Double Trouble Mutations Underlie Mitochondrial Dynamics Disorders in a Severe Form of Charcot-Marie-Tooth Disease
Article Information
Angela Puma1,2, Samuel Guilbault2, Emmanuelle C Genin3, Christophe Duranton2, Isabelle Rubera2, Nathalie Bonello- Palot4, Véronique Paquis-Flucklinger3, Sabrina Sacconi1, Saïd Bendahhou2*
1Université Côte d'Azur, Centre Hospitalier Universitaire de Nice, Système Nerveux Périphérique & Muscle, Hôpital Pasteur 2, 30 voie Romaine CS, Nice, France
2Université Côte d’Azur, CNRS, LP2M, Labex ICST, Faculté de Médecine, Nice, France
3Université Côte d'Azur, Inserm U1081, CNRS UMR7284, IRCAN, CHU de Nice, Nice, France
4APHM, CHU Timone, Département de Génétique Médicale, Marseille, France, INSERM, MMG,
U 1251, Marseille, France, Aix Marseille Univ, Marseille, France
*Corresponding author:Saïd Bendahhou, Université Côte d’Azur, CNRS, LP2M, Labex ICST, Faculté de Médecine, Nice, France.
Received: July 17, 2023;Accepted: July 24, 2023;Published: October 11, 2023
Citation: Angela Puma, Samuel Guilbault, Emmanuelle C Genin, Christophe Duranton, Isabelle Rubera, Nathalie Bonello-Palot, Véronique Paquis- Flucklinger, Sabrina Sacconi, Saïd Bendahhou. Double Trouble Mutations Underlie Mitochondrial Dynamics Disorders in a Severe Form of Charcot- Marie-Tooth Disease. Journal of Biotechnology and Biomedicine. 6 (2023): 468-475.
View / Download Pdf Share at FacebookAbstract
Charcot-Marie-Tooth disease type 2A (CMT2A) is an inherited axonal peripheral neuropathy mainly caused by mutations in the mitofusin 2 (MFN2) gene encoding for the MFN2 protein, a GTPase involved in the mitochondrial dynamics and bioenergetics. We identified a novel mutation, that was inherited from her mother, in the MFN2 protein (Met234Ile) in a Charcot-Marie-Tooth patient. She has also a variant of uncertain significance (Thr36Ala), in the PMP22 gene, encoding for peripheral myelin protein 22, inherited from her father. The patient presented severe sensorimotor neuropathy with early onset. The mother presented a distal muscle atrophy in the legs, the father was asymptomatic. To show the pathogenicity of these mutations, we characterized the structure, adenosine triphosphate (ATP) content, bioelectric characteristics and functions of mitochondria in cultured primary fibroblasts obtained from the proband and her parents. Under normal culture conditions, mitochondria showed normal morphology. Under oxidative stress conditions, ATP production was reduced and the proband cells showed a decrease of the mitochondrial fusion with small connected networks and a decrease of the mitochondrial volume. The alteration of the mitochondrial network only when cells are challenged in aerobiosis testifies to the fragility of mitochondria, which are unable to meet the metabolic needs of neurons. Interestingly, fibroblasts derived from the two parents did not show any change. These results support the hypothesis that the mutation in the MFN2 gene altering mitochondrial bioenergetics and fusion causes axonal sensory-motor neuropathy. We speculate that PMP22 may promote mitochondrial dysfunction, myelin having a role in mitochondrial metabolism.
