Dual-Task Performance Testing As an Indicator of Cognitive Deterioration in Parkinson's Disease: A Pilot Study
Article Information
Dalma Szögedi1†, Trevor W. Stone 2†, Elek Dinya 3 † , Judit Málly 4*†
1University of Physical Education, Budapest, Hungary
2Kennedy Institute of Rheumatology, University of Oxford
3Digital Health Department, University of Semmelweis, Budapest, Hungary
4Department of Rehabilitation, Institute of Neurorehabilitation, Sopron, Hungary
*Corresponding Author: Judit Málly, Department of Rehabilitation, Institute of Neurorehabilitation, Sopron, Hungary
Received: 26 July 2023; Accepted: 04 August 2023; Published: 14 August 2023
Citation: Dalma Szögedi, Trevor W. Stone, Elek Dinya , Judit Málly. Dual-task performance testing as an indicator of cognitive deterioration in Parkinson's disease: A pilot study. Journal of Psychiatry and Psychiatric Disorders. 7 (2023): 104-117
View / Download Pdf Share at FacebookAbstract
Introduction: The reaction times of Parkinson's disease (PD) patients in dual-task accuracy tests depend on their cognitive ability.
Objective: The non-disabled PD patients’ cognitive ability assessed by dual-task tests deteriorates and is recovered by a short training with dual-task activity. Dual-task training was found to produce improvement sustained for several months in the cognitive function of PD patients.
Method: Forty-six PD patients were compared with 47 age matched healthy controls and 26 patients were followed for one year. Five dual-task tests consisting of a primary cognitive task performed simultaneously with a secondary motor task were repeated for five consecutive days. Testing was repeated after 6 and 12 months. Participants? reaction times, number of Hits and Misses, and other cognitive and motor tests were quantified.
Results: In the initial tests slower reaction times, fewer Hits and more Misses were indicated in the patients? group especially over 65 years, while the other cognitive and movement tests were similar to the normal controls. The decayed cognition of the early PD patients was primarily characterized by the increased number of Misses. The dual-task performances were significantly improved by the training within 3 days, except for Misses. No deterioration up to 6 months was observed.
Conclusion: It is concluded that dual-tasks are objective and sensitive tests reflecting the global cognitive deterioration arising before the appearance of clinical symptoms when the movement is unaffected. Dual-task testing is recommended as an objective measure of cognitive function and as training to reduce cognitive deterioration
Keywords
Parkinson’s disease; Cognitive training; Dual-task testing; Attention; Executive function; Rehabilitation
Parkinson?s disease articles; Cognitive training articles; Dual-task testing articles; Attention articles; Executive function articles; Rehabilitation articles
Parkinson’s disease articles Parkinson’s disease Research articles Parkinson’s disease review articles Parkinson’s disease PubMed articles Parkinson’s disease PubMed Central articles Parkinson’s disease 2023 articles Parkinson’s disease 2024 articles Parkinson’s disease Scopus articles Parkinson’s disease impact factor journals Parkinson’s disease Scopus journals Parkinson’s disease PubMed journals Parkinson’s disease medical journals Parkinson’s disease free journals Parkinson’s disease best journals Parkinson’s disease top journals Parkinson’s disease free medical journals Parkinson’s disease famous journals Parkinson’s disease Google Scholar indexed journals Cognitive training articles Cognitive training Research articles Cognitive training review articles Cognitive training PubMed articles Cognitive training PubMed Central articles Cognitive training 2023 articles Cognitive training 2024 articles Cognitive training Scopus articles Cognitive training impact factor journals Cognitive training Scopus journals Cognitive training PubMed journals Cognitive training medical journals Cognitive training free journals Cognitive training best journals Cognitive training top journals Cognitive training free medical journals Cognitive training famous journals Cognitive training Google Scholar indexed journals Dual-task testing articles Dual-task testing Research articles Dual-task testing review articles Dual-task testing PubMed articles Dual-task testing PubMed Central articles Dual-task testing 2023 articles Dual-task testing 2024 articles Dual-task testing Scopus articles Dual-task testing impact factor journals Dual-task testing Scopus journals Dual-task testing PubMed journals Dual-task testing medical journals Dual-task testing free journals Dual-task testing best journals Dual-task testing top journals Dual-task testing free medical journals Dual-task testing famous journals Dual-task testing Google Scholar indexed journals Attention articles Attention Research articles Attention review articles Attention PubMed articles Attention PubMed Central articles Attention 2023 articles Attention 2024 articles Attention Scopus articles Attention impact factor journals Attention Scopus journals Attention PubMed journals Attention medical journals Attention free journals Attention best journals Attention top journals Attention free medical journals Attention famous journals Attention Google Scholar indexed journals Executive function articles Executive function Research articles Executive function review articles Executive function PubMed articles Executive function PubMed Central articles Executive function 2023 articles Executive function 2024 articles Executive function Scopus articles Executive function impact factor journals Executive function Scopus journals Executive function PubMed journals Executive function medical journals Executive function free journals Executive function best journals Executive function top journals Executive function free medical journals Executive function famous journals Executive function Google Scholar indexed journals Rehabilitation articles Rehabilitation Research articles Rehabilitation review articles Rehabilitation PubMed articles Rehabilitation PubMed Central articles Rehabilitation 2023 articles Rehabilitation 2024 articles Rehabilitation Scopus articles Rehabilitation impact factor journals Rehabilitation Scopus journals Rehabilitation PubMed journals Rehabilitation medical journals Rehabilitation free journals Rehabilitation best journals Rehabilitation top journals Rehabilitation free medical journals Rehabilitation famous journals Rehabilitation Google Scholar indexed journals bradykinesia articles bradykinesia Research articles bradykinesia review articles bradykinesia PubMed articles bradykinesia PubMed Central articles bradykinesia 2023 articles bradykinesia 2024 articles bradykinesia Scopus articles bradykinesia impact factor journals bradykinesia Scopus journals bradykinesia PubMed journals bradykinesia medical journals bradykinesia free journals bradykinesia best journals bradykinesia top journals bradykinesia free medical journals bradykinesia famous journals bradykinesia Google Scholar indexed journals disorders articles disorders Research articles disorders review articles disorders PubMed articles disorders PubMed Central articles disorders 2023 articles disorders 2024 articles disorders Scopus articles disorders impact factor journals disorders Scopus journals disorders PubMed journals disorders medical journals disorders free journals disorders best journals disorders top journals disorders free medical journals disorders famous journals disorders Google Scholar indexed journals dementia articles dementia Research articles dementia review articles dementia PubMed articles dementia PubMed Central articles dementia 2023 articles dementia 2024 articles dementia Scopus articles dementia impact factor journals dementia Scopus journals dementia PubMed journals dementia medical journals dementia free journals dementia best journals dementia top journals dementia free medical journals dementia famous journals dementia Google Scholar indexed journals Neurorehabilitation articles Neurorehabilitation Research articles Neurorehabilitation review articles Neurorehabilitation PubMed articles Neurorehabilitation PubMed Central articles Neurorehabilitation 2023 articles Neurorehabilitation 2024 articles Neurorehabilitation Scopus articles Neurorehabilitation impact factor journals Neurorehabilitation Scopus journals Neurorehabilitation PubMed journals Neurorehabilitation medical journals Neurorehabilitation free journals Neurorehabilitation best journals Neurorehabilitation top journals Neurorehabilitation free medical journals Neurorehabilitation famous journals Neurorehabilitation Google Scholar indexed journals
Article Details
1. Introduction
Parkinson’s Disease (PD) has been primarily considered to be a disorder of motor function, notably affecting gait and balance performance largely as a result of striatal dopaminergic dysfunction or an imbalance in the dopaminergic and cholinergic pathways [1-3]. However, it has become clear that in many, probably most, patients the progressive neuronal deterioration and loss is accompanied by psychological and psychiatric issues. Prominent among these is the presence of cognitive dysfunction [4-7]. Whether part of this decline is related to normal deterioration with age is an issue under discussion [8] but there is no doubt about the existence of primary links with PD pathology. Indeed, some aspects of cognitive deterioration have been demonstrated in other neurological disorders including traumatic brain injury and epilepsy in addition to PD [9], and may indicate that cognitive deficits are likely to be associated with any condition in which neuronal loss or damage have occurred. The cognitive loss can clearly be an additional, major source of difficulty for patients and their careers and therefore demands therapeutic efforts to minimize it, but at present there are no reliable methods of preventing or slowing cognitive decline. More information is being accumulated on the neurochemical factors involved in cognitive dysfunction [10.11], with suggestions of potential pharmacological and metabolic treatments. For example, there are deficits in glutamatergic neurotransmission and possible links with glucose metabolism which could be targeted pharmacologically [12, 13]. At a more fundamental level, deficits have been found in the expression of growth factors such as glial cell derived growth factor, leading to a reduction in dopaminergic transmission [14]. A range of pharmacological options have been proposed and discussed for their psychiatric use in PD, but with the recognition that none have been able to convincingly or reliably hinder the development of cognitive dysfunction [15]. Since these approaches currently have little impact on cognition in PD, better ways are needed to promote the halt of cognitive dysfunction and facilitate rehabilitation. Several commentators have detailed the need for further studies, agreeing that cognition is indeed a significant problem in patients with PD, but that it is amenable to treatment [16]. Potential treatments include the use of dual-task exercises [17]. These are tasks involving the simultaneous use of two distinct areas of brain activity such as, in this study, a motor task and a cognitive task. Compared with performance on single tasks, the distractive influence of a second task places a greater demand on the first [18]. Thus, the cognitive activity is being challenged more than in a single task test, potentially improving it or slowing its decline. The advantages of dual-task training are supported by the fact that there are mutual, bi-directional influences between motor control and cognition, such that improving one may be beneficial to improvements in both. This is also to be expected since, despite some variations, cognition is correlated with aspects of motor function [19-21], with a decline in executive function showing the highest association [22]. Conversely, the strongly positive effect of improving cognition on motor performance has also been reported [19]. It is well established that even focused, single task physical activity itself can stabilize or improve the motor deficits in PD [23] and there are several previous studies on the effect of dual-task training in PD, with the emphasis on improving gait, balance or other motor functions, which show significant functional improvements [18, 24, 25]. In some cases, cognitive ability has also been assessed but with relatively unclear or inconsistent results [26-30] although the ability of dual-task training to improve both motor performance and aspects of cognitive function has been presented [31]. The variability in results may be related to the fundamental differences between studies, as in the focus on limited, specific tasks, or in the choice of treatment duration.
