Early Risk Factors for Severe COVID-19 in Adults With Hypertension
Article Information
Denggao Peng*, Yanzhang Gao, Zhenyu Zhou, Huan Wang, Anjue Tang
The Third People's Hospital of Shenzhen, Shenzhen, China
*Corresponding author: Denggao Peng, Department of Emergency Medicine, The Third People's Hospital of Shenzhen, Longgang district, Shenzhen, 518112, China.
Received: 03 December 2021; Accepted: 14 December 2021; Published: 21 December 2021
Citation: Peng D, Gao Y, Zhou Z, Wang H, Tang A. Early Risk Factors for Severe COVID-19 in Adults With Hypertension. Cardiology and Cardiovascular Medicine 5 (2021): 726-738.
View / Download Pdf Share at FacebookAbstract
Objective Aimed to identify the early risk factors for severe COVID-19 in adults with hypertension, and provide a basis for risk stratification and treatment decisions.
Methods A retrospective study was performed. Adults were defined as being more than 18 years old. Multivariate binary logistic regression analysis and Receiver Operating Characteristic (ROC) curve were used to evaluate the risk factors.
Results A total of 68 COVID-19 cases with hypertension were included, 27 (39.7%) were severe and 41 (60.3%) were non-severe. Between the non-severe group (n=41) and severe group (n=27), number of elevated B-Type Natriuretic Peptide (BNP) and abnormal renal function, and albumin, lactate dehydrogenase, ultrasensitive troponin I, plasma PH value, arterial carbon dioxide partial pressure, sodium, plasma osmolality, blood glucose and ratio of arterial oxygen partial pressure (PaO2) and fraction of inspired oxygen (FiO2) were significantly different. BNP (Odds ratio [95%CI] 12.307[1.349-112.251];P=0.026), plasma osmolality (0.345[0.169-0.705];P=0.004), blood glucose (2.262[1.158-4.422];P=0.017) and PaO2/FiO2 ratio (0.267[0.124-0.577]; P=0.001) were significant independent risk factors. The area under ROC curve (AUC) of model was 0.904 ([95%CI] [0.832-0.976];P<0.001), with excellent performance. The sensitivity, specificity, positive and negative predictive value, and accuracy of predicting were 78.6%, 87.5%, 81.5%, 85.4% and 83.8%, respectively. BNP, plasma osmolality, blood glucose or PaO2/FiO2 ratio as single predictors, their AUCs were 0.624, 0.726, 0.687 and 0.75, respectively.
Conclusion Decreased plasma osmolality and PaO2/FiO2 ratio, elevated BNP and blood glucose may be the early risk factors for severe COVID-19 with hypertension. Targeting osmolality regulation, early use of diuretics may be a promising treatment.
Keywords
COVID-19; Hypertension; Plasma osmolality; Risk factors; Severe
COVID-19 articles; Hypertension articles; Plasma osmolality articles; Risk factors articles; Severe articles
Article Details
List of Abbreviations:
ACE: Angiotensin-converting enzyme; ADH: Antidiuretic hormone; ALF: Abnormal liver function; ARDS: Acute respiratory distress syndrome; ARF: Abnormal renal function; ASCVD: Atherosclerotic cardiovascular disease; AUC: Area under curve; BNP: B-type natriuretic peptide; CI: Confidence interval; COVID-19: Coronavirus disease 2019; FiO2: Fraction of inspired oxygen; NT-proBNP: N-terminal pro-brain natriuretic peptide; PaCO2: Arterial carbon dioxide partial pressure; PaO2: Arterial oxygen partial pressure; RAS: Renin-angiotensin -aldosterone system; ROC: Receiver operating characteristic curve; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2; SD: Standard deviation; SE: Standard error; TnIUltra: Ultrasensitive troponin I.