Keywords
MFN2; CMT2A; PMP22; Fibroblasts; Mitochondrial network; Fusion
MFN2 articles MFN2 Research articles MFN2 review articles MFN2 PubMed articles MFN2 PubMed Central articles MFN2 2023 articles MFN2 2024 articles MFN2 Scopus articles MFN2 impact factor journals MFN2 Scopus journals MFN2 PubMed journals MFN2 medical journals MFN2 free journals MFN2 best journals MFN2 top journals MFN2 free medical journals MFN2 famous journals MFN2 Google Scholar indexed journals CMT2A articles CMT2A Research articles CMT2A review articles CMT2A PubMed articles CMT2A PubMed Central articles CMT2A 2023 articles CMT2A 2024 articles CMT2A Scopus articles CMT2A impact factor journals CMT2A Scopus journals CMT2A PubMed journals CMT2A medical journals CMT2A free journals CMT2A best journals CMT2A top journals CMT2A free medical journals CMT2A famous journals CMT2A Google Scholar indexed journals PMP22 articles PMP22 Research articles PMP22 review articles PMP22 PubMed articles PMP22 PubMed Central articles PMP22 2023 articles PMP22 2024 articles PMP22 Scopus articles PMP22 impact factor journals PMP22 Scopus journals PMP22 PubMed journals PMP22 medical journals PMP22 free journals PMP22 best journals PMP22 top journals PMP22 free medical journals PMP22 famous journals PMP22 Google Scholar indexed journals Fibroblasts articles Fibroblasts Research articles Fibroblasts review articles Fibroblasts PubMed articles Fibroblasts PubMed Central articles Fibroblasts 2023 articles Fibroblasts 2024 articles Fibroblasts Scopus articles Fibroblasts impact factor journals Fibroblasts Scopus journals Fibroblasts PubMed journals Fibroblasts medical journals Fibroblasts free journals Fibroblasts best journals Fibroblasts top journals Fibroblasts free medical journals Fibroblasts famous journals Fibroblasts Google Scholar indexed journals Mitochondrial network articles Mitochondrial network Research articles Mitochondrial network review articles Mitochondrial network PubMed articles Mitochondrial network PubMed Central articles Mitochondrial network 2023 articles Mitochondrial network 2024 articles Mitochondrial network Scopus articles Mitochondrial network impact factor journals Mitochondrial network Scopus journals Mitochondrial network PubMed journals Mitochondrial network medical journals Mitochondrial network free journals Mitochondrial network best journals Mitochondrial network top journals Mitochondrial network free medical journals Mitochondrial network famous journals Mitochondrial network Google Scholar indexed journals Fusion articles Fusion Research articles Fusion review articles Fusion PubMed articles Fusion PubMed Central articles Fusion 2023 articles Fusion 2024 articles Fusion Scopus articles Fusion impact factor journals Fusion Scopus journals Fusion PubMed journals Fusion medical journals Fusion free journals Fusion best journals Fusion top journals Fusion free medical journals Fusion famous journals Fusion Google Scholar indexed journals neuropathy articles neuropathy Research articles neuropathy review articles neuropathy PubMed articles neuropathy PubMed Central articles neuropathy 2023 articles neuropathy 2024 articles neuropathy Scopus articles neuropathy impact factor journals neuropathy Scopus journals neuropathy PubMed journals neuropathy medical journals neuropathy free journals neuropathy best journals neuropathy top journals neuropathy free medical journals neuropathy famous journals neuropathy Google Scholar indexed journals mitochondrial articles mitochondrial Research articles mitochondrial review articles mitochondrial PubMed articles mitochondrial PubMed Central articles mitochondrial 2023 articles mitochondrial 2024 articles mitochondrial Scopus articles mitochondrial impact factor journals mitochondrial Scopus journals mitochondrial PubMed journals mitochondrial medical journals mitochondrial free journals mitochondrial best journals mitochondrial top journals mitochondrial free medical journals mitochondrial famous journals mitochondrial Google Scholar indexed journals ankle twisting articles ankle twisting Research articles ankle twisting review articles ankle twisting PubMed articles ankle twisting PubMed Central articles ankle twisting 2023 articles ankle twisting 2024 articles ankle twisting Scopus articles ankle twisting impact factor journals ankle twisting Scopus journals ankle twisting PubMed journals ankle twisting medical journals ankle twisting free journals ankle twisting best journals ankle twisting top journals ankle twisting free medical journals ankle twisting famous journals ankle twisting Google Scholar indexed journals muscles articles muscles Research articles muscles review articles muscles PubMed articles muscles PubMed Central articles muscles 2023 articles muscles 2024 articles muscles Scopus articles muscles impact factor journals muscles Scopus journals muscles PubMed journals muscles medical journals muscles free journals muscles best journals muscles top journals muscles free medical journals muscles famous journals muscles Google Scholar indexed journals
Article Details
Introduction
Charcot-Marie-Tooth disease (CMT) also known as hereditary sensory- motor neuropathies (HMSN) is the most common inherited neuropathy, with a prevalence of 1:2500 [1]. Depending on the motor conduction velocity (MCV) found on the median nerve at electrodiagnostic evaluation (EDX), CMTs can be classified into three principal groups, demyelinating (MCV ≤35 m/sec), intermediaries (MCV between 35 and 45 m/sec) or axonal (≥45 m/sec)[2]. As far as demyelinating forms are concerned, CMT1A is the most frequent form. It is due to a 1.4 Mb duplication on chromosome 17p11.2 that includes the PMP22 gene encoding for the peripheral myelin protein 22 [3]. More rarely (<5% of cases) is caused by a point mutation in the same gene [4]. CMT1A has a classic CMT phenotype, i.e. length- dependent. The clinical severity is highly variable. The onset age is in the first decade in 75% of cases. Clinically, patients present with distal weakness/atrophy, sensory loss and cavus feet. Deep reflexes are abolished. [5].
