Early prediction of Gestational Diabetes Mellitus: Evaluation of Fasting blood Sugar in the first Trimester of Pregnancy in Likasi in The Democratic Republic of the Congo
Article Information
Kabala Tshasuma Hénoch1,2, Cham Lubamba Chamy1, Nsambi Bulanda Joseph1, Mpoy Wembonyama Charles1
1Department of Gynecology and Obstetrics of the University of Lubumbashi, Democratic Republic of Congo
2Faculty of Medicine of the University of Likasi, Democratic Republic of Congo
*Corresponding author: Kabala Tshasuma Hénoch, Department of Gynecology and Obstetrics of the University of Lubumbashi, Democratic Republic of Congo. Email: henochkabala4@gmail.com
Received: 19 June 2024; Accepted: 26 June 2024; Published: 03 July 2024
Citation: Kabala Tshasuma Hénoch, Cham Lubamba Chamy, Nsambi Bulanda Joseph, Mpoy Wembonyama Charles. Early prediction of Gestational Diabetes Mellitus: Evaluation of fasting blood sugar in the first trimester of pregnancy in Likasi in The Democratic Republic of the Congo. Fortune Journal of Health Sciences. 7 (2024): 360-363.
View / Download Pdf Share at FacebookAbstract
Objective: To determine a glycemic threshold in the first trimester of pregnancy predictive of Gestational Diabetes Mellitus (GDM) in Likasi City in the Democratic Republic of the Congo.
Material and Methods: Prospective cohort study carried out in 5 hospital in Likasi. Fasting blood glucose was taken in the first trimester of pregnancy and orally induced hyperglycemia (OGTT) performed between the 24th and 28th weeks with application of the International Association of Diabetes and Pregnancy Study Group (IADPSG) criteria for the diagnosis of GDM. This was non-probability convenience sampling. ROC curve characteristics were performed with STATA 16 software to predict gestational diabetes.
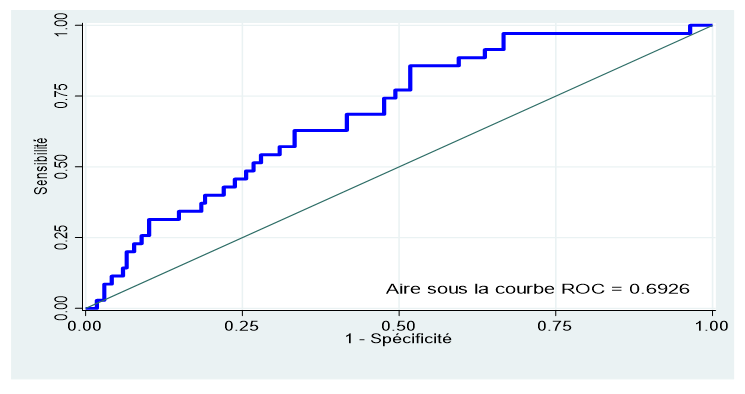
Result: The fasting glycemic threshold in the 1st trimester of pregnancy predictive of GDM was 81.5 mg/dl with an area under the ROC curve of 0.6926 (95% CI: 0.6034 - 0.7818), a sensitivity of 62, 86%, a specificity of 66.67%, and a negative predictive value (NPV) of 89.6%.
Conclusion: A simple and less expensive model based on fasting blood sugar levels could make it possible to early identify and monitor pregnant women at risk of developing GDM.