1. Introduction
Since the outbreak of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), the number of confirmed cases has increased and spread rapidly to most countries around the world [1]. Emerging data have suggested that hypertension may be associated with an up to 2.5-fold higher risk of severe and fatal COVID-19, especially among older patients [2]. However, the exact pathogenesis and early risk factor of severe COVID-19 in patients with hypertension were rarely reported [3,4], which undoubtedly has a serious impact on the early realization of risk stratification and optimal treatment. It is well known that SARS-CoV uses the host protein Angiotensin Converting Enzyme 2 (ACE2) as a entry receptor into human alveolar epithelial cells [5,6]. ACE2 is a key enzyme component of Renin-Angiotensin-Aldosterone System (RAS), which degrades angiotensin II (a peptide with multiple effects that promote hypertension) and produces angiotensin 1-7, thereby antagonizing the role of angiotensin II [7]. In fact, ACE2 expression is down-regulated after SARS-COV-2 infection. In addition to blood pressure regulation, RAS also plays an important role in water and sodium metabolism and osmolality regulation. Clinically, the vast majority of severe COVID-19 are caused by Acute Respiratory Distress Syndrome (ARDS). Our research aimed to identify the early risk factors for severe COVID-19 in adults with hypertension from the perspective of imbalance of water and sodium metabolism and osmolality regulation disorder, and provide a basis for clinical risk stratification and treatment decisions.
2. Materials and Methods
2.1. Definition and classification
Referring to the National Institute for Health and Clinical Excellence (NICE) 2019 guidelines for hypertension [8], hypertension is based on the following two definitions: (a) self-reported previous hypertension; (b) newly comfirm diagnosed hypertension: clinic systolic/diastolic blood pressure of 140/90 mmHg or higher. Adults were defined as being more than 18 years old. The PaO2/FiO2 was calculated as the ratio of arterial oxygen partial pressure (PaO2) and fraction of inspired oxygen (FiO2). We followed the guidelines on the diagnosis and treatment of 2019 novel coronavirus infected pneumonia (the sixth edition draft) issued by the National Health Commission of China [9], patients were clinically classified. The details were as follows: (a) Mild: clinical symptoms are mild, and there is no pneumonia manifestation in imaging. (b) Ordinary: it has fever, respiratory tract and other symptoms. Pneumonia can be seen on imaging. (c) Severe: meets any of the following: shortness of breath; respiratory rate >30 times/min at rest; refers to oxygen saturation < 93%; PaO2/FiO2 < 300 mmHg (l mmHg = 0.133 kPa); lung imaging shows the progress of lesion was > 50% at 24-48 h. (d) Critical: one of the following conditions: respiratory failure and mechanical ventilation; shock; combined with failure of other organs should be treated in the Intensive Care Unit. Abnormal liver function (ALF) was defined as total bilirubin > 21 µmol/L (reference range: 1.7-21) and/or alanine aminotransferase > 45 U/L (reference range: 0-45) and/or aspartate aminotransferase > 45 U/L (reference range: 0-45). Abnormal Renal Function (ARF) was defined as creatinine above the upper limit (reference range:0.6-1.2 for male, 0.55-1.1 mg/dL for female). Elevated B-Type Natriuretic Peptide (BNP) was defined as N-terminal pro-brain natriuretic peptide (NT-proBNP) (reference range: 0-125 for age under 75, 0-450 ng/L for age over 75) or BNP (reference range: 0-23 pmol/L) above the upper limit.
2.2. Data collection and review
For all included cases, we retrieved electronic medical records and conducted a retrospective study. The clinical and laboratory data on the first day of admission were collected and reviewed. According to the final clinical diagnosis classification, all included cases were divided into two groups: a non-severe group (mild + ordinary) and a severe group (severe + critical).
Inclusion criteria: all confirmed COVID-19 adult cases with hypertension.
Exclusion criteria: cases with missing data or withdrawing from other diseases.
3. Statistical Analysis
All analyses were conducted by using of IBM Statistical Product and Service Solutions software Version 24 (SPSS Inc, Chicago, IL). Continuous variables were summarized as the median with their Interquartile Ranges (IQRs) or mean with Standard Deviations (SDs), Median [IQR] or [mean±SD], depending on whether their distributions were normal or not. Comparisons of categorical variables were performed using the Pearson Chi-square test or Fisher exact test. 95% Confidence Intervals (CIs) were calculated if applied. The parametric tests (independent sample Student t-test) or non-parametric tests (Mann-Whitney U test) were used to analyze variables. Variables with P < 0.1 were entered into a multivariate binary logistic regression model. Model fitness was assessed with the Hosmer-Lemeshow goodness-of-fit test. Analysis of the Area Under Curve (AUC) of Receiver Operating Characteristic (ROC) was constructed to assess the predicting performance. Sensitivity, specificity, Positive Predictive Value (PPV), Negative Predictive Value (NPV) and accuracy were also determined. P < 0.05 was considered as statistically significant in all tests if applied.