PMP22 is a 22-kD protein that makes up 2-5% of myelin in the peripheral nervous system. Its function is unclear. It probably plays a role in Schwann cell growth and differentiation. It has been hypothesized that a point mutation of PMP22 makes the protein dysfunctional while duplication may compromise stoichiometry and interactions with other myelin proteins [6].
Axonal CMTs, known as CMT2A, are mostly associated with mutations in the Mitofusin 2 (MFN2) gene. MFN2 gene, located on chromosome 1p36.22, is encoding for MFN2 protein. [7]. CMT2A has an autosomal dominant transmission in 90% of cases. [8]. The age of onset varies within the same family and ranges from one year to the sixth decade, although most individuals develop manifestations in the first and second decades of life [9]. Its clinical phenotype is more complex than that of CMT1A, as it may involve the optic nerve [10] and the pyramidal tract [11]. Distal weakness and atrophy predominate over sensory signs. Deep tendon reflexes are usually absent, but can be present. Lesions of the cerebral white matter have been described [10,12,13]. Additional signs are postural tremor [14] and vocal cord paralysis with hoarse voice [15]. Some individuals with pathogenic variants of MFN2 are asymptomatic [16]; however, the disease may manifest later in life [17].
MFN2 is an ubiquitous protein of 757 amino acids belonging to the mitochondrial transmembrane GTPases family. It contains a GTPase domain, two transmembrane domains and two coiled-coil regions that mediate binding to other mitofusin molecules [18]. This protein is crucial for mitochondrial dynamics and is involved mainly in the processes of mitochondrial fusion and displacement of mitochondria within the cell [19], [20].
Although MFN2 is ubiquitously expressed, mutations in this gene cause mainly neuronal dysfunction. Cases of double trouble have been described in the literature to indicate the presence of two different mutations in genes involved in CMT that can interact to give new, sometimes more pronounced phenotypes [21,22,23]. Here, we report two genetic mutations in the heterozygous state in a patient, one inherited from the mother on the MFN2 gene ((NM_014874): c.702G>A (p.Met234Ile)) described as probably pathogenic, the other inherited from the father on the PMP22 gene ((NM_000304): c.106A>G (p.Thr36Ala)) described as a variant of uncertain significance (VUS). The concomitant presence of the two mutations seems to have facilitated the appearance of the clinical phenotype, a severe form of axonal CMT caused by mitochondrial dysfunction.
We used an in vitro model of the disease using fibroblasts to study in detail the mitochondrial dynamic
Materials and Methods
Patients
The proband is a 16-year-old female patient who presented to our department with slowly progressive gait disorders since childhood. The first symptoms reported were difficulties with running and sporting activity and recurrent episodes of ankle twisting. At the age of 12, she noticed a real deficit of the foot lift muscles with steppage and more recently, the onset of quadridistal paresthesias and weakness of the upper limbs with difficulties in hand dexterity movements.
Clinical examination confirms a significant deformity with cavus varus foot and bilateral achilles retraction. She has generalized hyperreflexia. There is amyotrophy in all four limbs, predominantly in distal muscles and in low limbs, bilateral steppage on walking and attitudinal tremor of the upper limbs. Romberg's sign is positive.