The present study has therefore been designed with features which address this question. Firstly, a battery of cognitive tasks has been employed, so that overall performance can be judged across a range of cognitive demands. Secondly, we have not just performed testing on single days, but have repeated testing on up to five consecutive days, to assess a possible synergistic, ‘wind-up’ of performance, or a more effective consolidation of the neuronal changes. Thirdly, patients have been re-tested at six months and 12 months after the training period, to assess the persistence of improvement. The overall objective is to determine whether an extended period of dual task training can produce a lasting improvement in cognitive function and whether that improvement is sustainable for several months. Since several variables are being examined, this work can only be regarded as a pilot study. However, as the results show a very encouraging, clear increase in cognitive ability lasting up to one year after one week training, it is hoped that more detailed and sophisticated studies will be performed in the future.
2. Materials and Methods
2.1 Ethics
Permission for the present trial was provided by the Regional Ethics Committee of the Petz Aladár County Hospital in Gyor, Hungary,(The number of permission: 76-1- 6/2019). A written informed consent at the onset of the trials according to the Helsinki Declaration was provided by each patient. The study was listed on the ISRCTN registry with study ID ISRCTN49538525 (www- isrctn.com/ISRCTN49538525). The concept of the study was arranged by JM and the delivery of the protocol was supervised by an independent committee. The recruitment was started in June of 2019 and the study was finished in December 2021.
2.2 Study Design
The first part of this work was a comparative study with age-matched healthy, control subjects. The second part was a self-controlled study, where each individual participated as their own control. The 5-day, 6-month and 1-year test values were compared with the baseline values. The study design is shown in Figure 1 (Figure 1).
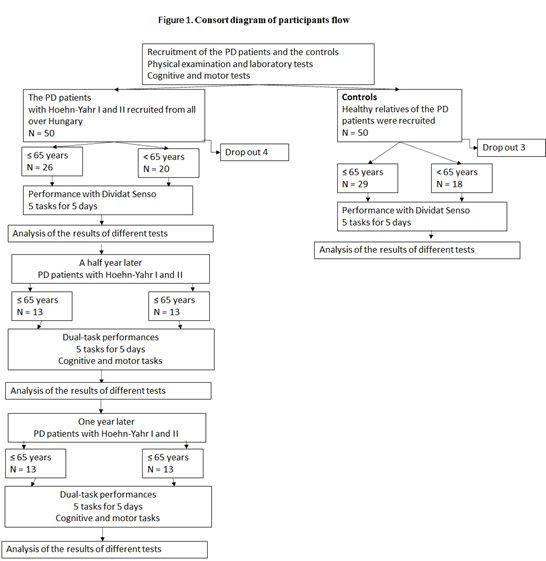
Figure 1: Consort diagram of participants’ flow. The diagram shows the interventions and the changes in the number of participants in both groups. The study focused on the maintenance of the improved global cognitive function of the PD patients in a follow-up study.
2.3 Participants
Participants were enrolled in the study after coming from different regions of Hungary and visiting the recruiting ambulance of the Institute of Neurorehabilitation (Sopron, Hungary). The study was not randomized statistically but involved sequential recruitment of patients from around Hungary. All of the examinations, and the tests were carried out by the staff of the Institute. Healthy relatives of the PD patients were asked to take part in this study as controls. Fifty patients with Parkinson’s disease and 50 age-matched healthy controls were recruited. The number of participants was considered large enough to draw initial, provisional conclusions, because they were selected according to the severity of their disease and were not based on inter-personal comparisons Four patients and 3 controls dropped out because of their business schedule (Figure 1). Thus, 46 patients with PD (F/M:23/23) were compared with 47 (F/M: 26/21) age-matched healthy controls. This number of participants has been generally used for studying patients with a larger range of disability at Hoehn-Yahr I- III but in this study, patients were rigorously selected according to a narrow range of disability (Hoehn-Yahr I and II), where the motor disability was negligible. The inclusion criteria were based on the UK PD Brain Bank, as follows: - (a) the presence of Parkinson’s disease responding well to levodopa; (b) no evidence of dementia or minimal mental impairment according to different cognitive tests (c) no other chronic disease. Patients with PD in H-Y I and II without any functional deterioration were included in this study although some patients. had a slight tremor and bradykinesia. Two independent neurologists, specialized in movement disorders, made the decision according to Hoehn-Yahr stages and UPDRS for the inclusion of the patients. The control subjects were selected from the relatives of the patients and from the staff of the Institute of Neurorehabilitation. The subjects were divided into two groups according to their age (under and over 65 years) (Table1). Patients were given levodopa retard at a low dose.
Table 1: The demographic data of the comparative study with dual-task tests
|
Control |
Parkinson's |
Control |
Parkinson's |
|
|
≤ 65 years |
> 65 years |
|||
|
Number |
29 |
26 |
18 |
20 |
|
Age (yrs) |
54.6 ± 8.8 |
58.3 ± 7.1 |
68.1 ± 2.6 |
71.5 ± 4.1 |
|
Female; male |
16; 13 |
14; 12 |
10; 8 |
9; 11 |
|
Duration of disease (yrs) |
5.6 ± 3.0 |
5.8 ± 3.5 |
||
|
H-Y stages |
1.5 ± 0.55 |
1.7 ± 0.58 |
||
|
UPDRS total. |
21.94 ± 11.46 |
23.2 ± 7.1 |
||
|
T/H |
15-Nov |
11-Sep |
||
|
Dose of levodopa (mg/d) |
357.8 ± 161.8 |
320.5 ± 182.0 |
||
Table 1 shows the demographic data. The controls and the patients with Parkinson’s disease were divided into two groups according to their ages (≤ 65 years and > 65 years). There was no significant difference between their duration of disease (p = 0.9110), dose of levodopa (p = 0.3805), total score of UPDRS (p = 0.6339), Hoehn-Yahr stage (H-Y stage) (p = 0.2766). T = tremor H = hypokinesia. Twenty-six patients with PD (N = 13 ≤ 65 years, N = 13 > 65 years) were followed for one year. The reduction in the number of patients compared to the initial number was due to the distance of their dwelling place and their busy schedule and the fact that participation was voluntary, with no pressure for follow-up. Although the number of cases in the study was lower, similar scientific parameters were shown in this group and in the initial group. As for the p values, no difference could be seen between the initial and the followed-up group. In the severity in UPDRS total between the initial group (I) ≤ 65 years and followed patients (F) ≤ 65 years (p = 0.4681) and H-Y stages in I group ≤ 65 years and F group ≤ 65 years (p = 0.3308) no significant difference could be seen between the groups. There was no significant difference in UPDRS total (p = 0.7366) and in H-Y (p= 0.6545) above 65 years. Their detailed demographic data are involved in Table 2.
