Echocardiography and 6-Minute Walk Test for Assessing Disease Progression in Systemic Lupus Erythematosus
Article Information
Brygida Przywara-Chowaniec1, Dominika Dyrcz1*, Marcin Bere?1, Jan Harpula1, Agnieszka Nowak2, Andrzej Tomasik1
1II Chair and Clinical Ward of Cardiology in Zabrze, School of Medicine with Division of Dentistry in Zabrze, Medical University of Silesia in Katowice, Poland
2Department of Chemistry, School of Medicine with the Division of Dentistry in Zabrze, Medical University of Silesia, Katowice, Poland
*Corresponding author: Dominika Dyrcz, II Chair and Clinical Ward of Cardiology in Zabrze, School of Medicine with Division of Dentistry in Zabrze, Medical University of Silesia in Katowice, Poland
Received: 24 July 2019; Accepted: 02 August 2019; Published: 05 August 2019
Citation: Brygida Przywara-Chowaniec, Dominika Dyrcz, Marcin Bereś, Jan Harpula, Agnieszka Nowak, Andrzej Tomasik. Echocardiography and 6-Minute Walk Test for Assessing Disease P rogression in Systemic Lupus Erythematosus. Cardiology and Cardiovascular Medicine 3 (2019): 221-235.
View / Download Pdf Share at FacebookAbstract
Introduction: Systemic lupus erythematosus is an autoimmune disease characterized by the production of antinuclear and cytoplasmic antibodies that can affect many organs. Cardiovascular diseases occurring in patients with SLE include myocardial infarction, coronary heart disease, stroke, transient cerebral ischemia, or thromboembolic disorders. Early identification of heart dysfunction in SLE is important in determining the course of the disease.
Aim of the study: The aim of this study was to show the relationship between the presence of SLE and changes in echocardiographic parameters, in particular LVEF, 6MWT results and complete blood count. We also aimed to show the correlation between disease duration and LVEF.
Material and methods: The subjects of this study were patients with SLE. The study results from October 2014 to February 2019 were analyzed. The analysis included 45 patients with SLE, comprising 40 women aged 55.67 ± 11.34 years and 5 men aged 62.6 ± 8.43 years. The study group consisted of patients with an SLE diagnosis and an average disease duration of 12.58 ± 8.18 years.
Results: In the echocardiographic study, the left ventricular end-diastolic diameter in the parasternal long-axis view was significantly larger in the SLE group than in the non-SLE group (45.60 ± 6.72 vs. 40.75 ± 6.83 mm, p = 0.0247). In the 6MWT, the distance covered by the SLE group was statistically smaller than that of the non-SLE group (547.86± 87.59 vs. 595.37 ± 78.56 m, p = 0.0472). The disease duration was important in left ventricular systolic dysfunction (r = 0.4516, p = 0.0001). Disease duration correlated with MPV (r = -0.4057, p = 0.0017).
Conclusions: Echocardiographic examination may significantly influence t
Keywords
Systemic Lupus Erythematosus; 6 Minute Walk Test; Echocardiography; Complete blood count; Autoimmune disease; SLEDAI-2K
Systemic Lupus Erythematosus articles, 6 Minute Walk Test articles, Echocardiography articles, Complete blood count articles, Autoimmune disease articles, SLEDAI-2K articles
Article Details
Introduction
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease characterized by the production of antinuclear and cytoplasmic antibodies that can affect many organs. SLE presents with a wide spectrum of clinical symptoms. The symptoms may manifest from the cardiovascular and respiratory systems, among other systems, and as hematologic and dermatologic disorders. However, SLE is a disease of a still unknown etiology [1]. SLE occurs worldwide, and the incidence varies depending on the geographic location. The highest and lowest incidence rates have been reported in North America and Africa, respectively. On the other hand, European countries are among the nations with a lower incidence of SLE. The disease more often affects women than men, and the peak of incidence in women occurs between the third and seventh decade of life. In the case of men, the peak of incidence occurs in the fifth to the seventh decade of life [2]. There has been an increase in the survival rate of patients with SLE [3]. The diagnosis of SLE is based on meeting at least 4 of the classification criteria established by the American College of Rheumatology [4].
Patients with SLE have an increased risk of developing cardiovascular diseases, which are among the most common causes of death in this patient population [5,6]. Cardiovascular diseases occurring in patients with SLE include myocardial infarction, coronary heart disease, stroke, transient cerebral ischemia, or thromboembolic disorders. The risk factors that contribute to the development of the above diseases include components of the metabolic syndrome (obesity, dyslipidemia, hypertension, and insulin resistance) and smoking. Immunologic factors increasing the risk of cardiovascular complications in SLE have also been identified, such as the presence of antiphospholipid antibodies, proinflammatory cytokines, and factors related to the disease (e.g., glucocorticosteroid therapy, antimalarial drugs, immunosuppressive drugs, and kidney diseases) [7,8]. Therefore, it is important to identify patients with an increased risk of developing cardiovascular disease. Transthoracic echocardiography (TTE) is used as a noninvasive screening examination for detecting structural and functional disorders of the myocardium in asymptomatic patients with lupus [9]. Assessment of generalized and segmental contractility is important in estimating myocardial efficiency according to left ventricular ejection fraction (LVEF). Such an assessment enables the measurement of myocardial parameters and comparing the results with follow-up measurements, allowing for the accurate observation of patients [10–12]. Early identification of heart dysfunction in SLE is important in determining the course of the disease [13].
