Effect of Short Term High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease
Article Information
Maheshwar Lakkireddy1, Srikanth Goud Gadiga2, RD Malathi3, Madhu Latha Karra4*, ISSV Prasad Murthy Raju2, Ragini2, Sangeetha Chinapaka2, Sai Baba KSS5, Manohar Kandakatla6
1Additional Professor, Department of Orthopaedics, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana, India
2Department of General Medicine, Gandhi Medical college, Secunderabad, Telangana, India
3Assistant Professor, Department of Biochemistry, Gandhi Medical college, Secunderabad, Telangana, India
4Assistant Professor, Department of Biochemistry, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana, India
5Professor and HOD, Department of Biochemistry, Nizam’s Institute of Medical sciences, Punjagutta, Hyderabad, Telangana, India
6Professor of Internal Medicine, Director- Nizam’s Institute of Medical Sciences, Punjagutta, Hyderabad, Telangana, India
*Corresponding author: Madhu Latha Karra, Assistant Professor, Department of Biochemistry, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana, India.
Received: 02 July 2022; Accepted: 12 July 2022; Published: 21 July 2022
Citation: Maheshwar Lakkireddy, Srikanth Goud Gadiga, RD Malathi, Madhu Latha Karra, ISSV Prasad Murthy Raju, Ragini, Sangeetha Chinapaka, Sai Baba KSS, Manohar Kandakatla. Effect of Short Term High Dose Oral Vitamin D Therapy on the Inflammatory Markers in Patients with COVID 19 Disease. Archives of Clinical and Biomedical Research 6 (2022): 623-630.
View / Download Pdf Share at FacebookAbstract
Introduction: COVID 19 is known to cause immune dysregulation and vitamin D is a known immunomodulator. This study aims to objectively investigate the effect of short term high dose vitamin D therapy in reducing the inflammatory markers of COVID-19.
Materials and Methods: Consented COVID-19 patients with hypovitaminosis D were evaluated for inflammatory markers (N/L ratio, CRP, LDH, IL6, Ferritin) along with vitamin D on 0th day and 9th / 11th day as per their respective BMI category. Subjects were allotted to VD and NVD groups. VD group received Pulse D therapy (a short term high dose supplementation of 60,000 IUs of vitamin D for 8 or 10 days depending upon their BMI) in addition to the standard treatment. NVD group received standard treatment alone. Differences in the variables between the two groups were analysed for statistical significance.
Results: Eighty seven out of one hundred and thirty subjects have completed the study (VD:44, NVD:43). Vitamin D level has increased from 15 ng/ml to 81 ng/ml in VD group and highly significant (p<0.01) reduction of all the measured inflammatory markers was noted. Reduction of markers in NVD group was insignificant (p>0.05). The difference in the reduction of markers between the groups (NVD vs VD) was highly significant (p<0.01).
Conclusions: Therapeutic improvement in vitamin D to 80-100 ng/ml has significantly reduced the inflammatory markers associated with COVID-19 without any side effects. Hence, adjunctive short term high dose vitamin D therapy can be added safely to the existing treatment protocols of COVID-19 for improved outcomes.
Keywords
Biomarkers; COVID-19; Cytokine storm; Respiratory Infection; Vitamin D
Article Details
1. Introduction
COVID-19 pandemic caused by SARS-CoV-2 virus has created an unprecedented hardship in the recent times [1,2]. Serious consequences of COVID-19 were attributed to the immune dysregulation leading to the enhanced production of pro inflammatory mediators (cytokine storm) [3-7]. In the absence of a specific vaccine or a treatment, strategies to minimize the effects of COVID-19 have become extremely important. Recent observational studies have reported that the patients with higher levels of serum vitamin D (vit.D) had less severe symptoms and vice versa and have postulated the usefulness of vit.D in prevention and treatment of COVID-19 [3,8-12]. The beneficial effects of vit.D in COVID-19 were attributed to be mediated through its multiple actions on the immune system. Vit.D is known to enhance the production of various anti-microbial peptides by the immune cells and modulates the immune system according to the internal milieu. It reduces the dysregulated production of self-damaging pro-inflammatory cytokines and promotes the expression of anti-inflammatory cytokines by immune cells [13-18]. The dynamic role of vit.D can be of immense value in the context of immune dysfunction observed in COVID-19 patients with cytokine storm and acute respiratory distress syndrome [2-6]. Though the protective immuno-modulatory effects of vit.D were explored in many autoimmune diseases and respiratory tract infections, there is a dearth of information from the randomised clinical trials in COVID-19. Pulse D therapy (short term high dose oral supplementation of vitamin D) is a targeted approach to increase the serum vit.D level by daily oral supplementation of 60,000 IUs of vit.D for a specific period of time determined by the individual’s BMI, initial level of vit.D and the formulation [19]. This study aims to objectively investigate the role of vit.D and the effect of Pulse D therapy in reducing the inflammatory biomarkers of COVID-19.