4. Results
From Jan 22nd to Mar 31st 2020, a total of 70 COVID-19 adult patients with hypertension were admitted to the designated hospital, 1 (1.4%) case with cerebellar infarction and 1 (1.4%) case with uremia combined with acute heart failure during hospitalization was excluded. Of the 68 included cases, the final clinical diagnostic classification was 27 (39.7%) for severe and 41 (60.3%) for non-severe. Between the non-severe group (n=41) and severe group (n=27), number of fever (78% vs 96.3%; P=0.043) and elevated BNP (4.9% vs 29.6%;P=0.011), albumin ([42.7±3.1] vs [40.5±3.7];P=0.008), lactate dehydrogenase (269[188-408] vs 385[243.5-532.5]; P=0.031), ultrasensitive troponin I (TnIUltra) (0.012[0.006-0.012] vs 0.012[0.012-0.015];P=0.004), PH value ([7.42±0.02] vs [7.44±0.03];P=0.016), arterial carbon dioxide partial pressure (PaCO2) ([37.9±3.4] vs [35.8±4.3];P=0.025), sodium ([139.5±2.9] vs [136.5±3.2];P<0.001), plasma osmolality ([285.9±5.4] vs [281.2±5.8];P=0.001), blood glucose (5.9[5.5-6.7] vs 7.7[6.3-8.5];P=0.009) and PaO2/FiO2 ratio ([395±62] vs [326±95]; P=0.002) had significant differences. While age ([59.8±11.3] vs [63.6±8.4];P=0.136), male gender (56.1% vs 70.4%;P=0.315), comorbidities with diabetes or atherosclerotic cardiovascular disease, smoking history, number of ALF and ARF (9.8% vs 25.9%;P=0.099), heart rate, respiratory rate, systolic/diastolic blood pressure, white blood cell count, hematocrit, potassium and lactic acid were statistically insignificant (Table 1).
|
Items |
Non-severe (n=41) |
Severe (n=27) |
P |
|
Age: (years) |
59.8±11.3 |
63.6±8.4 |
0.136 |
|
Male gender: n(%) |
23 (56.1%) |
19 (70.4%) |
0.315 |
|
Diabetes: n(%) |
5 (12.2%) |
5 (18.5%) |
0.502 |
|
ASCVD: n(%) |
24 (58.5%) |
21 (77.8%) |
0.122 |
|
Smoking history: n(%) |
3 (7.3%) |
1 (3.7%) |
1 |
|
Number of elevated BNP: n(%) |
2 (4.9%) |
8 (29.6%) |
0.011 |
|
Number of ALF: n(%) |
10 (37%) |
5 (22%) |
0.269 |
|
Number of ARF: n(%) |
4 (9.8%) |
7 (25.9%) |
0.099 |
|
Heart rate: (times/min) |
86±15 |
90±8 |
0.136 |
|
Respiratory rate: (times/min) |
20 (20-20) |
20 (19-22) |
0.747 |
|
Systolic blood pressure: (mmHg) |
139.2±16.4 |
143.3±20.8 |
0.363 |
|
Diastolic blood pressure: (mmHg) |
85.8±11.5 |
86.9±13.3 |
0.72 |
|
White blood cell: (×10^9/L) (3.5-9.5) |
5.1 (3.8-6.9) |
5.1 (3.8-6.3) |
0.666 |
|
Hematocrit: (%) (37-52) |
39.7±4.5 |
39.9±3.3 |
0.899 |
|
Albumin: (g/L) (40-55) |
42.7±3.1 |
40.5±3.7 |
0.008 |
|
Lactate dehydrogenase: (U/L) (120-250) |
269 (188-408) |
385 (243.5-532.5) |
0.031 |
|
TnIUltra: ( µg/L) (0-0.034) |
0.012 (0.006-0.012) |
0.012 (0.012-0.015) |
0.004 |
|
PH Value: (7.35-7.45) |
7.42±0.02 |
7.44±0.03 |
0.016 |
|
PaCO2: (mmHg) (35-45) |
37.9±3.4 |
35.8±4.3 |
0.025 |
|
Sodium: (mmol/L) (135-145) |
139.5±2.9 |
136.5±3.2 |
<0.001 |
|
Potassium: (mmol/L) (3.5-5.5) |