Table 2: Demographic data of patients with Parkinson’s disease included in this follow-up study.
|
≤ 65 YEARS PD |
> 65 YEARS PD |
|||||
|
Baseline |
Half year |
One year |
Baseline |
Half year |
One year |
|
|
Number of patients |
13 |
13 |
13 |
13 |
13 |
13 |
|
Age |
57.6 ± 7.5 |
71.3 ± 3.5 |
||||
|
Duration of the disease |
4.8 ± 2.7 |
5.6 ± 3.6 |
||||
|
UPDRS total |
18.9 ± 11.0 |
20.3 ± 12.9 |
21.0 ± 11.4 |
21.3 ± 7.3 |
20.4 ± 10.5 |
21.4 ± 9.6 |
|
H-Y stages |
1.38 ± 0.5 |
2.00 ± 0.7 |
1.96 ± 0.66 |
1.65 ± 0.55 |
1.69 ± 0.59 |
1.80 ± 0.63 |
|
T/H |
07-Jun |
06-Jul |
||||
|
Dose of levotopa (mg/day) |
315.3 ± 158.6 |
376.9 ± 183.2 |
369.2 ± 201.5 |
269.2 ± 163.9 |
284.6 ± 189.7 |
288.4 ± 188.3 |
Table 2 shows the demographic data of patients with Parkinson’s disease (PD) (N = 26). Comparing patients ≤ 65 years and > 65 years in the categories of “Baseline (B)”, “Half year (H)” and “One year (O)” time points, there were no significant differences in the duration of disease (p = 0.5302), total score of Unified Parkinson Disability Rating Scale (UPDRS) (B: p = 0.5719; H: 0.9708; O: 0.9272), the dose of levodopa (B: p = 0.5203;H: 0.2018;O: 0.2629) and Hoehn-Yahr stages (H-Y stages) (B: p = 0.2676; H: 0.2059; O: 0.5248). T = tremor H = hypokinesia.
2.4 Assessments
Dual-tasks performances were examined using Dividat Senso equipment (HUR, Finland), with subjects standing wearing no-slip socks on a glass platform (106 x 106 cm) overlying 20 force sensors. The platform was surrounded on three sides by a railing that could be held on to. The person stood in the middle of the platform. The force sensors made it possible to detect the sole pressure. A monitor was placed at 104 cm from the floor. The diameter of the monitor was 108 cm. A cognitive task was combined with a motor activity task in each test period, with subjects asked to focus on a game presented on a visual monitor. For the motor task, patients were asked to detect an object appearing at one edge (top, bottom, right or left) of the screen and were required to react immediately using leg movements. Focusing on the cognitive tasks, involving attention and decision-making, the patients were required to make four-way leg movements. Five dual-task tests were applied. The tasks were selected in such a way as to include a visual, auditory perception, and an abstraction task. For cognitive testing, at first a ‘Simple’ task was used, where red spots were shown at different positions. It was the easiest game, where the participants could learn the process of the activity. A ‘Bird’ task was performed in which a bird had to be selected from different colored figures. In this game the participants’ ability to differentiate between similar forms and colors was detected. In the game ‘Divided’, red spots were interrupted with high and low-pitched sounds. The difficulty of the task was that the participants were required to make quick changes between the visual and auditory impulses and to react for them with leg movements. In the game ‘Habitat’ four different animals had to be allocated to their appropriate living area. If the animal was not in its right living area, the patient had to take a step. This negative reply was the most difficult task. In the game of ‘Target’, black bullets were moving with different speeds around on the monitor, and when they reached the target the subject was required to make a step. The number of correct (Hits) and incorrect (Misses) responses were recorded. Dual-task interactions, namely Simple, Bird, Divided and Target were quantified by the average reaction times appearing immediately on the screen at the end of the game. All figures randomly appeared on the screen to disclose the automatic movement. The tasks lasted for one and a half minutes and were repeated each day for five consecutive days. The training with these dual-task performances were repeated after 6 and 12 months. The following traditional tests were also applied; the Mini Mental Rating Scale (32), the Ziehen Ranschburg Word Pair Test, the Trail Making Test (33), the Clock Drawing Test (34), and the Hamilton Depression Scale
Tests (35). For the detection and quantitation of Parkinsonian symptoms the Hoehn- Yahr Stages were used (36) together with the Unified Parkinson Disability Rating Scale (37). Walking ability was measured as distance walked in 6 mins (in m), and time taken to walk 10 m (in sec). The walking tests were performed on the first and fifth days of training. The test took a total of one hour. The cognitive test was administered by a psychologist, and the dual-task test with Dividat Senso were controlled by a physiotherapist. The tests took a total of one hour. The mathematical statistical analysis was made by a mathematician. Participants had a good compliance, because performance was enhanced by the competitive nature of the tests.
2.4 Statistical analysis
Results are expressed as the mean ± standard deviation of mean and sample size for each age group with Parkinson. The normality of data was checked by applying the Shapiro-Wilk’s test and the homogeneity of variances was assessed through the Levene’s test. For baseline values, we performed the necessary statistical analysis with the nonparametric Mann-Whitney test to determine significant differences for the PD age groups examined (< 65, > 65 years), but no significant differences were found. The means of different date (baseline, half year, one year data) were compared by nonparametric Friedman ANOVA, significance values have been adjusted by the Bonferroni correction for multiple tests. The analysis was two sided with a level of significance of α = 0.05. All statistical analyses were performed using the SAS 9.4 (SAS Institute Inc., Cary, NC, USA) software package.
3. Results
There was no difference in symptom severity or cognitive performance in PD patients under or over 65 years of age assessed by UPDRS total, H-Y stages and cognitive tests (Table 1). However, there was a significant difference between the age matched healthy controls and the patients with PD ≤ 65 years in their dual-task tests of Bird (p < 0.001), Divided (p < 0.05) and Misses (p < 0.05) (Figure 2).
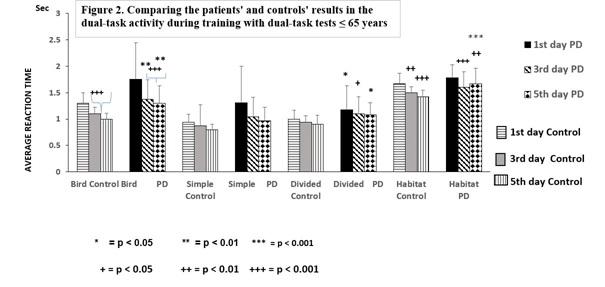
Figure 2. Comparing the patients’ and the controls' results during training with dual- task tests ≤ 65 years Figure 2 demonstrates the differences between the controls and the patients with Parkinson’s disease (PD) ≤ 65 years during the training with dual- task tests for 5 days. The asterisks (*) indicate significant difference when controls were compared with PD patients ≤ 65 years on the first, third and fifth days. A remarkable delay in reaction time was observed in the ‘Bird’ test and in the difference between results of first days and the others. Columns represent the mean ± S.D. The columns were labeled as follows: 1st days with horizontal stripes, control 3rd days with gray, control 5th days with vertical lines, results of PD patients on the 1st days with black, 3rd days with oblique striped lines, and values on the 5th days with dotted columns.