The electrodiagnostic evaluation (EDX) shows a predominantly motor, axonal, length-dependent neuropathy. The motor conduction velocity of the median nerves is between 56 and 60 m/sec. The overall clinical-electrical picture is compatible with axonal Charcot-Marie-Tooth disease (CMT 2) (Table 1). Cerebral MRI and ocular evaluation) were normal. The mother of the proband, originally from Italy, showed on clinical examination atrophy of the distal muscles of the lower limbs. The father, of Vietnamese origin, had a normal clinical examination. For both, the ENMG was normal except for the presence, on detection, of chronic neurogenic tracings on the two tibialis anterior muscles in the mother.
The pedigree is shown in Figure 1. The clinical and EDX data are shown in Table 1.
Genetic analyses
The proband and her parents gave written informed consent in accordance with French law prior to blood sampling and skin biopsy. NGS was performed using an in-house designed panel containing the coding exons and flanking intronic sequences of neuropathy disease-associated genes. Then, we interpreted variant with the help of database as Online Mendelian Inheritance in ClinVar (https://www.ncbi.nlm.nih. gov/clinvar/), gnomAD (https://gnomad.broadinstitute.org/) and HGMD (http://www.hgmd.cf.ac.uk). Finally, following the American College of Medical Genetics and Genomics (ACMG) recommendations (Richards’ classification, 2015), we classified the variants into five categories: pathogenic, likely pathogenic, variant of unknown significance (VUS), likely benign, and benign. Variants identified were confirmed by Sanger sequencing. Primer sequences and conditions for PCR and sequencing are available from the authors on request. The sequences were analyzed using Sequencher software.
Table 1: Clinical and neurophysiological data.
|
P0 |
PI |
PII |
|
|
Age |
16 |
62 |
53 |
|
Age of onset (years) |
6 |
N |
N |
|
Distal Weakness |
|||
|
Upper limbs |
+++ |
N |
N |
|
Lower limbs |
+ |
N |
N |
|
Proximal Weakness |
|||
|
Upper limbs |
N |
N |
N |
|
Lower limbs |
+ |
N |
N |
|
Distal Atrophy |
|||
|
Upper limbs |
+ |
N |
N |
|
Lower limbs |
+++ |
N |
+ |
|
Proximal Atrophy |
|||
|
Upper limbs |
N |
N |
N |
|
Lower limbs |
+ |
N |
N |
|
Senbility |
|||
|
Upper limbs |
N |
N |
N |
|
Lower limbs |
N |
N |
N |
|
Deep reflexs |
+++ |
N |
+ |
|
Pes cavus |
++ |
N |
+ |
|
MOTOR NERVES : |
|||
|
Right Median Nerve |
|||
|
Distal latency (ms) |
2.8 |
3.9 |
3.1 |
|
Distal cMAP (mV) |
8.7 |
9 |
7.6 |
|
MCV (m/s) |
60 |
56.4 |
53.8 |
|
Right Peroneal Nerve |
|||
|
Distal latency (ms) |
7 |
3.6 |
4.1 |
|
Distal cMAP (mV) |
0.7 |
4.4 |
3.9 |
|
MCV (m/s) |
36 |
51.9 |
47 |
|
SENSORY NERVES |
|||
|
Right Median Nerve |
|||
|
Distal SNAP (µV) |
42 |
28.2 |
42 |
|
SCV (m/s) |
63 |
54 |
57.8 |
|
Right Sural Nerve |
|||
|
Distal SNAP(µV) |
8.5 |
17.4 |
19.8 |
|
SCV (m/s) |
36 |
56.6 |
51.4 |
Cell Culture
After informed consent, skin samples were taken from the patient and from her parents. Primary cultures of fibroblasts were established using standard cell culture procedures. Briefly, the skin biopsy sample was cut into to small pieces then deposited directly on the surface of 60-mm culture dish in 600 µL culture medium (RPMI-1640 medium supplemented with 20% fetal calf serum, 50 µM 2-mercaptoethanol, 100 µM asparagine, 2 mM glutamine, and 1% penicillin/streptomycin) in a humidified atmosphere with 5% CO2 at 37°C. After 3 days, as all skin samples are well attached to the bottom of the dish, 4 mL complete medium was added and allowed the culture to proceed. After 3-4 days of culture, the cells start to proliferate. When confluent, cells are transferred to a100-mm culture dish for expansion. Medium was changed every other day.