Imaging examinations can be supplemented with the 6-min walk test (6MWT) to assess the clinical capacity of the cardiovascular system. 6MWT is an easy-to-perform, repeatable, and low-cost method that allows monitoring the clinical status of a patient with SLE [14,15]. This test can be used in daily clinical practice.
Common hematologic disorders in patients with SLE can also be used as prognostic factors in the course of the disease [16]. Peripheral blood parameters such as the platelet-to-lymphocyte ratio (PLR), red blood cell distribution width, neutrophil-to-lymphocyte ratio, and mean platelet volume (MPV) can be used as markers of inflammation [17–19].
Aim of the study
The aim of this study was to show the relationship between the presence of SLE and changes in echocardiographic parameters, in particular LVEF, and 6MWT results. We also aimed to show the correlation between disease duration and LVEF. The parameters of complete blood count were compared between patients with SLE and controls.
Material and methods
The subjects of this study were patients with SLE. The study was conducted in the cardiology clinic of the Silesian Medical University in Katowice. The study results from October 2014 to February 2019 were analyzed, and the study is still ongoing. Informed consent was obtained from patients for inclusion in this study. The study protocol was approved by the local bioethical committee. The analysis included 45 patients with SLE, comprising 40 women aged 55.67 ± 11.34 years and 5 men aged 62.6 ± 8.43 years. The research group was selected in terms of sex and age. The control group consisted of 39 people, including 34 women aged 49.5 ± 12.02 years and 5 men aged 51.8 ± 4.31 years, who did not have serious cardiovascular diseases, especially those of atherosclerotic origin. All patients from this group voluntarily decided to take part in the presented study. Subjects diagnosed with autoimmune diseases, pregnancy and metabolic disorder were excluded from the study. Furthermore, the medical history was obtained retrospectively from the medical records. In this group the diabetes mellitus type 2 occurred in 5.13% of patients and the arterial hypertension occurred in 25.64%. The chronic diseases which had been diagnosed previously were: spine or lower limbs osteoarthritis (12.82%). None of patients from the control group had cancer or was treated with glucocoricosteroids before.
SLE was diagnosed using the American College of Rheumatology (ACR) criteria. Afterwards activity of the disease among patients was assessed using Systemic Lupus Erythematosus Disease Activity Index (SLEDAI-2K). Scores of above 6 were a threshold to recognize active disease. The measurements were carried out by qualified personnel under the same conditions. All examinations were conducted in the clinic as part of a planned visit to a specialist. The study group consisted of patients with an SLE diagnosis and an average disease duration of 12.58 ± 8.18 years. Glucocorticosteroid treatment (prednisone dose ≤7.5 mg/day) was used in 42.22% of patients, antimalarial drugs in standard doses in 37.78%. 6.67% of patients were treated with combination of glucocorticosteroids and antimalarial drugs. Biologic in standard doses was used in 8,89% of patients and azathioprine in standard doses in 8.89%. The exclusion criteria were the presence of an overlap syndrome and organ failure.
Echocardiographic examination was performed with the patient lying on the left side. The LVEF was estimated using the Simpson’s biplane method based on echocardiographic imaging from apical two- and four-chamber views. Each record included at least three cardiac cycles. The results of the three measurements were averaged and given as a percentage. The study was performed with a GE Healthcare Vivid 7 ultrasound machine equipped with a 3.6-MHz probe. All tests were performed by one qualified doctor (a cardiology specialist).
The 6MWT was performed according to a standard protocol, administered by a qualified nurse. The test was carried out in a ward corridor specially prepared for testing. The patients walked on a flat surface without obstacles, with markers placed on the wall with the amount of distance shown in meters. Each patient was informed about the purpose of the examination and the method of implementation. Before and after the study, blood saturation, arterial blood pressure, heart rate, and subjective parameters were measured for assessing dyspnea and fatigue according to the Borg scale. The patients performed the test by walking as far as possible within 6 min.
Blood samples for laboratory tests were obtained from each patient on an empty stomach in the morning. Morphologic blood tests were performed, and parameters such as erythrocytes (RBC), leukocytes (WBC), hematocrit, hemoglobin, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular hemoglobin (MCH), platelets, mean platelet volume (MPV) and C-reactive protein (CRP) were measured. The platelet-to-lymphocyte ratio (PLR) was calculated as the platelet count divided by the lymphocyte count.