Keywords
Gestational diabetes mellitus, OGTT, fasting blood glucose in first trimester of pregnancy
Gestational diabetes mellitus articles, OGTT articles, fasting blood glucose in first trimester of pregnancy articles
Article Details
1. Introduction
Gestational diabetes mellitus (GDM) is a carbohydrate intolerance of varying severity that began or was first revealed during pregnancy, regardless of its evolution postpartum [1]. The work of the IADPSG (International Association of Diabetes and Pregnancy Study Group), on the HAPO (Hyperglycemia Adverse Pregnancy Out comes) 2008 study, made it possible to modify the screening and diagnosis criteria for gestational diabetes [2]. In addition to the Osullivan and Mahan test and the Carpenter and Coustan criteria, the IADPSG recommendations emerged in 2010 and were adopted by the WHO (World Health Organization) in 2013, JDS (Japan Diabetes Society) in 2013, ADIPS (Australasian Diabetes in Pregnancy Society) in 2014 and ADA (American Diabetes Association) in 2014. This was an Orally Induced Hyperglycemia (OGTT) with 75g of glucose, carried out between the 24th and the 28th week of amenorrhea. Pathological valuse were defined by venous blood glucose at Time Zero (T0) ≥ 92 mg/dl, at Time One (T1) ≥ 1.80 mg/dl and at Time Two (T2) ≥ 1.53 mg/dl. These thresholds were set based on maternal blood glucose levels measured between the 24th and 32nd weeks, associated with a 75% increased risk of fetal macrosomia, hyperinsulinism and fetal adiposity. With these new criteria, a single positive value confirms the diagnosis of GDM [2].
Controversy exists over the benefit of early identification of gestational diabetes. Some studies have linked early diagnosis of gestational diabetes to regression of complications such as polyhydramnios, prematurity and fetal macrosomia [3,4]. Some studies carried out recently around the world have found glycemic thresholds at an early stage of pregnancy, predictive of gestational diabetes. This could constitute a beneficial alternative to early detection of this pathology. In France a glycemic threshold predictive of GDM of 80 mg/dl was found [5]. This threshold was 82.8 mg/dl in China in 2017 and in Israel in 2010[6,7]. In Italy in 2013, it was 79.2 mg/dl [8]. In the Democratic Republic of Congo (DRC), in Haut Katanga and particularly in Likasi, studies on GDM prediction strategies are almost non-existent. The objective of this study was to determine a fasting glycemic threshold in the first trimester of pregnancy predictive of GDM.
2. Material And Method
This was a prospective observational cohort study carried out in five health structures in the city of Likasi, namely: SNCC Hospital of Likasi, SHEKINAH Medical Center, LA CONSOLATION Medical Center, ASVIE Medical Center and the University Hospital Center of Likasi. The city of Likasi covers an area of 245 km², and it has an estimated population size of 635,463 inhabitants [9]. This study extended over a period of 16 months, from December 1, 2020 to April 30, 2022. The study population consisted of all pregnant women who consulted the various health structures selected during the study period. The sampling was non-probabilistic for convenience, including all pregnant women encountered in the different health facilities in the first trimester of pregnancy and who were seen again between the 24th and 28th weeks; All pregnant women who were carrying pregnancies beyond 3 months at the first consultation, those who had multiple pregnancies and diabetics were excluded.
Summary of the study protocol
We measured fasting blood sugar levels in the 1st trimester (FBS1) of pregnancy in all pregnant women included in this work. When FBS was ≥ 126 mg/dl, the diagnosis of overt diabetes was made.
Those who had a FBS< 126mg/dl were divided into two groups:
- The FBS < 92mg/dl group was subjected to OGTT at 75g of sugar and the IADPSG criteria were applied for the diagnosis of GDM;
- The FBS ≥ 92mg/dl group, where a 2nd fasting blood sugar level was carried out between 24 and 28 weeks (FBS2):
- When the FBS2 was ≥ 92 mg/dl, the diagnosis of GDM was retained;
- When FBS2 was < 92 mg/dl, OGTT with 75g of sugar was performed according to the IADPSG criteria [2].
The sampling was carried out between 7 a.m. and 8 a.m. The pregnant woman, who had been fasting for at least 8 hours, was seated on a chair under sufficient lighting, after having placed a tourniquet on the forearm, and disinfected downstream using a swab soaked in denatured alcohol, 3ml of venous blood was taken and placed in a dry tube.
The sample was centrifuged for 5 minutes at 4000 revolutions per minute at the collection site. The removed serums were placed in the cryovials then stored in the refrigerator between 2 and 8°C for a maximum of 2 hours, before being transported, under a cold chain formed by a glacier, to the laboratory for direct analysis.
For the OGTT, three samples were taken:
- At T0: it was a FBS before ingestion of sugar;
- At T1: one hour after ingestion for 5 to 10 minutes, anhydrous glucose mixed with 0.5 ml of drinking water;
- At T2: two hours after the same ingestion.