There was a significant difference between the two Parkinsonian groups in the dual-task activities to the detriment of the older group of patients, namely: Bird (p < 0.05), Habitat (p < 0.05), Hits (p < 0.05), Misses (p < 0.05) (Figure 2 and 3), although the controls exhibited age-related differences in the dual-task test of Bird (p < 0.05). Dual-task training effectively influenced the results of dual-task activities in controls and PD patients (Figure 2, 3).
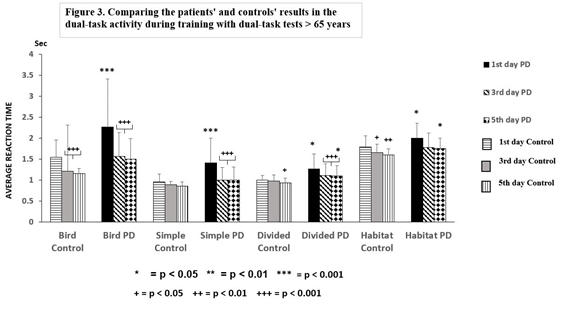
Figure 3. Comparing the patients’ and the controls' results during training with dual-task tests > 65 years Figure 3 shows the differences between the controls and patients with Parkinson’s disease (PD) > 65 years during training with dual-task tests for 5 days. The asterisks (*) indicate significant difference when controls were compared with PD patients ≤ 65 years on the first, third and fifth days. A notable delay in reaction time was observed in the ‘Bird’ test and between the results of first day and the others test. Columns represent the mean ± S.D. The columns were labeled as follows: 1st days with horizontal stripes, control 3rd days with gray, control 5th days with vertical lines, results of PD patients on the 1st days with black color, 3rd days with oblique striped lines, values on the 5th days with dotted columns.
Our data were analyzed in two ways, comparing the results on the first day to the 3rd and the 5th days (Figure 2, 3). In controls ≤ 65 years, the reaction times on dual-task Bird (p < 0.001), Divided (p < 0.01) and Habitat (p < 0.001) were decreased, and the Hits elevated (p < 0.001), but the Simple and Misses tests did not change on the 3rd day of training compared to the results on the 1st day (Figure 2). A similar improvement was detected on the 5th day of training.
PD patients ≤ 65 years showed reduced reaction times in Bird (p < 0.001), Simple (p ≤ 0.001) and Habitat (p < 0.01), but an elevation in Hits (p < 0.001) when results were taken on the 1st day, but the Divided and Misses tests showed no alteration during the training. Similar favorable changes were also obtained on the 5th day. Significant changes were noted with results on the 1st day, although they did not reach control values. The patients’ results on day 5 were significantly different from the controls, in the Bird (p ≤ 0.01), Divided (p < 0.05), Habitat (p < 0.001), and Misses (p < 0.05) paradigms (Figure 2). The number of Hits in the PD patients ≤ 65 years did not differ significant from the controls and the elevation of the number of Hits after training was remarkable elevation in both groups of participants (Figure 4).
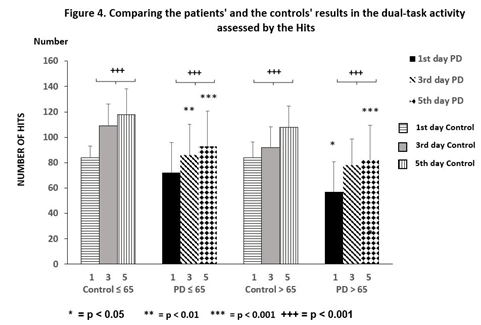
Figure 4 depicts the performances of training with the ‘Hits’ test for 5 days. There was no significant difference between the controls and the PD patients ≤ 65 years, although the PD patients > 65 years were significantly different from the controls (p < 0.05). While both groups’ performances increase significantly (mark +), the PD groups showed significantly less (*) achievement than the controls after training with dual-tasks. Columns represent the mean ± S.D. The columns were labeled as follows: 1st days with horizontal stripes, control 3rd days with gray, control 5th days with vertical lines, results of PD patients on the 1st days with black color, 3rd days with oblique striped lines, values on the 5th days with dotted columns.
Comparable results were obtained in participants > 65 years, but the amount of change during dual-task training was more impressive than in the age matched group under 65 years. Decreased reaction times were demonstrated in the controls > 65 years in dual task performances; Bird (p < 0.001), Divided (p < 0.01), Habitat (p <0.05), with an increase in the number of Hits (p < 0.001) on the 3rd day of training. The changes were preserved on the 5th day. The dual-task tests Simple and Misses did not change as in the younger control group of participants. The greatest changes were observed in the Parkinsonian patients > 65 years. All dual-task results were changed on the 3rd day compared to the 1st day (Figure 3), and these changes further increased on the 5th day after training (p < 0.001). However, the values of PD patients > 65 years remained elevated compared to the controls, namely: Divided (p < 0.05), Habitat (p < 0.05), on the 5th day of training. The number of Hits showed a significant elevation (p < during the training, but their number was less than the values of the controls (Figure 4). The number of Misses was also decreased in this group of patients, but the results were significantly higher at the end of training than that of the controls (p < 0.05).
3.1 Repetition after 6 and 12 months
The short-term dual-task training was repeated after 6 and 12 months. After 6 months, average reaction times on the first day were lower than the baseline, but only the reaction times of ‘Bird’ (p < 0.05) and ‘Simple’ (p < 0.01) were decreased significantly. After one year these changes further increased on the first day of training (p < 0.001) compared to the baseline (Figure 5).
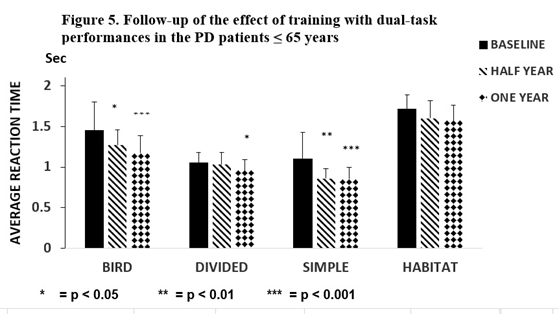
Figure 5. Follow-up of the effect of training in the PD ≤ 65 years Figure 5 represents the results of the follow-up study after training with dual-task activities in patients with Parkinson’s disease ≤ 65 years (N = 13), which was repeated a half year and one year later. After one half year the average reaction times on the first day were lower than that of the baseline, but only the reaction time in the ‘Simple’ (p < 0.01) and the Bird (p < 0.05) tests were statistically significant. The repetition of the dual-task activities after a half year led to a significant decrease in the average reaction time of ‘Bird’ (p < 0.001), ‘Divided’ p < 0.05), and ‘Simple’ p< 0.001). The columns represent the mean ± S.D: The black column indicates baseline values, the obliquely striped column shows the results after one half year, and the dotted column shows data after one year.
The number of ‘Hits’ was elevated (Baseline (B): 85.1 ±16.1; Half year (H): 99.5 ±16.5 p < 0.05; one year (O): 110.7 ±11.3 p < 0.001) in PD patients ≤ 65 years. No changes were noted in the 5-day dual-task tests of ‘Habitat’ and ‘Misses’. In our study, improvement was maintained in the PD patients > 65 years in ‘Bird’ and ‘Simple’ as in the younger group of patients, although with higher significance (p < 0.001) (Figure 6).
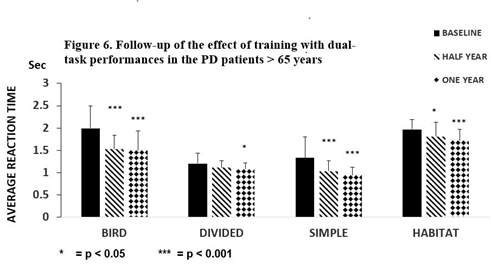
Figure 6. Follow-up of the effect of training with PD > 65 years Figure 6 depicts the results of the follow-up study after training with dual-task activities in the patients with Parkinson’s disease > 65 years (N = 13). Training for five days was repeated after one half and one year. The average reaction times on the first days were compared with baseline results. A remarkable decrease was detected in dual-task activity in ‘Bird’, ‘Simple’ and ‘Habitat’ tasks (p < 0.001) after a half year, but not for ‘Divided’ (p< 0.05). The columns represent the mean ± S.D. The black column is for the baseline, the striped column is for one half year later, and the dotted column is after one year.