Statistical analysis
Microsoft Excel 2016 and Statistica version 13.3 (StatSoft Inc. 2014) were used to analyze the data. Student’s t-test was used to analyze variables with normal distribution, and the Mann-Whitney U-test was used for nonparametric values. To calculate the correlation between nonparametric variables, the Spearman method was used. The Pearson correlation test was used for parametric values. Variables are presented as average ± standard deviation or median, and categorical data are summarized as number (percentage). The level of statistical significance was set at p < 0.05.
Results
Table 1 shows the anthropometric characteristics of patients with SLE and the control (non-SLE) group. The average age was 56.57 ± 11.25 years for the SLE group and 50.54 ± 11.97 years for the non-SLE group. The average duration of illness of patients with SLE was 12.58 ± 8.18 years. The height was comparable between groups (165.15 ± 7.37 cm in the SLE group and 164.18 ± 5.81 cm in the non-SLE group, p = 0.7818). Patients with SLE had lower average body weight than the control group (72.59 ± 15.32 vs. 73.57 ± 12.20 kg, p = 0.5150). Body mass index was lower in the SLE group (26.71 ± 4.29 vs. 27.61 ± 4.95 kg/m2, p = 0.4627).
In this study, diabetes mellitus type 2 occurred in 8.89% of the SLE group and in 5.13% of the non-SLE group (p = 0.5103). Significantly higher occurrence of hypertension was observed in the SLE group than in the non-SLE group (46.67% vs. 25.64%, p = 0.0471).
|
Parameters |
SLE |
Non-SLE |
p-Value |
|
Age [years] (mean ± SD) |
56.57 ± 11.25 |
50.54 ± 11.97 |
0.3475 |
|
Sex (% females) |
88.88 |
87.18 |
0.8121 |
|
Height [cm] (mean ± SD) |
165.15 ± 7.37 |
164.18 ± 5.81 |
0.7818 |
|
Weight [kg] (mean ± SD) |
72.59 ± 15.32 |
73.57 ± 12.20 |
0.5150 |
|
BMI [kg/m2] (mean ± SD) |
26.71 ± 4.29 |
27.61 ± 4.95 |
0.4627 |
|
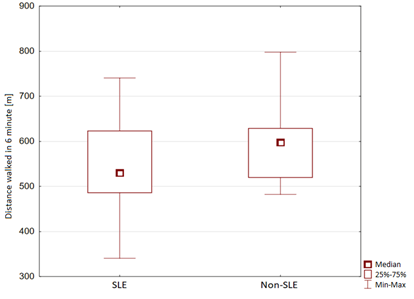
Distance walked in 6 min [m] (mean ± SD) |
547.86 ± 87.59 |
595.37 ± 78.56 |
0.0472 |
|
Borg dyspnea index (median) |
2 |
0.5 |
0.0036 |
|
Borg fatigue index (median) |
12 |
3 |
0.0001 |
|
SLEDAI-2K |
5.98 ± 5.43 |
0 |
0.0000 |
Table 1: Demographic characteristics, 6-MWT parameters and score SLEDAI-2K of the study and control groups
SLE: Systemic lupus erythematosus; BMI: Body mass index; SD: Standard deviation; SLEDAI-2K: Systemic Lupus Erythematosus Disease Activity Index. Source: Own studies.
Serologic tests, clinical symptoms of lupus and treatment are presented in the table number 2. There was no evidence of influence of using the glucocorticosteroids and changes of the LVEF (49.44 ± 7.14 vs. 48.38 ± 11.33, p=0.3450) and LVEDD (45.58 ± 5.56 vs. 46.04 ± 7.51, p =0.3649). Furthermore, the distance achieved during 6-MWT was lower among patients treated with glucocorticosteroids (531.57 ± 37.96 vs. 560.94 ± 104.63, p=0.1887). Presence of the dsDNA autoantibodies had no significant effect in lowering the LVEF (45.50 ± 12.75 vs. 51.12 ± 6.22, p=0.2541) and distance walked during the 6-MWT (531.93 ± 70.36 vs. 560.65 ± 89.86, p=0.3783). Similarly, presence of the Sm autoantibodies had no significant influence in lowering the LVEF (47.11 ± 12.35 vs. 50.01 ± 7.44, p=0.4408) and distance walked during 6-MWT (544.37 ± 67.38 vs. 551.20 ± 96.57, p=0.4595).
|
Parameters |
N (%) |
|
Serological |
|
|
ANAs (+) |
42 (93.33) |
|
dsDNA |
25 (55.56) |
|
RNP |
2 (4.44) |
|
SSA/Ro |
22 (48.89) |
|
SSB/La |
5 (11.11) |
|
Sm |
9 (20.00) |
|
Clinical |
|
|
Arthritis |
32 (71.11) |
|
Mucosal ulcers |
12 (26.67) |
|
Rash |
30 (66.67) |
|
Alopecia |
20 (44.44) |
|
Headache |
5 (11.11) |
|
Vasculitis |
2 (4.44) |
|
Pericarditis |
2 (4.44) |
|
Myositis |
1 (2.22) |
|
Pleurisy |
2 (4.44) |
|
Fever |
11 (24.44) |
|
Treatment |
|
|
≤ 7.5 mg of prednisone |
19 (42.22) |
|
Hydroxychloroquine |
17 (37.78) |
|
Azathioprine |
4 (8.89) |
|
Biologic |
4 (8.89) |
Table 2: Autoantibodies, treatment and organ manifestations in SLE group.