A skin sample was also taken from an unrelated, unaffected individual (with no mutation in MFN2 or PMP22). Cultured fibroblasts from the healthy individual were used throughout this work as a control sample.
Immunofluorescence
Fibroblasts were passaged in a 48-well plate containing a coverslip. Cells were grown on the coverslip until the confluency reached 50 %. Cells were then fixed with 4% paraformaldehyde, and washed twice with PBS solution. They were next permeabilized with a PBS solution containing 0.2% tween 20, then exposed to a rabbit monoclonal antibody against PMP2 protein (Life Technologies SAS). After washing with PBS, cells were treated with a goat anti-rabbit secondary antibody coupled to the Alexa Fluor 488. Prior to mounting the coverslip on a slide, nuclei were labelled with DAPI. Images were taken using ZEISS LSM 880 confocal microscope.
Electron microscopy
Cells were fixed in 1.6% glutaraldehyde in 0.1 M phosphate buffer (pH7.4). They were rinsed with cacodylate buffer 0.1 M, then post-fixed in osmium tetroxide (1% in cacodylate buffer) reduced with potassium ferricyanide (1%) during 1 h. Cells were washed once in water then dehydrated with several incubations in increasing concentrations of ethanol (from 30% to 100%) and embedded in epoxy resin (EPON). Eighty nanometer sections were contrasted with uranyl acetate (4% in water) then lead citrate and observed with a Transmission Electron Microscope (JEOL JEM 1400) operating at 100 kV and equipped with an Olympus SIS MORADA camera.
Mitochondrial membrane potential
To establish the mitochondrial membrane potential in live fibroblasts, tetramethylrhodamine ethyl ester (TMRE) was used. TMRE is a cell permeant fluorescent dye that accumulates inside the cells according to their state: depolarized mitochondrial membrane reduces the sequestration of the TMRE inside the cells. Cells (150 000), cultured in a 24-well plate, were incubated for 20 min at 37
°C with 200 nM TMRE, then washed with the regular culture medium, treated with 1% trypsin, and centrifuged at 2000 rpm for 10 min, resuspended in 200 µL PBS/FCS/EDTA medium, and analyzed with flow cytometry (using 488 nm laser for excitation and at emission 575 nm), with the FACS Canto II (BD Biosciences) and FACS Diva software (BD Biosciences). Each value represents the average of the wells per cell type.
Quantification of intracellular ATP content
ATP Measurements were performed using luminescent approach according to the manufacturer’s instructions (ATPlite assay kit, Perkin Elmer). Briefly, fibroblasts were plated in a 24-well plate until reaching confluency. The day of experiment, culture medium was removed, cells were scraped and resuspended in a lysis buffer (100 µL, included in the kit) to allow measurement of intracellular ATP content.
Proteins contents were simultaneous quantified in each well using DC protein assay kit (Bio-Rad, USA), according to the manufacturer’s instructions for data normalization: Values of ATP content are expressed in µmol of ATP per mg of proteins.
Mitochondrial network sizing
For mitochondrial staining, cells were incubated in a 100 nM solution of MitoTracker red (Invitrogen) for 15 min. Medium was replaced by fibroblasts culture medium, incubated 2 h at 37°C and washed in PBS. The samples were fixed with 4% paraformaldehyde (Electron Microscopy Sciences), washed with PBS and mounted on glass slides using ProLong Gold Antifade reagent (Molecular Probes). The images were acquired on a ZEISS LSM 880 confocal microscope and deconvolved with Huygens Essential SoftwareTM (Scientific Volume Imaging) using a theoretically calculated point spread function (PSF) for each of the dyes. All selected images were iteratively deconvolved with maximum iterations scored 40 and a quality threshold at 0.05. The deconvolved images were used for quantitative mitochondrial network analysis with Huygens Essentiel SoftwareTM with the following standardized set of parameters: threshold = 15%, seed = 10% and garbage = 10. The quantitative data were further analyzed in Microsoft Excel and GraphPad Prism 9 (GraphPad Software). Mitochondrial network length was quantified for 45 to 52 randomly-selected individual cells. Data are represented as mean ± S.E.M. Differences were considered as significant if p<0.05.