2. Material and Methods
This is an open label prospective therapeutic trial carried out at Gandhi Medical College, Hospital Secunderabad in collaboration with Nizam’s Institute of Medical Sciences, Hyderabad after receiving the approval of the Institutional Ethics Committee (IEC) of Gandhi Medical College (DCGI Regd. No: ECR/180/Inst/AP/2013/RR-19 dt.26-09-2019) vide Rc. No: IEC/GMC/2020/05/04 dt. 23-05-2020 with prior intimation, as per rules, to IEC Nizam’s Institute of Medical Sciences, Hyderabad. This trial was registered in Clinical Trials Registry of India (CTRI) vide Clinical Trial Registration No: CTRI /2020/12/030083 dated: 29/12/2020, Reference No: REF/2020/12/039236. Written informed consent was taken from all the subjects and all the relevant rules and regulations were followed. Confirmed COVID-19 patients above the age of 18 years with hypovitaminosis D (vit.D level below 30ng/ml) and mild to moderate illness (SpO2 >90%) as per the revised guidelines for COVID-19 issued by the Directorate General of Health Services, Government of India on 31-03-2020 were included. Patients with severe illness and patients who have taken high dose vit.D (60000 IUs) in the last 3 months, patients with active malignancy, chronic renal disease and HIV, pregnant and breastfeeding mothers were excluded. After admission, mild to moderately ill patients were allotted the serial numbers and were screened for serum vit.D level along with inflammatory markers of COVID-19. Haemogram with Neutrophil / Lymphocyte (N/L) ratio was performed on Mindray BC-6200 machine (Shenzhen Mindray Bio-Medical Electronics Co Ltd, Shenzhen, Guangdong sheng, China) using scatter fluorescence cube method. Vitamin D, serum Ferritin and Interleukin 6 (IL6) were estimated on Advia Centaur XPT machine (Siemens Healthineers, Erlangen, Germany) using direct chemiluminometric antibody competitive immunoassay method, direct chemiluminometric two-site sandwich immunoassay method and direct chemiluminometric one step immunoassay method respectively. Lactate Dehydrogenase (LDH) and C Reactive Protein (CRP) were estimated on AU5800 Beckman Coulter machine (Beckman Coulter Inc., Brea CA, USA) using photometric kinetic UV-IFCC and photometric immunoturbimetric methods respectively.
Patients with hypovitaminosis D were allotted to two groups vis a vis Experimental group/vit.D group (VD Group) and Active comparator/control group (NVD group) alternatively as per their pre allotment screening serial numbers duly following the predetermined allocation chart. As some of the intervening subjects in the screening serial order might have normal vitamin D and hence forth are excluded from the study, the allocating officer would be unaware of the subjects who will be allotted to either of the study groups thereby avoiding selection bias. Subjects of VD group received adjunctive Pulse D therapy (60,000 IUs of vit.D in the form of aqueol nano solution-Deksel® per day for 8 days for subjects with body mass index (BMI) of 18-25 and 10 days for subjects with BMI >25) along with the routine standard treatment for COVID-19. Subjects of NVD group received standard treatment for COVID-19 alone. After the completion of treatment with vit.D, repeat serum samples for vit.D and the inflammatory markers were collected on 9th or 11th day respectively for VD group. Similarly, samples were collected on 9th day for patients with BMI of 16-25 and 11th day for patients with BMI>25 in NVD group. Subjects in both the groups (VD and NVD) who have not received the drugs like Remdesivir, Favipiravir, Ivermectin or Dexamethasone were sub categorised into eVD and eNVD sub groups. Exclusive role of vit.D (without the influence of antiviral drugs or corticosteroids) in reducing the inflammatory markers of COVID-19 was studied in these subgroups. Differences in the serum parameters between the two groups were analysed for statistical significance using MedCalc (Ver.19.5.1). Descriptive statistics of parametric variables were represented by Mean ± SD and significance analysis by t test. Non parametric variables were represented by Median and IQR and comparative analysis by Mann-Whitney U test and Wilcoxon rank test. Decimal values are rounded off to the nearest whole number as per norms of statistical presentation. Decimal values >0.5 are rounded off to the next higher whole number and decimal values <0.5 are rounded off to the preceding whole number. Decimal values of 0.5 are presented as it is. P value <0.05 was considered statistically significant and p<0.01 as highly significant. Sample size calculation was done through openepi.com. Two sided confidence interval was taken as 95%, power as 80%, ratio of sample size as 1. It was assumed that vitamin D would decrease the inflammatory markers by 50% in the treatment group and there would be about 10% decrease (without vitamin D added to the treatment) in the control group. To overcome the non responder’s bias, sample size was adjusted by assuming an expected response proportion of 50%. Prefix “pre” was used for an analyte before treatment and Prefix “Post” was used for an analyte after treatment. Prefix “Diff.in” was used to denote the difference (i.e Pre/before treatment-Post/after treatment) in a given parameter.
3. Results
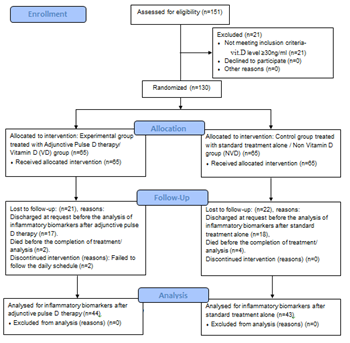
One hundred and thirty confirmed COVID-19 subjects were included and eighty seven subjects could complete the study. Details are enumerated in the flow diagram (Figure 1).