ANAs: Antinuclear antibodies; dsDNA: Anti-double-strand DNA antibodies; RNP: Anti-ribonucleoprotein antibodies; SSA/Ro: Anti-Ro antibodies; SSB/La: Anti-La antibodies; Sm: Anti-Smith antibodies. Source: Own studies.
In the 6MWT, the distance covered by the SLE group was statistically smaller than that of the non-SLE group (547.86 ± 87.59 vs. 595.37 ± 78.56 m, p = 0.0472). Study group with the active SLE (above 6 points in the SLEDAI-2K scale) showed statistically significant lower distance achieved during the 6-MWT when compared to patients in remission of the disease (505.57 ± 75.55 vs. 582.35 ± 71.84). The results are shown in Figure 1. The oxygen saturation (SpO2) measured before the test was 96.91 ± 1.95% in the SLE group and 97.49 ± 1.03% in the non-SLE group (p = 0.1330). The pre-test heart rate was 84.55 ± 13.42 beats/min in the SLE group and 86.41 ± 1.3 beats/min in the non-SLE group (p = 0.6897). The systolic blood pressure was 120.57 ± 22.38 mmHg in the SLE group and 118.53 ± 17.02 mmHg in the non-SLE group (p = 0.6897). The diastolic blood pressure was 77.32 ± 11.86 mmHg in the SLE group and 78.67 ± 9.77 mmHg in the non-SLE group (p = 0.6294). The median Borg dyspnea index was 0 in both the SLE and non-SLE groups (p = 0.0834). The median Borg fatigue index was 6 in the SLE group and 0 in the non-SLE group, and the difference was significant (p = 0.0001). The SpO2 measured after the test was 96.72 ± 1.45% in the SLE group and 96.67 ± 2.23% in the non-SLE group (p = 0.9008). The heart rate after the test was 104.1 ± 18.98 beats/min in the SLE group and 111.14 ± 18.79 beats/min in the non-SLE group (p = 0.1577). The systolic blood pressure after the test was 132.46 ± 23.72 mmHg in the SLE group and 128.91 ± 23.46 mmHg in the non-SLE group (p = 0.5336). The diastolic blood pressure was 80.17 ± 11.87 mmHg in the SLE group and 83.88 ± 12.97 mmHg in the non-SLE group (p = 0.2575). The median Borg dyspnea index was 2 in the SLE group and 0.5 in the non-SLE group, and the difference was statistically significant (p = 0.0036). The median Borg fatigue index was significantly higher in the SLE group (12) than in the non-SLE group (3) (p = 0.0001). Figure 1 presents the 6MWT results of the study and control groups.
SLE, systemic lupus erythematosus. Source: Own studies.
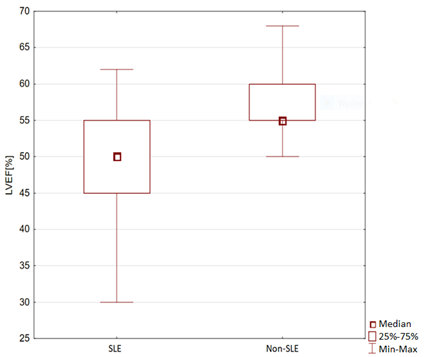
In the echocardiographic study, the left ventricular end-diastolic diameter (LVEDD) in the parasternal long-axis view was significantly larger in the SLE group than in the non-SLE group (45.60 ± 6.72 vs. 40.75 ± 6.83 mm, p = 0.0247). In the group with active SLE the LVEDD was higher than in patients in remission of the disease (47.48 ± 8.30 vs. 44.58 ± 4.95, p=0.1773). The measurement of the right ventricle in the parasternal long-axis view was statistically higher in the SLE group than in the non-SLE group (27.26 ± 7.44 vs. 23.04 ± 3.27 mm, p = 0.0026). The average LVEF was statistically lower in the SLE group than in the control group (50.82 ± 7.01% vs. 57.68 ± 4.56%, p = 0.0001). In the group with active SLE the LVEF was statistically lower when compared with patients in remission of the disease (44.89 ± 7.58 vs. 51.54 ± 10.31, p=0.0002). The results are shown in Figure 2. In the study group, 2.22% of patients with SLE showed left ventricular systolic dysfunction. LVEF <30% was present in 2.22% of the patients in the SLE group. LVEF 31%–40% was significantly more often observed in patients with SLE (20% vs. 0%, p = 0.0027). LVEF 41%–50% was present in 31.11% of patients with SLE (p = 0.0001). LVEF 51%–60% was significantly more often observed in the non-SLE group than in the SLE group (94.87% vs. 44.55% p = 0.0001). LVEF >60% was present in 2.22% of the SLE group and in 5.13% of the non-SLE group (p = 0.8851). Table 3 shows the LVEF of patients with SLE.