Venous blood glucose analysis was performed with the CYANSmart Spectrophotometer. We insisted on regular checking of the analysis device with the control serum. Calibration of the machine was carried out every 6 months by the supplier. Statistical analysis was carried out with STATA 16 software. We evaluated the predictive performance of FBS1, using the area under the ROC curve with its 95% confidence interval. Delong test was used to compare the areas under the ROC curve. Negative (VR-) and positive (VR+) resemblance values, sensitivity and specificity were calculated concomitantly with the other predictive values for FBS1. We submitted this project to the ethics committee of the University of Lubumbashi, and obtained its approval and favorable opinion under the number UNILU/CEM/127/2022.
Results
Prevalence of gestational diabetes
Out of a total of 203 pregnant women who had performed the OGTT between 24 and 28 weeks, we found 35 cases of gestational diabetes, representing a prevalence of 17.24% (95% CI: 12.31%-23.14%).
Table I: Sociodemographic characteristics
|
Profession |
n |
% |
|
House wife |
138 |
67.98 |
|
Civil servant |
29 |
14.29 |
|
Shopkeeper |
25 |
12.32 |
|
Without profession |
2 |
0.98 |
|
Farmer |
9 |
4.43 |
|
Marital status |
n |
% |
|
Single |
3 |
1.48 |
|
Bride |
200 |
98.52 |
|
Maternal age (year) |
n |
% |
|
<20 |
6 |
2.96 |
|
20-34 |
155 |
76.35 |
|
≥35 |
42 |
20.69 |
|
Parity |
n |
% |
|
0 |
51 |
25.12 |
|
01 04 |
108 |
53.2 |
|
≥5 |
44 |
21.68 |
Sixty-seven point ninety-eight percent of pregnant women were housewives. Almost all of the pregnant women were married (98.52%). 76.35% of pregnant women had an age between 20 – 34 years old. The median age of pregnant women who developed gestational diabetes mellitus was 30 years (25-35). 53.20% of pregnant women had a parity between 1 – 4. The median parity of pregnant women who developed gestational diabetes mellitus was 3 (1 – 5).
Fasting glycemic threshold predicting gestational diabetes
The area under the ROC curve calculated with the Stata16 software was 0.6926 (95% CI: 0.6034 - 0.7818) for the predictive capacity of fasting blood glucose in the 1st trimester (figure 3). Based on this curve, the best cutoff for fasting blood glucose was ≥81.5 mg/dl with a sensitivity of 62.86% and a specificity of 66.67%, a positive resemblance value (VR+) of 1.88, a negative resemblance value (NRV-) of 0.56, a positive predictive value (PPV) of 28.2% and a negative predictive value (NPV) of 89.6%.
Discussion
We found that fasting blood glucose in the first trimester of pregnancy could be used to predict gestational diabetes in our setting. Referring to the ROC curve, the best glycemic threshold was 81.5 mg/dl with a sensitivity of 62.86% and a specificity of 66.67%. Several authors who used the IADPSG criteria found glycemic thresholds very close to ours, but with different sensitivities and specificities (table II). We believe that this variability could be linked to ethnic diversity, as well as the frequency of obesity and type 2 diabetes, which greatly influence the occurrence of gestational diabetes [10]. Lopez et al. had found a correlation between fasting blood sugar in the first trimester of pregnancy and the occurrence of gestational diabetes in Spain in 2018. However, their predictive threshold was quite high, i.e. 92 mg/dl with a sensitivity of 46.6% and a specificity of 88 .8% [11].