3.6±0.3 |
3.5±0.3 |
0.795 |
|
Plasma osmolality: (mOsm/L) (290-310) |
285.9±5.4 |
281.2±5.8 |
0.001 |
|
Blood glucose: (mmol/L) (3.9-6.1) |
5.9 (5.5-6.7) |
7.7 (6.3-8.5) |
0.009 |
|
Lactic acid: (mmol/L) (0.5-1.5) |
1.3 (1.1-1.5) |
1.4 (1.15-1.8) |
0.584 |
|
PaO2/FiO2 ratio: (mmHg) (400-500) |
395±62 |
326±95 |
0.002 |
|
Abbreviations: ALF: abnormal liver function; ARF: abnormal renal function; ASCVD: atherosclerotic cardiovascular disease; BNP: B-type natriuretic peptide; FiO2: fraction of inspired oxygen; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; TnIUltra: ultrasensitive troponin I. |
|||
Table 1: Univariate comparison between the non-severe and severe group
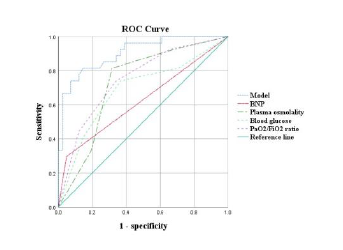
All variables with P < 0.1 were entered into a backward stepwise multivariate binary logistic regression model, and the last step was to obtain four independent risk factors of BNP (Odds ratio [95%CI] 12.307[1.349-112.251]; P=0.026), plasma osmolality (0.345[0.169-0.705];P=0.004), blood glucose (2.262[1.158-4.422];P=0.017) and PaO2/FiO2 ratio (0.267[0.124-0.577];P=0.001) (Table 2,3). Goodness of fit testing (Hosmer-Lemeshow test) was used to assess deviations between observed and expected values. A P value of > 0.05 implies no significant difference between the observed and expected values. The P value of the goodness of fit testing of our model was 0.739, and therefore it was acceptable (Figure 1). Analysis of the AUC of the ROC curve was constructed to assess the predicting performance. The AUC of model was 0.904 ([95%CI] [0.832-0.976];P<0.001), with excellent performance. The sensitivity, specificity, PPV, NPV and accuracy of predicting were 78.6%, 87.5%, 81.5%, 85.4% and 83.8%, respectively. BNP, plasma osmolality, blood glucose or PaO2/FiO2 ratio as single predictors, their optimal cut-offs were positive, 285 mOsm/L, 6.0 mmol/L and 350 mmHg, respectively; AUCs were 0.624, 0.726, 0.687 and 0.75, respectively; sensitivities were 80.0%, 66.7%, 53.8% and 59.1%, respectively; specificities were 67.2%, 85.7%, 79.3% and 69.6%, respectively; PPVs were 29.6%, 81.5%, 77.8% and 48.1%, respectively; NPVs were 95.1%, 73.2%, 56.1% and 78.1%, respectively; accuracies were 69.1%, 76.5%, 64.7% and 66.2%, respectively (Table 4).
|
Items |
P |
B |
Odds ratio |
95%CI |
|
BNP |
0.026 |
2.51 |
12.307 |
1.349-112.251 |
|
Plasma osmolality |
0.004 |
-1.064 |
0.345 |
0.169-0.705 |
|
Blood glucose |
0.017 |
0.816 |
2.262 |
1.158-4.422 |
|
PaO2/FiO2 ratio |
0.001 |
-1.319 |
0.267 |
0.124-0.577 |
|
Abbreviations: BNP: B-type natriuretic peptide; CI: confidence interval; COVID-19: coronavirus disease 2019; FiO2: fraction of inspired oxygen; PaO2: arterial oxygen partial pressure. |
||||
Table 2: Independent risk factors for severe COVID-19 patients with hypertension.