LVEF: Left ventricular ejection fraction; SLE: Systemic lupus erythematosus. Source: Own studies.
|
Echocardiography |
SLE |
Non-SLE |
|
n (%) |
45 |
39 |
|
Ejection fraction |
||
|
<30% |
1 (2.22) |
0 (0) |
|
31–40% |
9 (20.00) |
0 (0) |
|
41–50% |
14 (31.11) |
0 (0) |
|
51–60% |
20 (44.55) |
37 (94.87) |
|
>61% |
1 (2.22) |
2 (5.13) |
Table 3: Echocardiographic findings of the study and control groups
SLE: Systemic lupus erythematosus. Source: Own studies.
Table 3 presents the parameters of blood counts and CRP. A lower average hemoglobin concentration was observed in the SLE group (13.29 ± 1.49 vs. 13.47 ± 0.86 g/dL, p = 0.3594). A significantly higher average MCV was found in the SLE group (94.64 ± 13.92 vs. 86.48 ± 14.57 fL, p = 0.0019). A statistically lower average MCHC was observed in the SLE group (33.13 ± 2.01 vs. 37.27 ± 14.31 g/dL, p = 0.0022). Significantly higher CRP levels were observed in the SLE group (9.25 ± 33.24 vs. 1.23 ± 0.96 mg/L, p = 0.0169). Hematocrit was higher in the SLE group than in the non-SLE group (39.79 ± 5.25% vs. 38.00 ± 7.69%, p = 0.0973). MPV was statistically lower in the SLE group than in the non-SLE group (9.41 ± 1.31 vs. 10.42 ± 1.55 fL, p = 0.0002). In the group with active SLE the MPV was lower when compared with patients in the remission of the disease (9.89 ± 3.11 vs. 9.17 ± 2.67, p=0.2701), but on the other hand, the PLR values were significantly higher (181.30 ± 75.49 vs. 124.81 ± 49.41, p=0.0066). The remaining morphologic blood parameters are presented in Table 3.
|
Parameters |
SLE |
Non-SLE |
p-Value |
|
WBC [103/µL] |
6.02 ± 2.16 |
5.91 ± 2.54 |
0.7968 |
|
RBC [106/µL] |
4.45 ± 1.79 |
4.55 ± 0.66 |
0.5832 |
|
HGB [g/dL] |
13.29 ± 1.49 |
13.47 ± 0.86 |
0.3594 |
|
HCT [%] |
39.79 ± 5.25 |
38.00 ± 7.69 |
0.0973 |
|
MCV [fL] |
94.64 ± 13.92 |
86.48 ± 14.57 |
0.0019 |
|
MCHC [g/dL] |
33.13 ± 2.01 |
37.27 ± 14.31 |
0.0022 |
|
MCH [pg] |
30.73 ± 4.32 |
30.28 ± 1.87 |
0.3775 |
|
PLT [103/µL] |
242.12 ± 73.07 |
224.52 ± 84.17 |
0.2051 |
|
MPV [fL] |
9.41 ± 1.31 |
10.42 ± 1.55 |
0.0002 |
|
PLR |
150.59 ± 67.66 |
131.98 ± 36.96 |
0.0498 |
|
CRP [mg/L] |
9.25 ± 33.24 |
1.23 ± 0.96 |
0.0169 |
Table 4: Blood parameters and C-reactive protein level in the study and control groups
WBC: White blood cells; RBC: Red blood cells; HGB: Hemoglobin; HCT: Hematocrit; MCV: Mean corpuscular volume; MCHC: Mean corpuscular hemoglobin concentration; MCH: Mean corpuscular hemoglobin; PLT: Platelets; MPV: Mean platelet volume; CRP: C-reactive protein. Source: Own studies.
A significant correlation was found between the distance covered in 6MWT and the LVEF (r = 0.6585, p = 0.00001). The disease duration was important in left ventricular systolic dysfunction (r = 0.4516, p = 0.0001) and in the distance covered in 6MWT (r = -0.2201, p = 0.0001). Significant correlations between disease duration and blood counts were found. Disease duration also correlated with HCT (r = 0.4034, p = 0.0376), hemoglobin (r = 0.4280, p = 0.0266), MCV (r = 0.5126, p = 0.0007), and MPV (r = -0.4057, p = 0.0017). A correlation between disease duration and CRP was also observed (r = 0.4737, p = 0.0072). A correlation between disease duration and PLR was also observed (r = 0.4569, p = 0.0054).