Table II : Glycemic thresholds predictive of gestational diabetes according to the authors
|
FBS1 threshold (mg/dl) |
Sensitivity (%) |
Sensitivity (%) |
Authors (Country) |
|
81.5 |
62.86 |
66.67 |
Kabala |
|
80 |
72.9 |
64.3 |
Kanoun et al. (France) [5] |
|
82.8 |
53.89 |
70.9 |
Min Hao et al. (China) [6] |
|
82.8 |
65.2 |
67.6 |
Riskin et al. (Israel) [7] |
|
81 |
64.29 |
56.45 |
Pingli et al. (China) [12] |
|
>90 |
86 |
52 |
Reshma et al. (India) [13] |
|
79.2 |
78 |
38 |
Wei Wei et al. (China) [14] |
|
79.2 |
80 |
66 |
Pintaudi et al. (Italy) [8] |
FBS1: Fasting blood sugar in the first trimester of pregnancy
Conclusion
Maternal and perinatal morbidity and mortality constitute one of the best indicators of the health status of a society and its degree of development. It is in this context that the studies on gestational diabetes in 5 health structures in Likasi aimed to determine the fasting glycemic threshold in the first trimester of pregnancy predictive of GDM in our environment. It results from this study that this glycemic threshold was ≥81.5 mg/dl.
Limit of the study
The results of our study focused on five medical structures in the city of Likasi during prenatal consultations. It would have been interesting to extend our study to other health structures in the city, in order to have more representativeness of the population.
Proposal
That a more representative study with a larger sample be conducted in the future in Likasi.
Conflict of interest:
none
References
- Organization WH. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. World health organization; (1999).
- AI Panel. International Association of Diabetes and Pregnancy Study Groups Recommendations on the Diagnosis and Classification of Hyperglycemia in Pregnancy. Diabetes Care. March 33 (2010): 676-682.
- Bartha JL, Martinez-Del-Fresno P, Comino-Delgado R. Early diagnosis of gestational diabetes mellitus and prevention of diabetes-related complications. European Journal of Obstetrics & Gynecology and Reproductive Biology 109 (2003): 41-44.
- Seshiah V, Cynthia A, Balaji V, Balaji MS, Ashalata S, Sheela R, et al. Detection and care of women with gestational diabetes mellitus from early weeks of pregnancy results in birth weight of newborn babies appropriate for gestational age. Diabetes research and clinical practice 80 (2008): 199-202.
- Kanoun D. Fasting blood sugar in the 1st trimester of pregnancy as a predictive factor of gestational diabetes. UNIVERSITY OF LIMOGES; 1983. Accessible on aurore.unilim.fr and consulted on October (2021).
- Hao M, Lin L. Fasting plasma glucose and body mass index during the first trimester of pregnancy as predictors of gestational diabetes mellitus in a Chinese population. Endocrine journal (2017): EJ16-0359.
- Riskin-Mashiah S, Damti A, Younes G, Auslender R. First trimester fasting hyperglycemia as a predictor for the development of gestational diabetes mellitus. European Journal of Obstetrics & Gynecology and Reproductive Biology 152 (2010): 163-167.
- Pintaudi B, Di Vieste G, Corrado F, Lucisano G, Pellegrini F, Giunta L, et al. Improvement of selective screening strategy for gestational diabetes through a more accurate definition of high-risk groups. European journal of endocrinology 170 (2013): 87-93.
- Development indicators analysis unit. Accessible at https://scholar.google.com and accessed (2022).
- Galtier F. Definitions, epidemiology, risk factors. Journal of Gynecology Obstetrics and Reproductive Biology 39 (2010): S144-S170.
- Lopez D, Val T. Fasting glucose in the first trimester: An initial approach to diagnosis of gestational diabetes. Endocrinology, Diabetes and Nutrition 66 (2018): 11-18.
- Li P, Lin S, Li L, Cui J, Zhou S, Fan J. First-trimester fasting plasma glucose as a predictor of gestational diabetes mellitus and the association with adverse pregnancy outcomes. Pakistan Journal of Medical Sciences 35 (2019): 95.
- Aravind RS, Maheshwari L, Chander A. Evaluation of first trimester fasting blood glucose as a predictor of gestational diabetes mellitus. Indian Journal of Obstetrics and Gynecology Research 4 (2017): 66-70.
- Zhu W wei, Yang H xia, Wei Y mei, Yan J, Wang Z lian, Li X lan, et al. Evaluation of the value of fasting plasma glucose in the first prenatal visit to diagnose gestational diabetes mellitus in China. Diabetes care 36 (2013): 586-590.