectively), and HS (p=0.237, p=0.366, p=0.083) (Figure 6). Figure 5B, showing representative dot plots from one FM+NCWS patient before and after GFD, highlights how GFD modifies the percentage of IFN-γ, TNF-α, IL-17 and IL-22 producing CD8+T cells.
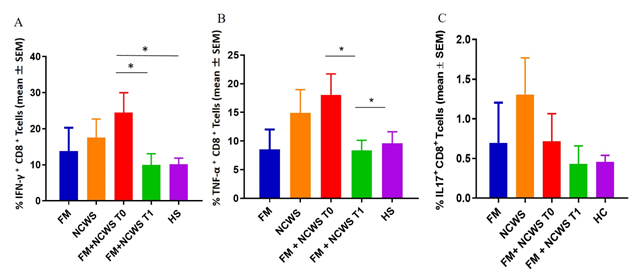
Figure 6: Cumulative data obtained by cytofluorimetric analyses of cytokines production from CD8+T cells of FM patients, NCWS patients, FM+NCWS patients before GFD (FM+NCWS T0), FM+NCWS patients after GFD (FM+NCWS T1), and HS. (A) IFN-γ+CD8+ T cells from patients and controls; (B) TNF-α+CD8+T cells from patients and controls; (C) IL17+CD8+T cells from patients and controls
* P value <0.05
6. Discussion
Our study showed that a very high percentage of FM patients, more than 47%, suffered from associated NCWS. This astonishing datum, is much more relevant considering that our data showed that GFD induces several clinical benefits, in terms of FM-related symptoms, quality of life, and gastrointestinal symptoms. A role of GFD in the modulation of the inflammatory stimuli was also addressed [14,36]. We demonstrated an increase of the CD4+/CD8+ ratio among FM+NCWS patients after GFD, suggesting that the resolution of the inflammatory process triggered in these patients by ingestion of gluten could be responsible of this modification. NCWS patients without any sign or symptoms of FM remain the main producers of IFN-γ and TNF-α confirming their inflammatory phenotype, while FM+NCWS patients before GFD produced higher levels of IL-17 and IL-22. After GFD there was a statistically significant reduction of cytokines’ production. This modification of the inflammatory profile was found among CD3+/CD8+ and CD4+ lymphocytes after GFD. It should be noted that the higher levels of proinflammatory cytokines at T0 underlines the intense systemic inflammatory state already described in FM patients [9]. This proinflammatory environment was massively reduced after GFD, reaching concentrations similar to those present in HS recruited as a control. Conversely, the increase of IL-5 producing Th2 lymphocytes after GFD compared to the levels present at T0 confirmed literature data in which FM subjects showed a reduction of Th2 percentages and their relative cytokines and an expansion of the Th1 T cells population [9,37]. We can speculate that, after GFD, the decrease of inflammatory stimuli and Th1 cell subset’s frequency, could justify the increase of IL-5 production and of Th2 population. Th2 suppression also provides strong evidence of immune dysregulation in FM patients. Moreover, FM+NCWS patients at T0 display very high percentage of Th17 cells respect to FM patients, suggesting a different phenotype between these two groups of patients (and then a possible diagnostic parameter to discriminate between FM+NCWS patients, and FM unrelated to NCWS). Additionally, a possible common etiopathogenesis among FM patients with gastrointestinal symptoms and NCWS ones could be supposed. In fact, recent published data identify a possible role for both IL-17 and TNF-a in NCWS pathogenesis, that were found highly expressed in rectal tissue and TNF-a in peripheral blood of NCWS patients. On the contrary, IL-22 production by cytotoxic cells was lower in NCWS patients than in healthy controls, suggesting that in NCWS patients, that received diagnosis after wheat challenge, show a complex immunological activation with a significant role for the adaptive response [38]. Although FM+NCWS patients at T0 have a lower production of TNF-α than NCWS patients, the release of this proinflammatory cytokine is significantly down-modulated by GFD after which we found a production comparable to HS. This result let speculate that TNF-α, in addition to promoting FM symptoms, could promote gastrointestinal symptoms in FM patients. Overall, a possible association between FM and NCWS might open to a dietary treatment (elimination diet with the exclusion of wheat) in FM as adjunctive, symptoms- and antinflammatory-effective treatment for at least a subgroups of FM patients. Although our study has some limitations, such as the small number of patients recruited, our data represent an important starting point to better understand the pathogenesis of FM and NCWS. Further studies will be necessary to understand if gluten/wheat could represent one of the starting factors of this inflammatory condition and could play an important role in the etiopathogenesis of both FM and NCWS, two diseases that are apparently different but which probably hide common pathogenetic elements.