Discussion
SLE is a rare autoimmune disease, the mortality of which is significantly associated with cardiovascular complications. Finding markers that can be used in daily clinical practice, which are also easy to use and access, is important in the development of clinical trials. Markers are important in the assessment of disease progression and in the comparison of results across various time points. In the study group, we proved a significant correlation between disease duration and reduction in LVEF, and this relationship is important owing to the possibility of subsequent complications in the form of heart failure. Lower values of the LVEF and distance walked during the 6-MWT correlated with the disease activity measured with SLEDAI-2K scale. TTE control will significantly delay the onset of severe cardiovascular complications after early treatment. Autoimmune mechanisms and a chronic inflammatory process are involved in the pathogenesis, leading to the development of atherosclerosis and the subsequent occurrence of cardiovascular disease or heart failure. Glucocorticosteroid therapy is also important in the development of heart failure [20,21]. We haven’t shown statistically significant impact of the glucocorticosteroids treatment and autoantibodies presence on the LVEF and 6-MWT changes in the study group.
Mavrogeni et al. described the significance of echocardiography as a noninvasive method of cardiovascular risk assessment in patients with SLE. Echocardiography should be performed both in patients with active disease and in asymptomatic individuals. The authors pointed out that patients with SLE, despite having good LVEF, might develop heart failure associated with diastolic dysfunction. Therefore, studies of patients with SLE require, in addition to echocardiography, other diagnostic methods to ensure adequate disease control [11]. An accessible test for patients with SLE can be 6MWT, which allows easily assessing the cardiopulmonary capacity of the patient and the progression of the disease. This study showed a negative correlation between the distance traveled in 6MWT by patients with SLE and the duration of the disease. During clinical progression, a smaller distance traveled was observed in the SLE group; a similar relationship was observed in relation to the control group.
In their study, Huang, Yao and Huang “Role of echocardiography for early detection of cardiac lesions in asymptomatic SLE,” described echocardiography as a noninvasive diagnostic test enabling the early detection of cardiovascular complications, thus resulting in earlier treatment and in reductions in mortality and morbidity. They analyzed 40 patients with SLE, and left ventricular systolic dysfunction was diagnosed in 25% of these patients. The study showed that, in many cases of a serologically active disease, there were no clinical symptoms and changes were only detected on TTE [22]. Echocardiography has an important role in the detection and control of cardiovascular disease. In addition to LVEF assessment, it is possible to demonstrate regional and general systolic dysfunction, and LVEDD measurements also provide important information on the enlargement of the left ventricle muscle.
Huang et al. investigated 34 patients with SLE and 34 controls. There were no significant differences in LVEF between the SLE group and the control group (67.0 ± 5.7% vs. 65.2 ± 7.8%). The study was performed using the two-dimensional TTE technique [23], and showed that it is important to gather as many study subjects as possible for meaningful results. Because SLE is a rare disease, performing research in a large number of patients from one center is often not possible.
Balsamo et al. presented a study involving 25 patients with SLE and 25 controls. Patients with SLE covered a significantly shorter distance in 6MWT (598 ± 45 m) than the control group (642 ± 14 m). The heart rate after the test was higher in patients with SLE than in the control group (134 ± 15 vs. 123 ± 23 beats/min). The results demonstrated that patients with SLE had a poorer quality of life [14]. Leite presented a 6MWT study in 42 patients with SLE. The average distance traveled by the patients was 478 ± 82 m. A significant correlation was found between decreased saturation and the distance traveled by the patients in 6MWT [24]. In both studies, a significantly smaller distance in 6MWT was covered by patients with SLE. Similar results were obtained in the study group. There were no important differences between the SLE and control groups in blood pressure and heart rate. Significantly higher values of parameters were obtained in a subjective study of fatigue and dyspnea according to the Borg scale.
Hematologic abnormalities are a common complication of SLE. Anemia is present in almost half of the patients. It is mostly associated with chronic inflammation and changes in iron levels, as well as with reduced erythropoietin activity [25–28]. In the group of patients with SLE, the hemoglobin concentration was lower than that in the control group. Almost 20% of patients with SLE were found to have anemia.
SLE assessment should be comprehensive and should include laboratory tests, such as measurements of MPV and CRP, which are indicators of inflammation and significantly correlate with the disease duration and results of imaging tests (e.g., TTE), which are used to assess cardiac dysfunction. In later phases, magnetic resonance imaging should be considered to more accurately observe the structure of the heart [11]. In the present study, the significantly higher measurements of MPV and CRP in the SLE group indicate active inflammation in the body. The correlation between disease duration and MPV and CRP may significantly contribute to the clinical evaluation of patients with SLE. In the study group the MPV values were lower among patients with active SLE disease but they weren’t statistically significant.