The mean age of patients who have completed the study (n= 87) was 45±13 years, range: 20 to 83 years. The mean age of patients in VD group (n= 44) was 47 ±12 years, range: 20 to 70 years and in NVD group (n= 43) was 44± 14 years, range: 20 to 83 years. There was no significant difference in age between the two groups (p= 0.23). There was no significant difference in median BMI between the patients in VD (25) and NVD (24) groups (Z= -0.8, p= 0.4). There was no significant difference in the median duration of symptoms between the patients in VD (5 days) and NVD (5days) groups (Z=0.9, p=0.4). There was no significant difference (p>0.05) in vital parameters between NVD and VD groups (median systolic blood pressure: p=0.9, mean diastolic blood pressure: p=0.4, median heart rate: p=0.3, median SpO2: p=0.8) at the time of enrolment. 34 out of the 87 subjects who have completed the study had either diabetes or hypertension as co-morbidity (39%). Owing to allotment, 21 and 13 subjects with co-morbidities were allotted to VD and NVD group respectively. There was no significant difference (p>0.05) in levels of all the measured inflammatory markers in the subjects of both groups with and without co-morbidities before and after treatment. Out of the 87 subjects who have completed the study, 75% (n=65) were men and 25% (n=22) were women. Following the allotment of subjects, 37 men and 7 women got allotted to VD group and, 28 men and 15 women got allotted to NVD group. The difference in the inflammatory markers before treatment between the genders in VD and NVD groups was not significant (p>0.05) except for IL6 (p=0.02) in VD group and Ferritin (p=0.002) in NVD group with men having higher levels. The difference in the inflammatory markers after treatment between the genders in VD and NVD groups was not significant (p>0.05) except for higher CRP (p=0.02) in women and higher Ferritin (p=0.002) in men in NVD group. Despite various independent parameters being similar, significant difference (p<0.05) in all the inflammatory markers between VD and NVD groups was noted before treatment with all the markers being high in VD group. In view of the above, direct comparison of the parameters between the groups was not possible. Hence, difference between the parameters before and after the treatment in individual groups (Pre Vs Post) was initially carried out to see the effect of Pulse D therapy followed by the comparison of the differences (Pre-Post values) between the groups. Percentage difference among the parameters could not be calculated in view of zero values for some of the study parameters. As the distribution of data was non normal for most of the studied variables, median with interquartile range was used for all the parameters for uniformity in presentation. Analysis of inflammatory markers and vit.D in the VD group before versus after treatment has shown highly significant reduction (p<0.01) in all the measured inflammatory markers and a significant increase (p<0.01) in vit.D (Table 1).
|
Variable |
Pre (n=44) |
Post (n=44) |
Pre vs Post |
|||
|
Median (IQR) |
95% CI of Median |
Median (IQR) |
95% CI of Median |
z statistic |
p value |
|
|
Vit.D (ng/ml) |
15 (12 -19) |
13-18 |
81 (64-111) |
75-102 |
-6 |
<0.0001 |
|
CRP (mg/L) |
76 (20-122) |
49-103 |
6 (3-15) |
3-11 |
5 |
<0.0001 |
|
LDH (U/L) |
330 (267-472) |
284-384 |
241 (207-308) |
223-280 |
4 |
<0.0001 |
|
IL6 (pg/ml) |
15 (5-57) |
9-29 |
3 (0.9-8) |
2-5 |
4 |
<0.0001 |
|
Ferritin (ng/ml) |
431 (190-836) |
262-708 |
334 (154-508) |
203-433 |
4 |
0.0004 |
|
N/L Ratio |
5 (3-11) |
4-8 |
3 (2-5) |
3-5 |
4 |
0.0003 |
|
Vit.D: Vitamin D, CRP: C-Reactive protein, LDH: Lactate Dehydrogenase, Il-6: Interleukin-6, N/L ratio: Neutrophil/Lymphocyte ratio, IQR: Interquartile range, 95% CI: 95% Confidence Interval. |
||||||
Table 1: Values of various parameters studied in the VD group before and after treatment.
Unlike the VD group, analysis of inflammatory markers in the NVD group before and after treatment has not shown significant reduction (p>0.05) except CRP. On the contrary levels of IL6 and Ferritin have increased though they were not significant statistically (Table 2).