A similar change was observed in Hits (B: 60.3 ±21.6, H: 83.9 ± 26.1 p < 0.001 O: 89.4 ± 16.2 p < 0.001). The dual-task Habitat was not altered in PD patients ≤ 65 years, but results were significantly attenuated in PD patients > 65 years compared to the baseline (half year:
p < 0.05, 12 months p < 0001) (Figure 6). PD patients > 65 years was the only group where the Misses decreased after dual-task training for five days and this favorable tendency was observed 6 and 12 months later (B: 23.3 ±13.1 H: 16.8 ± 5.5 p < 0.05, O: 11.8 ± 6.0, p < 0.001).
3.2 Walking tests detecting lower limbs’ bradykinesia
During the dual task tests the participants used their lower limbs, so we tested their limb movements when walking as an objective assessment suitable for future comparisons, in spite of UPDRS III, which mainly measured bradykinesia on upper limbs. PD patients were compared to age-matched controls in different walking tests to detect their degree of bradykinesia. Patients under 65 years were significantly different from healthy controls during a walking test for 6 min (Control (C): 614 ± 125 m PD: 452 ± 143 m (p < 0.01) or along a 10 m distance (C: 5.8 ± 0.54 sec, PD: 8.3 ± 3.8 sec p < 0.01). Patients > 65 years tended to be slower, although not significantly, to the 6 min criterion: (C: 482 ± 97 m, PD: 392 ± 147 m), or walking 10 m: (C: 6.6 ± 1.6 sec, PD: 8.8 ± 2.9 sec p < 0.01). There were no differences between the first and
fifth day of testing in the 6 min test, or between the 6 min and 10 m tests at 6 and 12 months after training.
3.3 Cognitive tests
There were no significant differences in the scores of patients and control subjects in either age group (over or under 65 years) on the Mini Mental Rating Scale, the Ziehen- Ranschburg Word Pair Test, the Clock Drawing Test, the Hamilton Depression Scale, and the Trail Making Test, neither after half a year nor after a year later.
4. Discussion
The efficacy of dual-task tests was demonstrated in this study in the assessment of cognitive decline in PD patients with H-Y I and II in an age-dependent way. The deterioration of dual-task performances was higher in the PD groups than in the age matched healthy controls, with the greatest decline in PD patients > 65 years. Training with dual-task performance tests for a short period had a rapidly and beneficial effect on the delayed reaction times and the number of Hits. However, the level of the control participants was never reached by the values of the patients. Dual-task training proved to be the most effective in patients > 65 years and the cognitive improvements were retained for at least 6 months. The increased number of Misses in the ‘Target’ test may be the most sensitive parameter for the assessment of cognitive decline. A delay in the reaction time is induced by the simultaneous performance of two tasks as opposed to single task activities, suggesting the interference of the attention and executive function in dual-task testing which are diminished in patients with PD (38 - 41, 25, 30). Recently, a report noted a significant decline in the performance of treadmill walking with visuomotor and cognitive game dual-tasks has been described (42), and illustrated by Johansson et al. (43). The influence of dual-task performance on cognitive function were considered only in a few studies including walking tests, with a strong focus on the spatiotemporal parameters of walking (44, 45). It has been reported that the walking time component
of a cognitive dual-task performance was influenced by practice, but not with a motor dual-task activity (46). In addition, the cognitive ability of PD patients was improved by cycling with cognitive tasks (47, 48). The motor achievement was improved after training for several weeks and the spatiotemporal walking parameters of PD patients
were influenced by training with dual-tasks after just one session (49). Although attention has been drawn to the cognitive difference between the H-Y stage II and III (50), and the differences between PD patients with and without minimal cognitive impairment (MCI) were stressed in a later publication (43). The view that dual-task activities are strongly influenced by the cognitive ability has been confirmed by several studies. In the light of these observations, the number of Hits and Misses was examined for the assessment of the patients’ global cognitive ability, clearly showing differences between the patients and the age-matched healthy controls. The increased reaction time delays in PD patients was indicated in several studies, although later stages of the disease were examined than in the present study (51 - 53). The alterations in cognition assessed by dual-task tests between PD groups at different ages were also observed in our study and are consistent with earlier work studying an older population (54, 55). It is suggested the cognitive deficits are detectable with dual-tasks in a younger age than previously assumed thought. It has been stated that cognitive impairment was apparent subjectively in 20 % of PD patients at the time of diagnosis (56), even when cognitive tests appeared to be within normal limits. The data were interpreted as indicating a subjective cognitive decline with no discussion of this paradox (57). Recently cognitive-motor interference was studied in patients following strokes, where the number of correct responses (NCR) was highly correlated with the walking distance, but no correlation was found with cognitive domains (58). This influence of dual-task training on post-stroke cognition has been supported by objective measurements of the rate of oxygen utilization in the frontal lobe (59, 60). This raises a critical issue in the rehabilitation of PD patients: there is an urgent need to detect cognitive impairment as early as possible, since it may predict the future decline of PD patients (61). At present a detailed cognitive testing is not applied routinely to PD patients, since the psychological tests of memory, executive function and attention are time consuming and need special requirements and dedicated staff. Dual-task performances can be performed relatively quickly and easily and can reveal much about the global cognition of patients. Here, a motor activity was employed as a part of the dual-task test, and no difference was demonstrable between the motor tests during the short training with dual-task activity. Nevertheless, the parameters of every dual –task test slightly or significantly improved after training, mainly in PD patients over 65. In accordance with the work of Johansson et al. (43), we concluded that bradykinesia did not play a role in the improved results after dual task training.
4.1 Duration of training
Previous studies of dual task training required extended periods of time with one or two occasion per week, for several weeks, to achieve improvement (28, 44 – 46, 62 - 68). One of our objectives was to determine whether shorter periods would be sufficient. One of our most striking observations was the rapidity of improvement after dual-task training. We hypothesize that rapid neural re-organization led to decreased interaction between the dual tasks, a view which would underline the importance of cognition in dual-task tests, consistent with the poorer performances seen even in the early stages of PD. On the other hand, not every test in the dual-task paradigm was improved by training. This might suggest that neural network organization is related to the nature of the dual-task performances, with some tasks requiring a higher level of cognitive input. The idea would be consistent with the greater improvement after training in PD patients > 65 years. The greater cognitive deficiency is present, the more defective is the dual task performance (> 65 years) and the greater potential for improvement can be seen after even a minimal training as it happened here. In our present study it was confirmed that the delay observed in dual-task activity depends on the modality of the dual-task tests (55, 56). Some dual task tests are better than others for generating cognitive improvements. One question to be addressed is whether a positive outcome in dual- tasks training can be transferred to other cognitive tasks. A few earlier studies of dual-task training focused on walking parameters, showing a slight, non-significant improvement in executive function assessed by the Trail Making Test in the DUALGAIT trial (28). Evidence suggests that not only the trained dual tasks are improved, but also non- trained dual-tasks such as the auditory Stroop test (the DUALITY trial) (62). A greater improvement in cognitive performance was achieved by the highly challenging tests, and it could be transferred to daily activities (44). The rapidity of cognitive improvement after training might indicate the general increase demanded in the exercise of attention, concentration and executive function necessary for the successful completion of a dual task session (70). However, a diversion of attention to irregularly changing objects our observations suggested. Our trial was conducted over several consecutive days, therefore it is possible that the novelty of those extraneous factors declined, allowing greater focus on the dual tasks.
4.2 Resumption of testing at 6 and 12 months
In the second part of this study, patients with PD were followed for one year to determine the sustainability of the response improvement. This represents the longest study using dual-task performances, since former studies generally followed the patients for only a few weeks after training (28, 44, 62, 64 – 68, 71). The transferred effectiveness of dual-task training has been detected in a few dual- task studies (48, 48). Our extended successful testing at 12 months provides a strong argument that patients can be provided with a prolonged period of enhanced cognitive function by a relatively transient training. Importantly, the reproducibility of these results across a 12 - month time span is demonstrated by these results.