Uyar et al. analyzed 37 patients with SLE and 30 controls. The average disease duration was 4.96 years. The MPV was significantly lower in the SLE group (8.27 ± 1.68 vs. 9.16 ± 1.52 fL, p = 0.031). In all patients who were in SLE remission, the CRP, leukocyte, and complement levels were normal. The authors suggested that hydroxychloroquine therapy contributed to the lower MPV values [29]. The lower MPV values obtained in the study group were probably associated with higher disease activity. The CRP level was elevated, which indicates the progression of the disease. Similar results were obtained by Safak et al. in their study involving 44 patients, in which the MPV values were significantly lower in active SLE. The authors compared the state of remission and the active form of the disease. They concluded that MPV significantly decreased in the group with active arthritis, compared with the group in remission and the healthy control group [18]. Further studies with a larger sample are needed to demonstrate the possibility of using MPV as a biomarker for disease activity.
Soliman et al. have included 120 patients with the SLE and 30 adults as a control group in their research. Studied PLR values correlated significantly with the SLEDAI scale and CRP. Furthermore, they established a correlation of PLR with dsDNA and proposed a cut-off point above 132.9 for the PLR. [30] Similar results were showed in the study group – PLR was statistically related with SLEDAI-2K scale outcomes. Mean values for patients with above 6 points in the SLEDAI-2K were significantly higher than among patients with remission of the disease. PLR could have significant role in the determination of the SLE activity. It will be necessary to evaluate the PLR values above which disease progression will be recognized.
Thus far, the available studies on SLE in the literature involved small study populations. Most studies have <50 subjects, which is related to the difficulty in gathering a large group of patients from one research center or from a given region. It is an important issue that SLE is a rare disease, and research requires multi-year collections of data to allow performing numerous clinical trials. Moreover, aggregating data from several research centers is desirable.
SLE is a clinically variable autoimmune disease. Evaluation of disease activity and progression, choice of therapy, and early and accurate diagnosis are among the most important factors in proper disease control. The search for new markers is related to the possibility of assessing the future risk of the disease. Available and simple clinical tests can help evaluate the progression of SLE. Remission and low activity of the SLE have an influence of the life quality and are important factors for severe organ complications prevention [31,32].
Conclusions
Echocardiographic examination may significantly influence the further treatment and prevention of cardiovascular complications and the reduction of mortality in patients with SLE. This test especially should be carried out among patients who have above 6 points in the SLEDAI-2K scale. Noninvasive laboratory monitoring should be regularly performed for clinical evaluation. Easy-to-use and easily accessible methods such as TTE and 6MWT will allow the early detection of cardiovascular diseases and the introduction of appropriate treatment. The interpretation of 6MWT results should not be limited to the assessment of the cardiopulmonary system, but can also be used for assessing the activity and progression of the disease. MPV, PLR, CRP level and SLEDAI-2K may be helpful in assessing disease activity.
Data Availability
The data used to support the findings of this study are included within the article and have also been deposited in the PubMed database and the ClinicalKey repository.
Conflicts of Interest
The authors declare no conflict of interests.
Funding Statement
The article did not receive funding. It was performed as part of the employment of the authors. The employer is II Chair and Clinical Ward of Cardiology in Zabrze, School of Medicine with Division of Dentistry in Zabrze, Medical University of Silesia in Katowice and Head of the Clinical Department Ewa Nowalany-Kozielska MD, PhD.
References
- Fortuna G, Brennan MT. Systemic lupus erythematosus: epidemiology, pathophysiology, manifestations, and management. Dent Clin North Am 57 (2013): 631-655.
- Rees F, Doherty M, Grainge MJ, et al. The worldwide incidence and prevalence of systemic lupus erythematosus: a systematic review of epidemiological studies. Rheumatology 56 (2017): 1945-1961.
- Mak A, Cheung MW, Chiew HJ, et al. Global trend of survival and damage of systemic lupus erythematosus: meta-analysis and metaregression of observational studies from the 1950s to 2000s. Semin Arthritis Rheum 41 (2012): 830–839.
- Gordon C, Amissah-Arthur MB, Gayed M, et al. The British Society for Rheumatology guideline for the management of systemic lupus erythematosus in adults. Rheumatology 57 (2018): 1-45.
- Urowitz MB, Bookman AA, Koehler BE, et al. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med 60 (1976): 221–225.
- Doria A, Iaccarino L, Ghirardello A, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med 119 (2006): 700-706.
- Benvenuti F, Gatto M, Laros M, et al. Cardiovascular risk factors, burden of disease and preventive strategies in patients with systemic lupus erythematosus: a literature review. Expert Opin Drug Saf 14 (2015): 1373–1385.
- Zeller CB, Appenzeller S. Cardiovascular Disease in Systemic Lupus Erythematosus: The Role of Traditional and Lupus Related Risk Factors. Curr Cardiol Rev 4 (2008): 116–122.
- Sitia S, Atzeni F, Sarzi-Puttini P, et al. Cardiovascular involvement in systemic autoimmune diseases. Autoimmun Rev 8 (2009): 281-286.