|
Variable |
Pre (n=43) |
Post (n=43) |
Pre vs Post |
|||
|
Median (IQR) |
95% CI of Median |
Median (IQR) |
95% CI of Median |
z statistic |
p value |
|
|
Vit.D (ng/ml) |
17 (13-22) |
15-19 |
16 (10 -20) |
14-18 |
0.4 |
0.7 |
|
CRP (mg/L) |
11 (3-43) |
5-31 |
5 (1-9) |
2-7 |
3 |
0.008 |
|
LDH (U/L) |
244 (172-298) |
189-263 |
207 (175-251) |
190-224 |
1 |
0.2 |
|
IL6 (pg/ml) |
3 (1-9) |
1-6 |
4 (1-11) |
1-7 |
-0.1 |
0.9 |
|
Ferritin (ng/ml) |
169 (63-526) |
87-329 |
196 (54-456) |
68-331 |
2 |
0.07 |
|
N/L Ratio |
3 (2-5) |
2-4 |
2 (2-4) |
2-3 |
0.3 |
0.8 |
|
Vit.D: Vitamin D, CRP: C-Reactive protein, LDH: Lactate Dehydrogenase, Il-6: Interleukin-6, N/L ratio: Neutrophil/Lymphocyte ratio, IQR: Interquartile range, 95% CI: 95% Confidence Interval. |
||||||
Table 2: Values of various parameters studied in the NVD group before and after treatment.
The difference in the reduction of inflammatory markers between the two groups (NVD vs VD) was highly significant (p<0.01) with the reduction in VD group being markedly higher than the NVD group (Table 3).
|
Variable |
NVD (n=43) |
VD (n=44) |
NVD vs VD |
|||
|
Difference (Pre –Post) |
Median (IQR) |
95% CI of Median |
Median (IQR) |
95% CI of Median |
z statistic |
p value |
|
Vit.D (ng/ml) |
-0.1 (-3 to 5) |
-1 to 3 |
-64 (-92 to 52) |
-81 to -58 |
8 |
<0.0001 |
|
CRP (mg/L) |
5 (-3 to 39) |
-0.003 to 21 |
51 (10 to 113) |
30 to 85 |
-4 |
0.0001 |
|
LDH (U/L) |
15 (-22 to 60) |
-13 to 40 |
72 (13 to 168) |
48 to 133 |
3 |
0.002 |
|
IL6 (pg/ml) |
0.5 (-7 to 4) |
-4 to 1 |
13 (2 to 46) |
8 to 25 |
-4 |
0.0002 |
|
Ferritin (ng/ml) |
11 (11 to 55) |
-6 to 38 |
84 (8 to 268) |
55 to 171 |
3 |
0.008 |
|
N/L Ratio |
0.05 (-1 to 2) |
-0.7 to 1 |
0.9 (0 to 5) |
0.3 to 2 |
-3 |
0.009 |
|
Vit.D: Vitamin D, CRP: C-Reactive protein, LDH: Lactate Dehydrogenase, Il-6: Interleukin-6, N/L ratio: Neutrophil/Lymphocyte ratio, IQR: Interquartile range, 95% CI: 95% Confidence Interval. |
||||||
Table 3: The values of difference in the inflammatory markers and vitamin D between the two groups (NVD vs VD).
Fifteen cases each in VD and NVD group have not received any drugs like Remdesivir, Favipiravir, Ivermectin or Dexamethasone. Analysis of inflammatory markers in the eVD sub group (before and after treatment) has shown highly significant reduction (p<0.01) in all the measured inflammatory markers after Pulse D therapy wherein significant increase in vit.D level was noted (p<0.01) (Table 4).
|
Variable |
Pre (n=15) |
Post (n=15) |
Pre vs Post |
|||
|
Median (IQR) |
95% CI of Median |
Median (IQR) |
95% CI of Median |
z statistic or smaller total of ranks |
p value |
|
|
Vit.D (ng/ml) |
13 (10-19) |
10-19 |
78 (58-90) |
58-89.5 |
0 |
0.0001 |
|
CRP (mg/L) |
57 (22-96) |
22-96 |
8 (3-16) |
3-16 |
3 |
0.0003 |
|
LDH (U/L) |
301.5 (268-507) |
268-506 |
238 (201-312) |
201-311 |
10 |
0.0026 |
|
IL6 (pg/ml) |
23 (6-55) |
6-55 |
2 (0-5) |
0-5 |
7 |
0.0012 |
|
Ferritin (ng/ml) |
207 (126-565) |
126-565 |
186 (94-423) |
94-423 |
13 |
0.005 |
|
N/L Ratio |
6 (3-13) |
3-12 |
3 (2-4) |
2-4 |
11 |
0.003 |
|
Vit.D: Vitamin D, CRP: C-Reactive protein, LDH: Lactate Dehydrogenase, Il-6: Interleukin-6, N/L ratio: Neutrophil/Lymphocyte ratio, IQR: Interquartile range, 95% CI: 95% Confidence Interval. |
||||||
Table 4: Values of various parameters analysed in the eVD sub group before and after treatment.