7. Conclusion
In the present study, we showed a very high frequency of the association between FM and NCWS, with the aim of better understand the possible correlation between FM and NCWS, and their pathogenesis. This study showed that PBMC of FM patients with NCWS produce high level of proinflammatory cytokines (IFN-γ, TNF-α, IL-17, and IL-22) at baseline (T0) and a poor Th2 population; GFD leads to an important reduction of the proinflammatory populations (Th1, Th17 and Th22, and CD8+ T cells), together with an increase of Th2 response, proved by higher IL-5 levels compared to those detected at T0. Additionally, the percentage of Th17 cells could help in the identification of FM patients with NCWS that could take advantage from a GFD. Therefore, gluten/wheat could represent one of the starting factors of this inflammatory condition and could play an important role in the etiopathogenesis of both FM and NCWS.
Abbreviations: anti-DGP: anti-deamidated gliadin peptide; anti-tTG: anti-tissue transglutaminase; CD: Celiac Disease; DBPC: double-blind placebo-controlled; EmA: anti-endomysial antibodies; FCS: fetal cow serum; FIQR: Revised Fibromyalgia Impact Questionnaire; FM: Fibromyalgia; GFD:gluten-free diet; GSRS: Gastrointestinal Symptom Rating Scale; HS: healthy subjects; IBS: irritable bowel syndrome; mAbS: monoclonal antibodies; NCWS: Non-Celiac Wheat Sensitivity; NCGS: Non-Celiac Gluten Sensitivity; PBMC: peripheral blood mononuclear cells; SD: standard deviation; SS: Symptom Severity Scale; TH: T helper; TP: Manual tender points; VAS: visual analog scale; WA: Wheat Allergy; WPI: Widespread Pain Index.
Ethics approval and consent to participate: This study was approved by the Ethical Committee of the University Hospital of Palermo and informed consent was obtained from each patient in accordance with the Helsinki Declaration.
Consent for publication: Consent for publication has been obtained from all patients.
Availability of data and materials: All data generated or analysed during this study are included in this published article. The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests: The authors declare that they have no competing interests.
Authors' contributions:G.G., A.C., C.S., D.D.L., F.C., and P.M. conceptualized the study and study design. C.S., D.D.L., A.C., P.M. and M.L.P performed the experiments and wrote the paper. G.G. and E.M. were responsible for the patients’ enrollment and follow-up. M.L.P, F.D., and D.D.L. ran the statistical analysis of the data. All authors contributed to the manuscript review and read and approved the final manuscript.
Acknowledgements:
Not applicable
Grants: This work was supported in part by a grant from Ministero dell’Istruzione dell’Università e della Ricerca, Italy, and in part by the Italian Health Ministry Grant PE-2016-02363692 “Non Celiac Gluten Sensitivity (NCGS): is the gluten the true culprit? A clinical and immunological study on the tolerability of different wheat grains in NCGS patients”. The study sponsor had no role in the study design, in the collection, analysis and interpretation of data, in writing the manuscript, or in the decicion to submit the manuscript of publication.
References
- Bennett RM, Friend R, Jones KD, et al. The Revised Fibromyalgia Impact Questionnaire (FIQR): validation and psychometric properties. Arthritis Res Ther 11 (2009): R120.
- Bigatti SM, Hernandez AM, Cronan TA, et al. Sleep disturbances in fibromyalgia syndrome: relationship to pain and depression. Arthritis Rheum 59 (2008): 961-967.
- Heidari F, Afshari M, Moosazadeh M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatology international 37 (2017): 1527-1539.
- Mease P. Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment. J Rheumatol Suppl 75 (2005): 6-21.
- Bellato E, Marini E, Castoldi F, et al. Fibromyalgia syndrome: etiology, pathogenesis, diagnosis, and treatment. Pain research and treatment 12 (2012): 42-61.
- Sarzi-Puttini P, Atzeni F, Mease PJ. Chronic widespread pain: from peripheral to central evolution. Best practice & research Clinical rheumatology 25 (2011): 133-139.
- Schmidt-Wilcke T, Clauw DJ. Fibromyalgia: from pathophysiology to therapy. Nature reviews Rheumatology 7 (2011): 518-527.
- Rodriguez-Pintó I, Agmon-Levin N, Howard A, et al. Fibromyalgia and cytokines. Immunology letters 161 (2014): 200-203.
- Guggino G, Schinocca C, Lo Pizzo M, et al. T helper 1 response is correlated with widespread pain, fatigue, sleeping disorders and the quality of life in patients with fibromyalgia and is modulated by hyperbaric oxygen therapy. Clin Exp Rheumatol Suppl 116 (2019): 81-89.
- Cabo-Meseguer A, Cerdá-Olmedo G, Trillo-Mata JL. Fibromyalgia: Prevalence, epidemiologic profiles and economic costs. Medicina clinica 149 (2017): 441-448.
- Clauw DJ, Arnold LM, McCarberg BH. The science of fibromyalgia. Mayo Clinic proceedings 86 (2011): 907-911.