4.3 Mechanisms
In spite of its effectiveness, the underlying central mechanisms of training by dual-tasktests remain unclear. The complexity of motor and cognitive networks during dual-task activities were confirmed by the comparison of mental parameters with the interaction of dual-task tests, although there is little association between them (46). Examination of functional networks relevant to dual-task activities was performed by functional MRI, showing positive activity in the precuneus nuclei of the parietal lobes during dual-task activities compared to that of single tasks (72). Furthermore, there was a shortage of activation in the right vermis of the cerebellum in PD patients, and this may be responsible for the integration of motor and cognitive networks (73). Some of these observations may be relevant to the present results. Overall, this study indicates the usefulness of dual-task tests in the detection of global cognitive decay in the early stage of PD, where other assays are normal. The cognitive deficit apparent in dual-task testing was rapidly improved by dual-task training, but not to the level of the controls. The cognitive improvement generated remained for at least 6 months.
4.4Limitations
The most important limitation of the study is the lack of classical randomization in the recruitment and treatment of patients and healthy subjects. Participants were recruited from the entire population of Hungary based on their ability and wish to attend our (mobile) recruiting office, while their relatives were used as controls. Although it was not a mathematically rigid randomization, however, a conventional analysis of the results generated statistically significant differences. It is hoped that this pilot study will therefore encourage others to expand the work in the continuing search for methods to promote cognitive function in PD patients. It should be noted that no distinction has been made between male and female participants in this study. We believe this aspect deserves to be the subject of a dedicated study in its own right in the future. It must be emphasised that the number of patients participating in this study is small, and no ‘a priori’ calculations of an ideal sample size were performed. Although the results show statistically significant differences between the study groups, therefore, it is not possible to extend our conclusions to a wider spectrum of patients. Nevertheless, we feel that the results are sufficiently encouraging – qualitatively – to indicate the potential importance and value of replication the study with larger cohorts.
5. Conclusion
Simultaneously performed dual task paradigms were used to monitor the cognitive functioning in PD patients. Considering the response delay and the increased number of Misses in the patients compared to the controls, a decline in global cognition was demonstrated in Hoehn-Yahr stages I and II, mainly in patients above 65 years of age (Tables 3 and 4). Cognitive decline assessed by dual-task performances coincided with the appearance of movement difficulties in patients with PD who had been without early clinical symptoms. Cognitive performance was improved by training with dual- task activities for only a few days, with greatest improvement in patients > 65 years. These favorable results were maintained for at least 6 months after the initial training (Figure 5 and 6). It must be emphasised that the number of patients participating in this study is small, and no ‘a priori’ calculations of an ideal sample size were performed. Although the results show statistically significant differences between the study groups, therefore, it is not possible to extend our conclusions to a wider spectrum of patients. Nevertheless, we feel that the results are sufficiently encouraging – qualitatively – to indicate the potential importance and value of replication the study with larger cohorts
Author Contributions
Dalma Szögedi: execution of research project
Trevor W. Stone: Review and critique of the manuscript
Elek Dinya: Execution of statistical analysis
Judit Málly: conception of research project, writing of the first draft
Authors’ declaration
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this work is consistent with those guidelines. All authors have substantially taken part in the study and the preparation of the manuscript. No undisclosed groups or persons have had a primary role in the study and/or in the manuscript preparation. All co-authors have read and approved the submission of the manuscript to the Frontiers in Neurology. There is no ghost author among us. The work has not been published earlier nor is being considered for publication in another journal. The study was conducted in accordance with the Helsinki Declaration of 1975. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Competing interest:
The authors have declared that no competing interests exist.
References
- Silva ABRL, de Oliveira RWG, Diogenes GP, Aguiar MFD, Sallem CC, et al. Premotor, nonmotor and motor symptoms of Parkinson's Disease: A new clinical state of the art. Aging Research Reviews 84 (2023): https://doi.org/10.1016/j.arr.2022.101834 PMID: 101834
- Xu DC, Yong C, Yang X, Chen-Yu S, Peng LHl. Signaling pathways in Parkinson's disease: molecular mechanisms and therapeutic interventions. Signal Transduction Targeted Therapy 8 (2023): 73 https://doi.org/10.1038/s41392-023-01353-3 PMID: 36810524
- Zheng ZJ, Zhang SS, Zhang HW, Gao ZZ, Wang XR, et al. Mechanisms of Autoimmune Cell in DA Neuron Apoptosis of Parkinson's Disease: Recent Advancement. Oxidative Medicine and Cellular Longevity (2022): 7965433 https://doi.org/10.1155/2022/7965433 PMID: 7965433
- Aarsland D, Batzu L, Halliday GM, Geurtsen GJ, Ballard C, et al. Parkinson disease-associated cognitive impairment. Nature Reviews Disease Primers 7 (2021): 47 https://doi.org/10.1038/s41572-021-00280-3 PMID: 34210995
- Carceles-Cordon M, Weintraub D, Chen-Plotkin AS. Cognitive heterogeneity in Parkinson's disease: A mechanistic view. Neuron 23 (2023): S0896-6273 00217-9. https://doi.org/10.1016/j.neuron.2023.03.021 in press.
- Weintraub D, Aarsland D, Biundo R, Dobkin R, Goldman J, et al. Management of psychiatric and cognitive complications in Parkinson's disease. British Medical Journal 379 (2022): e068718 https://doi.org/10.1136/bmj-2021-068718 PMID: 36280256
- Yu RL, Wu RM. Mild cognitive impairment in patients with Parkinson's disease: An updated mini-review and future outlook. Frontiers in Aging Neuroscience 14 (2022): https://doi.org/10.3389/fnagi.2022.943438 PMID: 36147702
- Sousa-Fraguas MC, Rodriguez-Fuentes G, Conejo NM. Frailty and cognitive impairment in Parkinson's disease: a systematic review. Neurological Sciences 43 (2022): 6693- 6706 https://doi.org/10.1007/s10072-022-06347-7 PIMID: 36147702
- Bauer J, Steiger BK, Kegel C, Eicher M, Konig, K, et al. A comparative study of social cognition in epilepsy, brain injury, and Parkinson's disease. PsyCh Journal (2023): https://doi.org/10.1002/pchj.650 PMID: 37127428 in press.