Analysis of inflammatory markers in the eNVD sub group (before and after treatment) has not shown any significant reduction (p>0.05). The levels of Ferritin (p>0.05) and N/L ratio (p<0.05) on the contrary have increased in the post samples when compared to the pre samples (Table 5).
|
Variable |
Pre (n=15) |
Post (n=15) |
Pre vs Post |
|||
|
Median (IQR) |
95% CI of Median |
Median (IQR) |
95% CI of Median |
z statistic or smaller total of ranks |
p value |
|
|
Vit.D (ng/ml) |
16 (11-20) |
11-20 |
18 (10-24) |
10-24 |
47 |
0.488 |
|
CRP (mg/l) |
8 (2-19) |
2-19 |
5 (2-8) |
2-9 |
42 |
0.3 |
|
LDH (U/L) |
244 (175-293) |
176-292 |
210 (177-267) |
178-266 |
48 |
0.524 |
|
IL6 (pg/ml) |
4 (2-9) |
2-9 |
1 (0-9) |
0-9 |
49 |
0.6 |
|
Ferritin (ng/ml) |
147 (65-230) |
65-229 |
189 (51-237) |
51-237 |
51 |
0.6 |
|
N/L Ratio |
1.67 (1-3) |
1-3 |
2.41 (2-5) |
2-5 |
24 |
0.04 |
|
Vit.D: Vitamin D, CRP: C-Reactive protein, LDH: Lactate Dehydrogenase, Il-6: Interleukin-6, N/L ratio: Neutrophil/Lymphocyte ratio, IQR: Interquartile range, 95% CI: 95% Confidence Interval. |
||||||
Table 5: Values of various parameters analysed in the eNVD sub group before and after treatment.
The difference in the reduction of inflammatory markers between the two sub groups (eNVD vs eVD) was significant (p<0.05) with the reduction in eVD sub group being markedly higher than the eNVD sub group except for Ferritin. Though the reduction of median Ferritin levels after Pulse D therapy was quite high in the VD group, it was not statistically significant (Table 6).
|
Difference in in variable (Pre-Post) |
eNVD (n=15) |
eVD (n=15) |
eNVD vs eVD |
|||
|
Median (IQR) |
95% CI of Median |
Median (IQR) |
95% CI of Median |
z statistic |
p value |
|
|
Vit.D (ng/ml) |
-1 (-6 to 4) |
-6 to 4 |
-63 (-75 to 48) |
-75 to 48 |
5 |
<0.0001 |
|
CRP (mg/L) |
0.7 (-2.35 to 12.98) |
2 to 13 |
39 (17 to 84) |
17 to 84 |
3 |
0.001 |
|
LDH (U/L) |
-0.6 (-22 to 64) |
-22 to 63 |
73 (42 to 204) |
42 to 203 |
2 |
0.02 |
|
IL6 (pg/ml) |
1 (-4 to 4) |
-4 to 4 |
23 (5 to 46) |
5 to 45 |
3 |
0.0107 |
|
Ferritin (ng/ml) |
1 (-12 to 55) |
-12 to 55 |
76 (6 to 159) |
6 to 158 |
2 |
0.065 |
|
N/L Ratio |
-0.5 (-1 to 0.01) |
-1 to 0.01 |
1.0 (0.07 to 5) |
0.08 to 5 |
3 |
0.0006 |
|
Vit.D: Vitamin D, CRP: C-Reactive protein, LDH: Lactate Dehydrogenase, Il-6: Interleukin-6, N/L ratio: Neutrophil/Lymphocyte ratio, IQR: Interquartile range, 95% CI: 95% Confidence Interval. |
||||||
Table 6: Values of difference in the inflammatory markers and vitamin D between the sub groups (eNVD vs eVD).
Difference in the mean hospital stay between VD vs NVD groups (13±5 days vs 14±5 days) was not significant (p=0.9). Intensive care support was required for 9 subjects (VD group: n= 4, NVD group: n=5) and 7 of them died (VD group: n=2, NVD group: n=5). 6 out of these 7 subjects (VD group: n=2, NVD group: n=4) died after 5 ± 1 day of enrolment without completing the study. One subject in NVD group died after 21 days of enrolment. All of them had very high levels of inflammatory markers at admission when compared to the survivors. The difference was highly significant (p<0.01) for IL6, CRP, Ferritin and significant (p=0.02) for N/L ratio and LDH. 2 of the 7 non survivor subjects (28.5%) had either diabetes or hypertension as co-morbidity. No adverse reactions attributable to vit.D toxicity were noted in any of the patients studied. Serum calcium level in VD group after treatment was within normal limits (9 ± 0.5 mg/dl).