- Plazak W, Kopec G, Tomkiewicz-Pajak L, et al. Heart structure and function in patients with generalized autoimmune diseases. Echocardiography with tissue Doppler study. Acta Cardiol 66 (2011): 159–165.
- Mavrogeni S, Koutsogeorgopoulou L, Dimitroulas T, et al. Complementary role of cardiovascular imaging and laboratory indices in early detection of cardiovascular disease in systemic lupus erythematosus. Lupus 26 (2017): 227-236.
- Buss SJ, Wolf D, Korosoglou G, et al. Myocardial left ventricular dysfunction in patients with systemic lupus erythematosus: New insights from tissue Doppler and strain imaging. J Rheumatol 37 (2010): 79–86.
- Barutcu A, Aksu F, Ozcelik F, et al. Evaluation of early cardiac dysfunction in patients with systemic lupus erythematosus with or without anticardiolipin antibodies. Lupus 24 (2015): 1019-1028.
- Balsamo S, Nascimento Dda C, Tibana RA, et al. The quality of life of patients with lupus erythematosus influences cardiovascular capacity in 6-minute walk test. Rev Bras Reumatol 53 (2013): 75-87.
- Minai OA, Nguyen Q, Mummadi S, et al. Heart rate recovery is an important predictor of outcomes in patients with connective tissue disease-associated pulmonary hypertension. Pulm Circ 5 (2015): 565-576.
- Abdel Galil SM, Edrees AM, Ajeeb AK, et al. Prognostic significance of platelet count in SLE patients. Platelets 28 (2017): 203-207.
- Xie S, Chen X. Red blood cell distribution width-to-platelet ratio as a disease activity-associated factor in systemic lupus erythematosus. Medicine 97 (2018): 12342.
- Safak S, Uslu AU, Serdal K, et al. Association between mean platelet volume levels and inflammation in SLE patients presented with arthritis. Afr Health Sci 14 (2015): 919–924.
- Uslu AU, Küçük A, S¸ahin A, et al. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. Int J Rheum Dis 18 (2015): 731–735.
- Dhakal BP, Kim CH, Al-Kindi SG et al. Heart failure in systemic lupus erythematosus. Trends Cardiovasc Med 28 (2018): 187-197.
- Urowitz MB, Gladman D, Ibanez D et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res 62 (2010): 881-887.
- Killi S, Bhyravavajhala S. Role of echocardiography for early detection of cardiac lesions in asymptomatic systemic lupus erythematosus (SLE). Indian Heart J 70 (2018): 22.
- Huang BT, Yao HM, Huang H. Left ventricular remodeling and dysfunction in systemic lupus erythematosus: a three-dimensional speckle tracking study. Echocardiography 31 (2014): 1085-1094.
- Leite MA, Pereira MC, Costallat LT, et al. Evaluation of respiratory impairment in patients with systemic lupus erythematosus with the six-minute walk test. Rev Bras Reumatol 54 (2014): 192-199.
- Tzioufas AG, Kokori SI, Petrovas CI, Moutsopoulos HM. Autoantibodies to human recombinant erythropoietin in patients with systemic lupus erythematosus: correlation with anemia, Arthritis Rheum 40 (1997): 2212-2216.
- Ganz T, Nemeth E. Hepcidin and iron homeostasis, Biochim Biophys Acta 1823 (2012): 1434-1443.
- Nemeth E, Rivera S, Gabayan V, Keller C et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin, J Clin Investig 113 (2004): 1271-1276.
- Schett G, Firbas U, Füreder W, Hiesberger H et al. Decreased serum erythropoietin and its relation to anti-erythropoietin antibodies in anaemia of systemic lupus erythematosus, Rheumatology (Oxford) 40 (2001): 424-431.
- Uyar S, Abanonu GB, Pehlevan SM, Karatoprak C et al. Elevated beta-thromboglobulin and mean platelet volume levels may show persistent platelet activation in systemic lupus erythematosus patients. Adv Clin Exp Med 27 (2018): 1279–1283.
- Soliman WM, Sherif NM, Ghanima IM, El-Badawy MA. Neutrophil to Lymphocyte and Platelet to Lymphocyte Ratios in Systemic Lupus Erythematosus: Relation With Disease Activity and Lupus Nephritis. Reumatol Clin 2018.
- Ugarte-Gil MF, Gamboa-Cárdenas RV, Reátegui-Sokolova C, Medina-Chinchón M et al. Low Disease Activity State/Remission Predicts a Better Health-Related Quality of Life in Systemic Lupus Erythematosus Patients. Arthritis Care Res 2019.
- Sharma C, Raymond W, Eilertsen G and Nossent, J. Achieving Lupus Low Disease Activity State (LLDAS-50) is associated with both reduced damage accrual and mortality in patients with Systemic Lupus Erythematosus. Arthritis Care Res 2019.