|
Items |
AUC |
SE |
P |
95%CI |
|
Model |
0.904 |
0.037 |
<0.001 |
0.832-0.976 |
|
BNP |
0.624 |
0.072 |
0.086 |
0.482-0.765 |
|
Plasma osmolality |
0.726 |
0.063 |
0.002 |
0.603-0.85 |
|
Blood glucose |
0.687 |
0.069 |
0.009 |
0.553-0.821 |
|
PaO2/FiO2 ratio |
0.75 |
0.06 |
0.001 |
0.632-0.869 |
|
Abbreviations: AUC: area under curve; BNP: B-type natriuretic peptide; CI: confidence interval; COVID-19: coronavirus disease 2019; FiO2: fraction of inspired oxygen; PaO2: arterial oxygen partial pressure; SE: standard error. |
||||
Table 3: Predicting performance for severe COVID-19 patients with hypertension.
|
Items |
Cut-off |
Sensitivity |
Specificity |
PPV |
NPV |
Accuracy |
|
Model |
N/A |
78.60% |
87.50% |
81.50% |
85.40% |
83.80% |
|
BNP |
positive |
80.00% |
67.20% |
29.60% |
95.10% |
69.10% |
|
Plasma osmolality |
285 mOsm/L |
66.70% |
85.70% |
81.50% |
73.20% |
76.50% |
|
Blood glucose |
6.1 mmol/L |
53.80% |
79.30% |
77.80% |
56.10% |
64.70% |
|
PaO2/FiO2 ratio |
350 mmHg |
59.10% |
69.60% |
48.10% |
78.10% |
66.20% |
|
Abbreviations: BNP: B-type natriuretic peptide; COVID-19: coronavirus disease 2019; FiO2: fraction of inspired oxygen; NPV: negative predictive value; PaO2: arterial oxygen partial pressure; PPV: positive predictive value. |
||||||
Table 4: Predicting characteristics of risk factors for sereve COVID-19 patients with hypertension
Figure 1: The P value of the goodness of fit testing of our model was 0.739. The area under receiver operating characteristic curve was 0.904, with excellent performance. The sensitivity, specificity, positive and negative predictive value, and accuracy of predicting were 78.6%, 87.5%, 81.5%, 85.4% and 83.8%, respectively.
5. Discussion
The pattern of lung injury caused by SARS-CoV-2 is called diffuse alveolar injury, which is a histological change associated with ARDS [10,11]. Undoubtedly, systemic water and sodium retention and reduced osmolality will lead to edema and exudation of alveolar epithelial cells, alveolar collapse, and oxygenation dysfunction. The body's water and sodium metabolism balance and osmolality regulation are mainly through the following three neuroendocrine systems: (a) RAS. (b) Antidiuretic Hormone (ADH). (c) Natriuretic peptide. Notably, SARS-CoV-associated pneumonia can aggravate water and sodium retention and osmolality reduction. The possible pathogenesis are as follows: (a) ACE2 is mainly produced in type II alveolar epithelial cells. The damage associated with SARS-CoV infection seriously impairs the production of ACE2. Down-regulated ACE2 can result in excessive activation of RAS and promoting the reabsorption of water and sodium from the kidney [12,13]. (b) SARS-CoV-associated pneumonia may be accompanied by excessive and inappropriate secretion of ADH [14,15]. In addition, accumulated angiotensin II can also promote secretion of ADH. The COVID-19 cases with hypertension we observed generally had different levels of decreased plasma osmolality, sodium and PaO2/FiO2 ratio, and elevated BNP at the beginning of admission. This suggests that water and sodium retention, osmolality regulation disorder and diffuse alveolar injury may occur in the early stage of COVID-19. Compared with the non-severe group, the severe group had lower plasma osmolality and higher BNP. These results indicate the imbalance of water and sodium metabolism and osmolality regulation disorder may be one of the initiating factors and play a key role in the development of ARDS; and elevated BNP may be a passive secondary change.