4. Discussion
COVID-19 caused by SARS-CoV-2 (novel corona virus) has not only incited intense adaptive immune response in the individuals who were affected by it but also has incited immense human response at various fronts to fight it all over the world [4,5]. As the immune dysregulation caused by COVID-19 lead to respiratory failure and multi organ dysfunction syndrome, many attempts were made to repurpose the available drugs to address the challenges posed by the novel corona virus [4,6,7 20,21]. Mortality and morbidity were recorded to be high in patients with significantly elevated inflammatory markers (surrogate markers of COVID-19 severity) such as N/L ratio, CRP, LDH, IL6, Ferritin, D dimer etc [3,6,7,11,22]. Similarly, Mortality and morbidity were also recorded to be high in patients with vit.D deficiency [9,11,12]. Low vit.D level was proposed to be an independent risk factor for acquiring COVID-19 infection, hospitalization and COVID related mortality [9,10]. Based on the earlier evidence that vit.D could decrease the incidence of flu and other respiratory infections and the observational studies in COVID-19, few hypothesis and recommendations have been published in support of supplementing vit.D to avert the serious consequences of COVID-19 [2,3,9-12,23, 24]. Kaufman HW et.al reported that SARS-CoV-2 positivity is strongly and inversely associated with serum vitamin D level and proposed that vitamin D supplementation could reduce the risk for SARS-CoV-2 infection and COVID-19 disease [25]. Vit.D has innumerable effects on human physiology. In addition to its endocrinal and calcitropic musculoskeletal effects, it is a potential immunomodulator. Depending upon the prevailing internal milieu and the level of 25 hydroxy vitamin D in the blood, intracrinal activation of 1α hydroxylase occurs in the immune cells to produce calcitriol locally and have its autocrine effects like promotion of innate immune response to infections and modulation of adaptive immune response. Vit.D acts as a smart switch to decrease the Th1 response and pro inflammatory cytokines while enhancing the production of anti-inflammatory cytokines in cases of immune dysregulation [13-16,23]. It is pertinent to note that SARS-CoV-2 virus activates Th1 response and suppresses Th2 response4. It was postulated that the levels of vit.D above 40-60 ng/ml could be protective to tide over the COVID crisis [8,11,26]. Annweiler G et.al reported that the hospitalised frail elderly patients who had regularly taken bolus vitamin D supplementation before hospitalisation with COVID-19 had significantly better survival rates than others [27]. In a retrospective analysis, Ling SF et.al reported a reduced risk of mortality in COVID 19 patients treated with high dose cholecalciferol booster therapy [28]. Owing to the paucity of evidence from prospective randomised clinical trials, high dose vit.D was not included in the existing treatment protocols of COVID-19. Few Randomised control trials using bolus doses of vitamin D in COVID-19 are yet to be completed and reported [29]. McNally JD et.al reported that rapid normalization of vitamin D levels can be achieved with loading therapy, duly considering the disease status, baseline vit.D level and weight but loading doses >300 000 IU were advised to be avoided until trials are conducted to evaluate the risk and benefit [30]. Intermittent bolus dose vit.D therapies with 3 monthly gaps have failed to achieve the target levels [31].
As the concentration dependent effects of vit.D on the immune system and the means to achieve such concentrations safely in the shortest possible time in a given individual is known [19, 30, 32], we have carried out this study to determine the effect of Pulse D therapy (short term high dose oral vit.D therapy) on the inflammatory markers of COVID-19. Despite various independent parameters being similar in both the study groups with respect to age, BMI, duration of symptoms, co-morbidities and vital parameters, significant difference in the inflammatory markers before treatment between the groups was intriguing. This difference can be attributed to chance alone. Male predominance (75% vs 25%) was noted akin to earlier reports [3]. Analysis of inflammatory markers before and after treatment in VD group has shown highly significant reduction (p<0.01) in all the inflammatory markers after adjunctive pulse D therapy. On the contrary insignificant reduction (p>0.05) of inflammatory markers was noted in the NVD group. The difference in reduction of inflammatory markers between the groups (NVD vs VD) was highly significant (p<0.01) with the reduction of markers being markedly high in VD group when compared to the NVD group. Hence, adjunctive Pulse D therapy targeted at a mean vit D level of 80-100ng/ml has effectively reduced the inflammatory markers associated with cytokine storm and COVID-19 severity. Rastogi A et.al reported that high dose vit.D supplementation orally for seven consecutive days has increased the vit.D level in a group of 16 patients from 8.6 to 42.4 ng/ml with significant reduction in fibrinogen levels and insignificant reduction in CRP. Early viral clearance in the form of negative RT-PCR after vit.D supplementation was also reported [1]. Entrenas Castillo M et al reported that oral administration of high dose calcifediol has significantly reduced the severity of COVID-19, need for ICU treatment and mortality. Though elevated levels of inflammatory markers at enrolment were reported, initial level of vitamin D or the follow up levels of vitamin D or inflammatory markers was not studied [33].