- Federico PP, Lorenzo G, Bellomo G, Chipi E, Salvadori N, et al. Neurochemical profile and cognitive changes in Parkinson's disease with mild cognitive impairment. NPJ Parkinsons Disease 9 (2023): 68 https://doi.org/10.1038/s41531-023-00509-w. PMID: 37095141
- Cousineau J, Plateau V, Baufreton J, Le Bon-Jego M. Dopaminergic modulation of primary motor cortex: From cellular and synaptic mechanisms underlying motor learning to cognitive symptoms in Parkinson's disease. Neurobiology of Diseases 167 (2022) : 105674 https://doi.org/10.1016/j.nbd.2022.105674. PMID: 35245676
- Weber MA, Sivakumar K, Tabakovic EE, Oya M, Aldridge GM, et al. Glycolysis-enhancing alpha (1)-adrenergic antagonists modify cognitive symptoms related to Parkinson's disease. NPJ Parkinsons Disease 9 (2023): 32 https://doi.org/:10.1038/s41531-023-00477-1 PMID: 36864060
- Knezovic A, Piknjac M, Barilar JO, Perhoc AB, Virag D, et al. Association of cognitive deficit with glutamate and insulin signaling in a rat model of Parkinson's Disease. Biomedicines 11 (2023): 683 https://doi.org/10.3390/biomedicines11030683 PMID: 36979662
- Tang CX, Chen J, Shao KQ, Liu YH, Zhou XY, et al. Blunt dopamine transmission due to decreased GDNF in the PFC evokes cognitive impairment in Parkinson's disease. Neural Regeneration Research 18 (2023): 1107-1117 https://doi.org/10.4103/1673-5374.355816 PMID: 36255000
- Mantovani E, Zucchella C, Argyriou AA, Tamburin S. Treatment for cognitive and neuropsychiatric non-motor symptoms in Parkinson's disease: current evidence and future perspectives. Expert Review Neurotherapeutics 23 (2023): 25 -43. https://doi.org/10.1080/14737175.2023.2173576 PMID: 36701529
- Monaghan AS, Gordon E, Graham L, Hughes E, Peterson DS, et al. Cognition and freezing of gait in Parkinson's disease: A systematic review and meta-analysis. Neuroscience Biobehavioral Reviews 147 (2023): 105068 https://doi.org/10.1016/j.neubiorev.2023.105068 PMID: 36738813
- Johansson H, Folkerts AK, Hammarström I, Kalbe E, Leavy B. Effects of motor- cognitive training on dual-task performance in people with Parkinson's disease: a systematic review and meta-analysis. Journal of Neurology (2023) https://doi.org/10.1007/s00415-023-11610-8 PMID: 36820916 in press
- de Souza Fortaleza AC, Mancini M, Carlson-Kuhta P, King LA, Nutt JG, et al. Gait Posture 56 (2017): 76–81. https://doi.org/10.1016/j.gaitpost.2017.05.00 PMID: 28521148
- Sasikumar S, Sorrento G, Lang AE, Strafella AP, Fasano A. Cognition affects gait adaptation after split-belt treadmill training in Parkinson's disease. Neurobiology of Disease. (2023) 181: 106109 https://doi.org/10.1016/j.nbd.2023.106109 PMID: 37019221
- Amin RM, Phillips JJ, Humbert AT, Cholerton BA, Short VD, et al. Associations between baseline cognitive status and motor outcomes after treadmill training in people with Parkinson's disease: a pilot study. Disability Rehabilitation (2023): in press https://doi.org/10.1080/09638288.2023.2189318 PMID: 37010072
- Meng D, Jin Z, Wang Y, Fang B. Longitudinal cognitive changes in patients with early Parkinson's disease and neuropsychiatric symptoms. CNS Neuroscience and Therapeutics (2023): in press. https://doi.org/10.1111/cns.14173 PMID: 36924300
- Gan Y, Xie H, Qin G, Shan M, Hu T, et al. Association between cognitive impairment and freezing of gait in patients with Parkinson's Disease. Journal of Clinical Medicine 12 (2023): https://doi.org/10.3390/jcm12082799 PMID: 37109137
- Ahmad SO, Longhurst J, Stiles D, Downard L, Martin S. A meta-analysis of exercise intervention and the effect on Parkinson’s Disease symptoms. Neuroscience Letters 801 (2023): https://doi.org/10.1016/j.neulet.2023.137162 PMID: 3686355
- Hvingelby VS, Glud AN, Sørensen JCH, Tai Y. Andersen ASM, et al. Interventions to improve gait in Parkinson’s disease: A systematic review of randomized controlled trials and network meta-analysis. Journal of Neurology (2022): https://doi.org/10.1007/s00415-022-11091-1 PMID: 35378605
- Li Z, Wang T, Liu H, Jiang Y, Wang Z, et al. Dual-task training on gait, motor symptoms, and balance in patients with Parkinson’s disease: A systematic review and meta-analysis. Clinical Rehabilitation. 34 (2020): 1355–1367. https://doi.org/10.1177/0269215520941142 PMID: 32660265
- Fernandes Â, Rocha N, Santos R, Tavares JM. Effects of dual-task training on balance and executive functions in Parkinson’s disease: A pilot study. Somatosensory and Motor Research 32 (2015): 122–127. https://doi.org/10.3109/08990220.2014.1002605
- Salazar RD, Ren X, Ellis TD, Toraif N, Barthelemy OJ, et al. Dual tasking in Parkinson’s disease: Cognitive consequences while walking. Neuropsychology 31 (2017): 613–623. https://doi.org/10.1037/neu0000331 PMID: 28414497
- Valenzuela CSM, Moscardó LD, López-Pascual J Serra-Añó P, Tomás JM. Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson’ Disease: Results of randomized controlled DUALGAIT trial. Archives of Physical Medicine and Rehabilitation 101 (2020): 1849–1856. https://doi.org/10.1016/j.apmr.2020.07.008 PMID: 32795562
- Pereira-Pedro KP, de Oliveira I.M, Mollinedo-Cardalda I, Cancela-Carral JM.. Effects of cycling dual-task on cognitive and physical function in Parkinson’s Disease: A randomized double-blind pilot study. International Journal of Environmental Research and Public Health 19 (2022): 7847. https://doi.org/10.3390/ijerph19137847 PMID: 35805505
- Xiao Y, Yang T, Shang H. The impact of motor-cognitive dual-task training on physical and cognitive functions in Parkinson's Disease. Brain Science 13 (2023): https://doi.org/10.3390/brainsci13030437 PMID: 36979247
- Wong PL, Cheng SJ, Yang YR, Wang RY. Effects of dual task training on dual task gait performance and cognitive function in individuals with Parkinson's disease: A meta-analysis and meta-regression. Archives of Physical Medicine and Rehabilitation (2022): https://doi.org/10.1016/j.apmr.2022.11.001 PMID: 36574531
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". „Mini-Mental State” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research12 (1975): 189–98. https://doi.org/10.1016/0022-3956(75)90026-6 PMID: 1202204.
- Reitan RM. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory: Tempe, AZ: USA (1992) Corpus ID:141448957
- Sunderland T, Hill JL, Mellow AM, Lawlor BA, Gundersheimer J, et al. Clock drawing in Alzheimer’s disease: a novel measure of dementia severity. Journal of the American Geriatric Society 37 (1989):725–729.
- Hamilton M. A rating scale for depression. Journal of Neurosurgery and Psychiatry 23 (1960): 56–62. http://dx.doi.org/10.1136/jnnp.23.1.56 PMID: 14399272
- Hoehn MM, Yahr MD. Parkinsonism: onset, progression, and mortality. Neurology 17 (1967): 427–442. https://doi.org/10.1212/WNL.17.5.427 PMID: 6067254
- Fahn S, Elton R. “Members of the UPDRS Development Committee”. In: Fahn S, Marsden CD, Calne DR, Goldstein M. eds. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health Care Information. (1987): pp. 153–163; 293–304. https://doi.org/10.1177/0269215520941142 PMID: 32660265
- Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Performance of simultaneous movements in patients with Parkinson’s disease. Brain 109 (1986):739– https://doi.org/10.1093/brain/109.4.739 PMID: 3730813
- Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Simple and complex movements off and on treatment in patients with Parkinson’s disease. Journal of Neurosurgery and Psychiatry 50 (1987): 296–303.