Results
Patients and genotypes:
Next generation sequencing was performed on the proband. We identified one variant probably pathogenic in the MFN2 gene (NM_014874): c.702G>A (p.Met234Ile), inherited from her mother and a variant of uncertain significance (VUS) in the PMP22 gene (NM_000304): c.106A>G (p.Thr36Ala) inherited from her father (Figure 1).
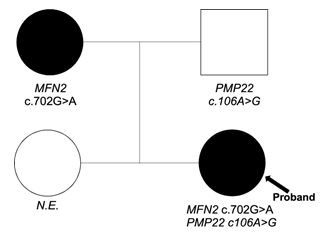
Figure 1: Pedigree and genotypes.
Symbols: squares for male, circles for females. Arrow: proband. Clinical status: n.e. = not examined; Genotypes: mutations indicated MFN2 and PMP22 are heterozygous
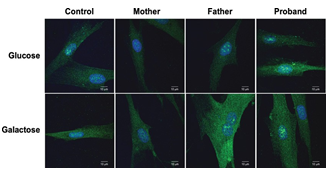
Figure 2: PMP22 expression profile is not affected in fibroblasts Images of fibroblasts, from all family members, that were cultured in normal proliferation medium containing either glucose (25 mM) or galactose (10 mM). Images are representative of 3 independent cultures.
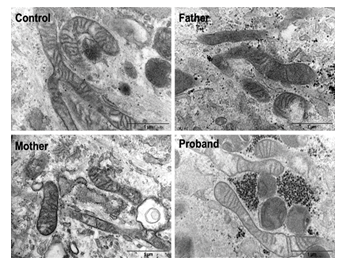
Figure 3: Fibroblasts have normal mitochondrial structure Electron microscopy images of fibroblasts of all family members and a control that were cultured in the presence of glucose (25 mM).
PMP22 expression in fibroblasts
The localisation of PMP22 in fibroblasts was examined by immunofluorescence. No pathological PMP22 aggregates were found in the proband and control fibroblasts cultured either in presence of glucose (25 mM) or galactose (10 mM) (Figure 2).
CMT fibroblasts have no phenotypical change
Mitochondria from normal and CMT cells, in glucose medium, visualized under electron microscopy showed no phenotypical difference (Figure 3). All genotypes showed normal phenotypical organization of the mitochondria structural compartments.
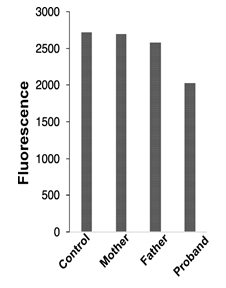
Figure 4: Mitochondrial membrane potential
Mitochondrial membrane potential was monitored in fibroblasts from all family members using TMRE as a membrane potential indicator
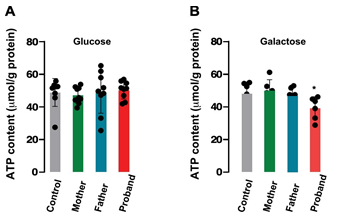
Figure 5: ATP content in patients’ fibroblasts
ATP content was measured in all patients’ fibroblasts either in the presence of 25 mM glucose (A) or 10 mM galactose (B). Values represent the mean ± SEM of three wells.
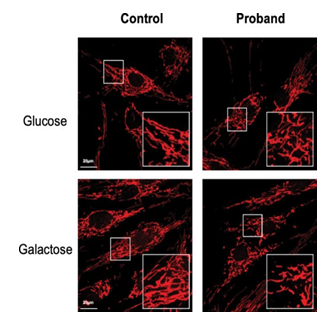
Figure 6: Mitochondrial network
Confocal images of mitochondrial network stained with MitoTracker Red for the control and the daughter fibroblasts, in glucose (25 mM) or galactose (10 mM) conditions. Culture in galactose medium allowed to observe a fragmentation of the mitochondrial network and less connected mitochondria in CMT patients compared to control fibroblasts.
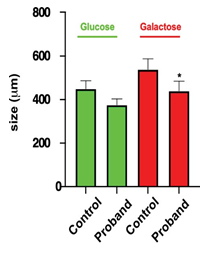
Figure 7: Mitochondrial network size
Size of the mitochondrial network was measured under glucose or galactose conditions for the control and the daughter fibroblasts.
*: using Anova test, only values between control and the proband cells were significantly different (p< 0.05).