It may be noted that the statistically significant reduction in all the inflammatory markers in this study may be attributed to the level of vit.D achieved i.e. median: 81 ng/ml (IQR: 64 to 111) and aqueol nano formulation (Deksel®) has facilitated the target levels to be achieved, akin to the earlier report [19]. Significant reduction in CRP was noted in our study when compared to the report of Rastogi A et.al [1]. This may be attributed to the difference in the level of vit.D after treatment. As per our knowledge, these finding are the first of its kind to be reported. We have analysed the inflammatory markers in a separate subset of cases (eVD and eNVD sub groups) derived from both the study groups who have not received any drugs like Remdesivir, Favipiravir or Ivermectin or Dexamethasone. Highly significant reduction (p<0.01) in all the measured inflammatory markers with significant increase in vit.D was noted in the eVD sub group unlike the eNVD sub group (p>0.05). The difference in reduction of inflammatory markers between the sub groups (eNVD vs eVD) was highly significant (p<0.01) with the reduction of markers being markedly high in eVD subgroup when compared to the eNVD sub group. Hence, improvement in serum vit.D level to near 80 ng/ml has shown to effectively reduce the levels of surrogate markers of COVID-19 severity/ cytokine storm independently. These findings are exclusive to our study as on date and could not be compared with others. DiNicolantonio JJ et.al reported that both magnesium and vitamin D are important to the immune system independently. Together, they may be beneficial in COVID-19 infection as magnesium is necessary to activate vitamin D. Results from our study can be compared with the results of future studies with and without magnesium in high dose vitamin D regimens to formulate effective dosing schedules [34]. Hospital stay was subjective and multifactorial in both the groups. It could not be attributed to the physical impact of the disease alone. Murai IH et al reported that, a single high dose vitamin D3 (2,00,000 IU) supplementation hasn’t reduced the length of hospital stay, mortality or ICU admission significantly when compared to with placebo. Their findings did not support the use of single bolus dose of vitamin D3 for treatment of moderate to severe COVID-19 [35]. At enrolment, significantly higher levels of all the inflammatory markers were noted in the non survivors compared to survivors. Similar relationship of mortality to the elevated levels of inflammatory markers was reported by Jain A et.al. in their observational study [3]. No adverse reactions to vit.D were reported in our study. Serum calcium levels were within the normal limits after treatment (9 ± 0.5.mg/dl) in VD group. Similar finding on the safety of short-term high dose vit.D supplementation were reported by Rastogi A et.al and long term by McCullough PJ et. al [1, 36]. De Carvalho JF et.al. reported that mega doses (6,00,000 IU) of vitamin D administered through intramuscular route even in cases of nephrolithiasis are safe [37].
5. Conclusions
Immune dysregulation in COVID-19 is marked by increased inflammatory biomarkers such as N/L ratio, CRP, LDH, IL6 and Ferritin. Vitamin D is a potential immunomodulator and its adjunctive role in the treatment of COVID-19 is established by this study. Improvement of serum vit.D level to 80-100 ng/ml has significantly reduced the inflammatory markers without any side effects. Hence, adjunctive Pulse D therapy (short term high dose oral vit.D supplementation) can be added safely to the existing treatment protocols of COVID-19.
Limitations of the study
This is an open labelled single centre therapeutic trial. It can be considered as a pilot for larger multicentric RCTs in future.
Acknowledgements
We express our gratitude to Dr. Raja Rao, Medical Superintendent, Dr. MD Suleman, Vice Principal, Dr. Vinay Shekar, HOD, Dept. of General Medicine, Dr. S U M Raju, Associate Professor of Pharmacology, Gandhi Medical College and Hospital for their kind support. We thank Mr. Mallesh Kothapally and Mr.Ravi Paili for their extensive support in carrying out this project.
Declaration of Interest/ Competing Interest
None.
Conflict of Interest
Nil
Funding Source
Sponsored by Pulse Pharmaceuticals. Funding source has no involvement in study design, collection, analysis, interpretation of data, writing the report or decision to submit the article for publication.
The Author Contributions Statement
|
S.No |
Contribution |
Author Name (First name, Last name ) |
|
1 |
Study Design |
Maheshwar Lakkireddy, Madhu Latha Karra, I S S V Raju |
|
2 |
Data Collection |
Srikanth Gadiga, R.D. Malathi, I S S V Raju, Ragini, Sangeetha Chinapaka, Maheshwar Lakkireddy, Madhu Latha Karra. |
|
3 |
Statistical Analysis |
Maheshwar Lakkireddy, Madhu Latha Karra, Sai Baba KSS |
|
4 |
Data Interpretation |
Maheshwar Lakkireddy, Madhu Latha Karra, Sai Baba KSS, Manohar Kandakatla |
|
5 |
Manuscript Preparation |
Maheshwar Lakkireddy, Madhu Latha Karra |
|
6 |
Literature Search |
Maheshwar Lakkireddy, Madhu Latha Karra, I S S V Raju, Sai Baba KSS, Manohar Kandakatla |
|
7 |
Funds Collection |
I S S V Raju, Manohar Kandakatla, Srikanth Gadiga, Maheshwar Lakkireddy |
References
- Rastogi A, Bhansali A, Khare N, et.al. Short term, high-dose vitamin D supplementation for COVID-19 disease: a randomised, placebo-controlled, study (SHADE study). Postgrad Med J (2020).
- Aygun H. Vitamin D can prevent COVID-19 infection-induced multiple organ damage. Naunyn Schmiedebergs Arch Pharmacol 393 (2020): 1157-1160.
- Jain A, Chaurasia R, Sengar NS, et al. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep 10 (2020): 20191.
- Huang C, Wang Y, Li X, et.al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395 (2020): 497-506.
- Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm' in COVID-19. J Infect 80 (2020): 607-613.
- Chen L, Liu HG, Liu W, et.al Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 43 (2020): E005.
- Chen G, Wu D, Guo W, et.al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 130 (2020): 2620-2629.
- Grant WB, Lahore H, McDonnell SL, et.al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 12 (2020): 988.
- De Smet D, De Smet K, Herroelen P, et al. Serum 25(OH)D Level on Hospital Admission Associated With COVID-19 Stage and Mortality. Am J Clin Pathol 25 (2020).
- Merzon E, Tworowski D, Gorohovski A, et.al. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J 287 (2020): 3693-3702.