Until now, there are accumulating evidences that diabetes is closely correlated with increased risk of COVID-19, as well as poor outcomes [16]. Our investigation found that there was no significant difference in the number of patients with diabetes but significantly higher blood glucose in the severe group compared with the non-severe group. This indicates that severe COVID-19 infection is significantly associated with increased blood glucose, which is consistent with related reports [17,18]. In addition to the effects of diabetes mellitus and adverse drug reaction (for instance, glucocorticoid induced hyperglycemia), insulin resistance and stress response may also be involved in the increased blood glucose in severe COVID-19 [19]. Furthermore, another research found SARS-CoV damaged the endocrine part of pancreas, indicating that SARS-CoV may cause acute insulin dependent diabetes mellitus [20]. These suggest that blood glucose should be strictly controlled for all COVID-19 patients during hospitalization to monitor the progress of illness and avoid aggravation regardless of whether they have diabetes mellitus or not [19]. RAS inhibitors are the first-line drugs widely used in patients with hypertension [21]. However, whether to continue to use RAS inhibitors in the setting of COVID-19 has caused widespread controversy [22,23]. The continuous management of RAS inhibitors may prevent excessive activation of RAS by preventing ACE2 down-regulation, which is beneficial to reduce the risk of ARDS [7,21]. In addition, most hypertension itself has high RAS activity. Both hypertension and pharmacological RAS inhibition will increase ACE2 levels, which may increase human susceptibility and theoretically promote SARS-CoV-2 invasion and infection proliferation [24,25]. This may be why COVID-19 patients with hypertension is more likely to increase severity.
In addition to traditional RAS inhibitors [7,21-25], some new drugs targeting ACE2 have appeared. Recombinant human ACE2 (rhACE2) protein may play an important role in protecting ARDS patients [26], and has achieved good therapeutic effects in animal models and has entered clinical trials [27,28]. The latest research data shows that recombinant human soluble (hrsACE2) can directly inhibit SARS-CoV-2 infection in engineered human blood vessel organoids and human kidney organoids, blocking the early stage of infection [29]. In theory, systemic administration of hrsACE2 can neutralize SARS-CoV-2 and avoid the damage of alveolar epithelial cells caused by direct virus invasion, which has significant clinical application value. Compared with RAS inhibitors, diuretics can not only reduce water and sodium retention to lower blood pressure, but also will not up-regulate ACE2 expression. More importantly, diuretics can be applied to COVID-19 patients who are not accompanied by underlying hypertension. Combined with our data analysis, targeting plasma osmolality, early use of diuretics may be a reasonable and effective treatment. In addition, tolvaptan as an ADH type-2 receptor antagonist [29], a diuretic that has been used clinically, may also have certain clinical application prospect. There are several limitations in our retrospective cohort study. First, due to the small sample size of the single-center research hospital, there may be sampling errors. Second, the patients may be in different stages of COVID-19 when they are admitted to the hospital. Third, the activity and concentration of RAS and ADH in plasma were not detected at the time of admission; comparison with COVID-19 patients without hypertension was also not performed. Therefore, these results should be carefully interpreted owing to potential selection bias and residual confounding. Large prospective randomized clinical trials in countries around the world may also be needed to provide further data support.
6. Conclusions
COVID-19 cases with hypertension generally have different levels of decreased plasma osmolality and PaO2/FiO2 ratio. Decreased plasma osmolality and PaO2/FiO2 ratio, elevated BNP and blood glucose may be the early risk factors for severe COVID-19 with hypertension. Targeting osmolality regulation, early use of diuretics may be a promising treatment.
7. Declarations
7.1. Ethics approval and consent to participate
The studies involving human participants were reviewed and approved by the Ethics Committee of The Third People's Hospital of Shenzhen (approval number: 2020-173). Permission in access to all raw data supporting the conclusions were granted by the Scientific Research Committee of The Third People's Hospital of Shenzhen. Written informed consent from the patients was not required to participate in this study in accordance with the national legislation and the institutional requirements. Verbal informed consent was obtained from all participants.
Consent for publication
Not Applicable. All data was recorded anonymously.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Funding
No funding was supported for this study. All authors have no financial relationships relevant to this article to disclose.
Authors' contribution
All authors have read and approved the final manuscript. YG was responsible for methodology, investigation, formal analysis, data curation, writing the original draft and visualization; AT for investigation, formal analysis, data curation; DP for conceptualization, investigation, review & editing, supervision. Both ZZ and HW worked on term, conceptualization, formal analysis, investigation, data curation, visualization.
Acknowledgements
Thanks for the approval of the Ethics Committee of The Third People's Hospital of Shenzhen. This manuscript has been released as a pre-print at [https://www.researchsquare.com/article/rs-31888/v1; DOI:10.21203/rs.3.rs-31888/v1] (Denggao Peng et al.)