- Clemente E, Efthymakis K, Carletti E, et al. An explorative study identifies miRNA signatures for the diagnosis of non-celiac wheat sensitivity. PLoS One 14 (2019): e0226478.
- Cha RR, Kim HJ. Non-celiac Gluten Sensitivity. The Korean journal of gastroenterology 75 (2020): 11-16.
- Isasi C, Colmenero I, Casco F, et al. Fibromyalgia and non-celiac gluten sensitivity: a description with remission of fibromyalgia. Rheumatology international 34 (2014): 1607-1612.
- Losurdo G, Principi M, Iannone A, et al. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World journal of gastroenterology 24 (2018): 1521-1530.
- D'Alcamo A, Mansueto P, Soresi M, et al. Contact Dermatitis Due to Nickel Allergy in Patients Suffering from Non-Celiac Wheat Sensitivity. Nutrients 9 (2017): 13-18
- Slim M, Calandre EP, Rico-Villademoros F. An insight into the gastrointestinal component of fibromyalgia: clinical manifestations and potential underlying mechanisms. Rheumatology international 35 (2015): 433-444.
- Carroccio A, Rini G, Mansueto P. Non-celiac wheat sensitivity is a more appropriate label than non-celiac gluten sensitivity. Gastroenterology 146 (2014): 320-321.
- Catassi C, Elli L, Bonaz B, et al. Diagnosis of Non-Celiac Gluten Sensitivity (NCGS): The Salerno Experts' Criteria. Nutrients 7 (2015): 4966-4977.
- Sapone A, Bai JC, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC medicine 10 (2012): 13-20.
- Barbaro MR, Cremon C, Stanghellini V, et al. Recent advances in understanding non-celiac gluten sensitivity. F1000Research 7 (2018): 13-15
- Cabrera-Chávez F, Dezar GV, Islas-Zamorano AP, et al. Prevalence of Self-Reported Gluten Sensitivity and Adherence to a Gluten-Free Diet in Argentinian Adult Population. Nutrients 9 (2017).
- Carroccio A, Giambalvo O, Blasca F, et al. Self-Reported Non-Celiac Wheat Sensitivity in High School Students: Demographic and Clinical Characteristics. Nutrients 9 (2017).
- Gils T, Nijeboer P, CE IJ, et al. Prevalence and Characterization of Self-Reported Gluten Sensitivity in the Netherlands. Nutrients 8 (2016).
- Volta U, Bardella MT, Calabrò A, et al. An Italian prospective multicenter survey on patients suspected of having non-celiac gluten sensitivity. BMC medicine 12 (2014): 85.
- Brottveit M, Beitnes AC, Tollefsen S, et al. Mucosal cytokine response after short-term gluten challenge in celiac disease and non-celiac gluten sensitivity. The American journal of gastroenterology 108 (2013): 842-850.
- Di Liberto D, Mansueto P, D'Alcamo A, et al. Predominance of Type 1 Innate Lymphoid Cells in the Rectal Mucosa of Patients With Non-Celiac Wheat Sensitivity: Reversal After a Wheat-Free Diet. Clinical and translational gastroenterology 7 (2016): e178.
- Sapone A, Lammers KM, Mazzarella G, et al. Differential mucosal IL-17 expression in two gliadin-induced disorders: gluten sensitivity and the autoimmune enteropathy celiac disease. International archives of allergy and immunology 152 (2010): 75-80.
- Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC medicine 9 (2011): 23.
- Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 144 (2013): 903-911.
- Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum 33 (1990): 160-172.
- Wolfe F, Clauw DJ, Fitzcharles MA, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis care & research 62 (2010): 600-610.
- Carroccio A, Mansueto P, Iacono G, et al. Non-celiac wheat sensitivity diagnosed by double-blind placebo-controlled challenge: exploring a new clinical entity. The American journal of gastroenterology 107 (2012): 1898-1906.
- Carroccio A, Giannone G, Mansueto P, et al. Duodenal and Rectal Mucosa Inflammation in Patients With Non-celiac Wheat Sensitivity. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 17 (2019): 682-690.
- Flaherty SA. Pain measurement tools for clinical practice and research. AANA journal 64 (1996): 133-140.
- Losurdo G, Piscitelli D, Pezzuto F, et al. T Helper Lymphocyte and Mast Cell Immunohistochemical Pattern in Nonceliac Gluten Sensitivity. Gastroenterology research and practice 20 (2017): 502-506.
- Häuser W, Bernardy K, Uçeyler N, et al. Treatment of fibromyalgia syndrome with antidepressants: a meta-analysis. Jama 301 (2009): 198-209.
- Mansueto P, Di Liberto D, Fayer F, et al. TNF-α, IL-17, and IL-22 production in the rectal mucosa of nonceliac wheat sensitivity patients: role of adaptive immunity. American journal of physiology Gastrointestinal and liver physiology 319 (2020): G281-G288.