- Maghbooli Z, Sahraian MA, Ebrahimi M, et.al. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One 15 (2020): e0239799.
- Carpagnano GE, Di Lecce V, Quaranta VN, et.al. Vitamin D deficiency as a predictor of poor prognosis in patients with acute respiratory failure due to COVID-19. J Endocrinol Invest 9 (2020): 1-7.
- Chambers ES, Hawrylowicz CM. The impact of vitamin D on regulatory T cells. Curr Allergy Asthma Rep 11 (2011): 29-36.
- Adams JS, Hewison M. Unexpected actions of vitamin D: new perspectives on the regulation of innate and adaptive immunity. Nat Clin Pract Endocrinol Metab 4 (2008): 80-90.
- Lemire JM, Adams JS, Kermani-Arab V, et al. 1,25-Dihydroxyvitamin D3 suppresses human T helper/inducer lymphocyte activity in vitro. J Immunol 134 (1985): 3032-3035.
- Gombart AF, Pierre A, Maggini S. A Review of Micronutrients and the Immune System-Working in Harmony to Reduce the Risk of Infection. Nutrients 12 (2020): 236.
- Meltzer DO, Best TJ, Zhang H, et al. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open 3 (2020): e2019722.
- Baeke F, Takiishi T, Korf H, et al. Vitamin D: modulator of the immune system. Curr Opin Pharmacol 10 (2010): 482-496.
- Lakkireddy M, Karra ML, Patnala C, et.al. Efficiency of vitamin D supplementation in patients with mechanical low back ache. J Clin Orthop Trauma 10 (2019): 1101-1110.
- Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA 323 (2020): 1824-1836.
- WHO Solidarity Trial Consortium, Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V et.al. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med (2020).
- Velavan TP, Meyer CG. Mild versus severe COVID-19: laboratory markers. Int J Infect Dis 95 (2020): 304-307.
- Liu G, Hong T, Yang J. A Single Large Dose of Vitamin D Could be Used as a Means of Coronavirus Disease 2019 Prevention and Treatment. Drug Des Devel Ther 14 (2020): 3429-3434.
- Giannini S, Passeri G, Tripepi G, et.al. Effectiveness of In-Hospital Cholecalciferol Use on Clinical Outcomes in Comorbid COVID-19 Patients: A Hypothesis-Generating Study. Nutrients 13 (2021): 219.
- Kaufman HW, Niles JK, Kroll MH, et al. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One 15 (2020): e0239252.
- Ebadi M, Montano-Loza AJ. Perspective: improving vitamin D status in the management of COVID-19. Eur J Clin Nutr 74 (2020): 856-859.
- Annweiler G, Corvaisier M, Gautier J, et.al. Vitamin D Supplementation Associated to Better Survival in Hospitalized Frail Elderly COVID-19 Patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 12 (2020): 3377.
- Ling SF, Broad E, Murphy R, et.al. High-Dose Cholecalciferol Booster Therapy is Associated with a Reduced Risk of Mortality in Patients with COVID-19: A Cross-Sectional Multi-Centre Observational Study. Nutrients 12 (2020): 3799.
- Mariani J, Tajer C, Antonietti L, et al. High-dose vitamin D versus placebo to prevent complications in COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial (CARED-TRIAL). Trials 22 (2021): 111.
- McNally JD, Iliriani K, Pojsupap S, et.al. Rapid normalization of vitamin D levels: a meta-analysis. Pediatrics 135 (2015): e152-66.
- Välimäki VV, Löyttyniemi E, Pekkarinen T, et al. How well are the optimal serum 25OHD concentrations reached in high-dose intermittent vitamin D therapy? a placebo-controlled study on comparison between 100 000 IU and 200 000 IU of oral D3 every 3 months in elderly women. Clin Endocrinol (Oxf) 84 (2016): 837-44.
- Wu Z, Camargo CA Jr, Reid IR, et.al. What factors modify the effect of monthly bolus dose vitamin D supplementation on 25-hydroxyvitamin D concentrations? J Steroid Biochem Mol Biol 201 (2020): 105687.
- Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, et. al. "Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study". J Steroid Biochem Mol Biol 203 (2020):105751.
- DiNicolantonio JJ, O'Keefe JH. Magnesium and Vitamin D Deficiency as a Potential Cause of Immune Dysfunction, Cytokine Storm and Disseminated Intravascular Coagulation in covid-19 patients. Mo Med 118 (2021): 68-73.
- Murai IH, Fernandes AL, Sales LP, et.al. Effect of a Single High Dose of Vitamin D3 on Hospital Length of Stay in Patients With Moderate to Severe COVID-19: A Randomized Clinical Trial. JAMA 325 (2021): 1053-1060.
- McCullough PJ, Lehrer DS, Amend J. Daily oral dosing of vitamin D3 using 5000 TO 50,000 international units a day in long-term hospitalized patients: Insights from a seven year experience. J Steroid Biochem Mol Biol 189 (2019): 228-239.
- de Carvalho JF, Churilov LP. Safety of megadose of vitamin D in patients with nephrolithiasis. Nutrition 87-88 (2021):111201.