References
- Wu C, Chen X, Cai Y, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med 180 (2020): 934-943.
- Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med 130 (2020): 304-309.
- Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395 (2020): 1054-1062.
- Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol 109 (2020): 531-538.
- South AM, Diz DI, Chappell MC. COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318 (2020): 1084-1090.
- Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579 (2020):270-273.
- Zhang P, Zhu L, Cai J, et al. Association of Inpatient Use of Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers With Mortality Among Patients With Hypertension Hospitalized With COVID-19. Circ Res 126 (2020): 1671-1681.
- Herrett E, Gadd S, Jackson R, et al. Eligibility and subsequent burden of cardiovascular disease of four strategies for blood pressure-lowering treatment: a retrospective cohort study. Lancet 394 (2019): 663-671.
- The guidelines for diagnosis and treatment of 2019-nCoV infected pneumonia (the sixth edition draft) issued by the National Health Commission of China In.
- Barton LM, Duval EJ, Stroberg E, et al. COVID-19 Autopsies, Oklahoma, USA. Am J Clin Pathol 153 (2020): 725-733.
- Adachi T, Chong JM, Nakajima N, et al. Clinicopathologic and Immunohistochemical Findings from Autopsy of Patient with COVID-19, Japan. Emerg Infect Dis 26 (2020): 2157-2161.
- Gagliardi I, Patella G, Michael A, et al. COVID-19 and the Kidney: From Epidemiology to Clinical Practice. J Clin Med 9 (2020): 2506.
- Cheng H, Wang Y, Wang GQ. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J Med Virol 92 (2020): 726-730.
- Gemcioglu E, Karabuga B, Ercan A, et al. A case of Inappropriate Antidiuretic Hormone Secretion Syndrome Associated with COVID-19 Pneumonia. Acta Endocrinol (Buchar) 16 (2020): 110-111.
- Habib MB, Sardar S, Sajid J. Acute symptomatic hyponatremia in setting of SIADH as an isolated presentation of COVID-19. IDCases 21 (2020): 00859
- Katulanda P, Dissanayake HA, Ranathunga I, et al. Prevention and management of COVID-19 among patients with diabetes: an appraisal of the literature. Diabetologia 63 (2020): 1440-1452.
- Chen J, Wu C, Wang X, et al. The Impact of COVID-19 on Blood Glucose: A Systematic Review and Meta-Analysis. Front Endocrinol (Lausanne) 11 (2020): 574541.
- Gao Y, Li T, Han M, et al. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 92 (2020): 791-796.
- Wang A, Zhao W, Xu Z, et al. Timely blood glucose management for the outbreak of 2019 novel coronavirus disease (COVID-19) is urgently needed. Diabetes Res Clin Pract 162 (2020): 108118.
- Yang JK, Lin SS, Ji XJ, et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol 47 (2010): 193-199.
- Vaduganathan M, Vardeny O, Michel T, et al. Renin-Angiotensin-Aldosterone System Inhibitors in Patients with Covid-19. N Engl J Med 382 (2020): 1653-1659.
- Kuster GM, Pfister O, Burkard T, et al. SARS-CoV2: should inhibitors of the renin-angiotensin system be withdrawn in patients with COVID-19? Eur Heart J 41 (2020): 1801-1803.
- Rico-Mesa JS, White A, Anderson AS. Outcomes in Patients with COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep 22 (2020): 31.
- Esler M, Esler D. Can angiotensin receptor-blocking drugs perhaps be harmful in the COVID-19 pandemic? J Hypertens 38 (2020): 781-782.
- Mancia G, Rea F, Ludergnani M, et al. Renin-Angiotensin-Aldosterone System Blockers and the Risk of Covid-19. N Engl J Med 382 (2020): 2431-2440.
- Zhang H, Baker A. Recombinant human ACE2: acing out angiotensin II in ARDS therapy. Crit Care 21 (2017): 305.
- Zou Z, Yan Y, Shu Y, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun 5 (2014): 3594.
- Khan A, Benthin C, Zeno B, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care 21 (2017): 234.
- Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell 181 (2020): 905-913.
- Gunderson EG, Lillyblad MP, Fine M, et al. Tolvaptan for Volume Management in Heart Failure. Pharmacotherapy 39 (2019): 473-485.