- Brown RG, Marsden CD. Dual task performance and processing resources in normal subjects and patients with Parkinson’s disease. Brain 114 (1991): 215–231. https://doi.org/10.1093/oxfordjournals.brain.a101858 PMID: 1998883
- Dalrymple-Alford JC, Kalders AS, Jones RD, Watson RW. A central executive deficit in patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 57 (1994): 360–367. https://doi.org/10.1136/jnnp.57.3.360 PMID: 8158188;
- Bhatt M, Mahana B, Ko JH, Kolesar TA, Kanitkar A, et al. Computerized dual-task testing of gait visuomotor and cognitive functions in Parkinson’s disease: Test-retest reliability and validity. Frontiers in Human Neuroscience 15 (2021): 706230. https://doi.org/10.3389/fnhum.2021.706230 PMID: 34335213
- Johansson H, Ekman U, Rennie L, Peterson DS, Leavy B, et al. Dual-task effects during a motor-cognitive task in Parkinson’s disease: Patterns of prioritization and the influence of cognitive status. Neurorehabilitation and Neural Repair 35 (2021): 356–366. https://doi.org/10.1177/1545968321999053 PMID: 33719728
- Conradsson D, Löfgren N, Nero H, Hangströmer M, Stahle A, et al. The effects of highly challenging balance training in elderly with Parkinson’s disease: A randomized controlled trial. Neurorehabilitation and Neural Repair 29 (2015): 827–836. https://doi.org/10.1177/1545968314567150 PMID: 25608520
- Zhang X, Fan W, Yu H, Li L, Chen Z, et al. Single- and dual-task gait performance and their diagnostic value in early-stage Parkinson’s disease. Frontiers in Neurology 13 (2022): 974985. http://doi.org/10.3389/fneur.2022.974985 PMID:3631349
- Yang YR, Cheng SJ, Lee YJ, Liu YC, Wang RY. Cognitive and motor dual task gait training exerted specific training effects on dual task gait performance in individuals with Parkinson’s disease: A randomized controlled pilot study. PLoS ONE 14 (2019): 1–7. https://doi.org/10.1371/journal.pone.0218180 PMID:31220121
- Hsiu-Chen C, Chiung-Chu C, Jiunn-Woei L, Wei-Da C, Yi-Hsin W, et al. The effects of dual-task in patients with Parkinson’s disease performing cognitive-motor paradigms. Journal of Clinical Neuroscience 72 (2020): 72–78. https://doi.org/10.1016/j.jocn.2020.01.024 PMID: 31952973
- Brauer SG, Morris ME. Can people with Parkinson's disease improve dual tasking when walking? Gait Posture 31 (2010): 229 - 233. http://doi.org/10.1016/j.gaitpost.2009.10.011 PMID: 19969461
- Malcolm BR, Foxe JJ, Butler JS De Sanctis P. The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: a mobile brain/body imaging (MoBI) study. Neuroimage 117 (2015): 230–242. https://doi.org/10.1016/j.neuroimage.2015.05.028 PMID: 25988225
- Bhatt M, Mahana B, Ko JH, Kolesar TA, Kanitkar A, et al. Computerized dual-task testing of gait visuomotor and cognitive functions in Parkinson’s disease: Test-retest reliability and validity. Frontiers in Human Neuroscience 15 (2021): 706230. https://doi.org/10.3389/fnhum.2021.706230 PMID: 34335213
- Salazar RD, Ren X, Ellis TD, Toraif N, Barthelemy OJ, et al. Dual tasking in Parkinson’s disease: Cognitive consequences while walking. Neuropsychology 31 (2017): 613–623. https://doi.org/10.1037/neu0000331 PMID: 28414497
- Raffegeau TE, Krehbiel LM, Kang N, Thijs FJ, Altmann LJP, et al, A meta-analysis: Parkinson’s disease and dual-task walking. Parkinsonism and Related Disorders 62 (2019): 28–35. https://doi.org/10.1016/j.parkreldis.2018.12.012 PMID:30594454
- Zhang Q, Aldridge GM, Naravanan NS, Anderson SW, UC E. Approach to cognitive impairment in Parkinson’s disease. Neurotherapeutics 17 (2020): 1495–1510. https://doi.org/10.1007/s13311-020-00963-x PMID: 33205381
- Brustio PR, Magistro D, Zecca M, Rabaglietti E, Liubicich ME Age-related decrements in dual-task performance: Comparison of different mobility and cognitive tasks. A cross sectional study. A cross sectional study. PLoS ONE (2017): https://doi.org/10.1371/journal.pone.0181698 PMID: 28732080
- Ehsani H, Mohler MJ, O'Connor K, Zamrini E, Tirambulo C, et al. The association between cognition and dual-tasking among older adults: the effect of motor function type and cognition task difficulty. Clinical Interventions in Aging 14 (2019): 659–669. https://doi.org/10.2147/CIA.S198697 PMID: 31040655
- Aarsland D, Creese B, Politis M, Chaudhuri KR, Ffytche DH, et al. Cognitive decline in Parkinson disease. Nature Reviews in Neurology 13 (2017): 217–231. https://doi.org/10.1038/nrneurol.2017.27 PMID: 28257128
- Burn D, Weintraub D, Robbins T. Introduction: The importance of cognition in movement disorders. Movement Disorders 29 (2014): 581–583. https://doi.org/10.1002/mds.25871 PMID: 24757107
- Tsang CSL, Chong DYK, Pang MYC. Cognitive-motor interference in walking after stroke: test-retest reliability and validity of dual-task walking assessments. Clinical Rehabilitation 33 (2019): 1066-1078. http://doi.org/10.1177/0269215519828146. PMID: 30722681
- Sun R, Li X, Zhu Z, Li T, Zhao M, et al. Effects of dual-task training in patients with post-stroke cognitive impairment: A randomized controlled trial. Frontiers in Neurology 13 (2022): 1027104 http://doi.org/10.3389/fneur.2022.1027104
- Sun R, Li X, Zhu Z, Li T, Zhao M, et al. Effects of combined cognitive and exercise interventions on poststroke cognitive function: A systematic review and meta-analysis. BioMed Research International 17 (2021): http://doi.org/10.1155/2021/4558279 PMID: 34840972
- Soliveri P, Brown RG, Jahanshahi M, Marsden CDl. Effect of practice on performance of a skilled motor task in patients with Parkinson's disease. Journal of Neurology, Neurosurgery and Psychiatry 55 (1992): 454–460. http://dx.doi.org/10.1136/jnnp.55.6.454 PMID: 1619411
- Strouwen C, Molenaar EALM, Münks L, Keus SHJ, Zijlmans JCM, et al. Training dual tasks together or apart in Parkinson’s disease: Results from the DUALITY trial. Movement Disorders 32 (2017): 1201–1210. https://doi.org/10.1002/mds.27014 PMID: 28440888
- Van Selst M, Ruthruff E, Johnston JC. Can practice eliminate the psychological refractory period effect? Journal of Experimental Psychology: Human Perception and Performance 25 (1999): 1268–1283. https://doi.org/10.1037/0096-1523.25.5.1268 PMID: 10531663
- Ruthruff E, Johnston JC, Van Selst M. Why practice reduces dual-task interference. Journal of Experimental Psychology: Human Perception and Performance 27 (2001): 3–21. https://doi.org/10.1037/0096-1523.27.1.3 PMID: 11248938
- Fritz NE, Cheek FM, Nickols-Larsen DS. Motor-Cognitive dual-task training in persons with neurologic disorders: A systematic review: Journal of Neurologic Physical Therapy 39 (2015): 142-153. https://doi.org/10.1097/NPT.0000000000000090
- Geroin C, Nonnekes J, de Vries NM, Strouwen C, Smania N, et al. Does dual-task training improve spatiotemporal gait parameters in Parkinson’s disease? Parkinsonism and Related Disorders 55 (2018): 86–91. https://doi.org/10.1016/j.parkreldis.2018.05.018 PMID: 29802080
- Wollesen B, Rudnik S, Gulberti A Cordes T, Gerloff C, et al. A feasibility study of dual-task strategy training to improve gait performance in patients with Parkinson’s disease. Scientific Reports 11 (2021): 12416. https://doi.org/10.1038/s41598-021-91858-0 PMID: 34127721
- Hazeltine E, Ruthruff E, Remington RW. The role of input and output modality pairings in dual-task performance: Evidence for content-dependent central interference. Cognitive Psychology 52 (2006): 291–345. https://doi.org/10.1016/j.cogpsych.2005.11.001 PMID: 16581054
- Halvorson KM, Hazeltine E. Separation of tasks into distinct domains, not set-level compatibility, minimizes dual-task interference. Frontiers in Psychology 10 (2018): 711. https://doi.org/10.3389/fpsyg.2019.00711 PMID: 30984091
- Beck EN, Intzandt BN, Almeida QJ. Can dual-task walking improve in Parkinson’s disease after external focus of attention exercise? A single blind randomized controlled trial. Neurorehabilitation and Neural Repair 32 (2018): 18–33. https://doi.org/10.1177/1545968317746782 PMID: 29262749
- De Freitas TB, Leite PHW, Doná F, Pompeu JE, Swarowsky A, et al. The effects of dual task gait and balance training in Parkinson's disease: a systematic review. Physiotherapy Theory and Practice 36 (2020): 1088-1096. http://doi.org/10.1080/09593985.2018.1551455 PMID: 30501424
- Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry 79 (2008): 760–766. https://doi.org/10.1136/jnnp.2007.126599 PMID: 18006652
- Gao L, Zhang J, Hou Y, Hallett M, Chan P, et al. The cerebellum in dual-task performance in Parkinson’s disease. Scientific Reports 7 (2017): 45662 https://doi.org/10.1038/srep45662 PMID: 28358358