Mitochondria membrane potential (ΔΨm)
Since a mitochondrial dysfunction is often correlated with a change in mitochondrial oxidative phosphorylation, we measured the mitochondrial potential using the ΔΨm-sensitive fluorescent dye TMRE and FACS analysis. Interestingly, the cells from the proband exhibits less fluorescence (reflecting a shift of ΔΨm towards of less-negative value, Figure 4) than cells from control or the patients carrying only one or the other mutation (mother, father).
Intracellular ATP content.
ATP production was quantified in all genotypes in the presence of glucose or galactose. This strategy has been used to force the mitochondrial oxidative phosphorylation rather than glycolysis by substituting galactose for glucose in the growth media and to reveal mitochondrial dysfunction [24] In the presence of glucose (Figure 5A) intracellular ATP content was not different in all cell type (n=6-9, independent experiments). However, when cultured in the presence of galactose, cells from the daughter showed a significant lower ATP content than the other cell types (39.20 ± 2.84 µmol/g protein, proband; 48.05 ± 2.92 µmol/g protein, Control; p<0.05) (Figure 5B).
Mitochondrial network sizing
Considering that MFN2 function is important for mitochondrial network organization, we compared the mitochondrial network of fibroblasts from CMT patient with that obtained from control fibroblasts. After staining with MitoTracker and examination by confocal microscopy, control fibroblasts displayed a typical filamentous interconnected network. In glucose medium, CMT patient fibroblasts presented with a mitochondrial network similar to control fibroblasts (Figures 5-6). Identical experiments were performed on fibroblasts grown in a glucose-free medium containing galactose. Culture in galactose medium allowed to observe a fragmentation of the mitochondrial network and less connected mitochondria in CMT patients compared to control fibroblasts. The length was in µm: 536.8 ± 48.5 (n= 47) for control, 438.4 ± 44.5 (n= 52) for the proband, 408.69 ± 26.3 (n= 38) for the mother, and 436.26 ± 42.5 (n= 29) for the father. Using Anova test, only values between control and the proband cells were significantly different (p< 0.05) (Figures 6-7).
Confocal images of mitochondrial network stained with MitoTracker Red for the control and the daughter fibroblasts, in glucose (25 mM) or galactose (10 mM) conditions. Culture in galactose medium allowed to observe a fragmentation of the mitochondrial network and less connected mitochondria in CMT patients compared to control fibroblasts.
Discussion
More than 100 pathogenic variants of the MFN2 gene are known, mostly missense mutations that selectively target the GTPase domain and the coil-coiled region. We report for the first time the case of a patient with a heterozygous missense MFN2 mutation (leading to the amino acid change Met234Ile) in the GTPase domain, which is likely to be associated with
CMT2A. Our study represents to our knowledge, the first analysis of changes in mitochondrial structure and function in a cell line of a patient carrying this genetic mutation.
From a clinical point of view, our patient presents a disabling neuropathy with an early onset, a progressive evolution and a motor impairment that predominates over sensory symptoms, which can be considered the classical phenotype of CMT2A, in its severe form. We did not find optic atrophy or brain lesions on MRI, but we did find pyramidal involvement and postural hands tremor. EDX shows preserved MCV, with variable reduction in the amplitude of compound muscle action potentials and distal sensory potentials, mainly in the lower limbs.
The mechanism underlying the pathogenesis of CMT2A has not yet been fully elucidated. Mitochondria play a key role in ensuring the efficient energy metabolism necessary for the proper functioning of peripheral nerves. The continuous process of fusion and fission, underlying mitochondrial dynamics, is crucial for the maintenance of the shape, size and number of mitochondria, as well as their axonal transport and their interaction with other organelles. Our CMT2A fibroblasts show no alterations in morphology but we found a decrease in mitochondrial bioenergetics. Similar findings have already been reported previously [25]. The absence of morphological changes could also be explained by the fact that fibroblasts express MFN1 which could compensate for some of the MFN2 deficit. In order to further stress the cell and reveal mitochondrial dysfunction, we replaced glucose with galactose in the growth medium, forcing fibroblasts to rely on mitochondrial oxidative phosphorylation [26]. Culture in galactose medium revealed a fragmentation of the mitochondrial network and less connected mitochondria in CMT patient compared to control fibroblasts corroborating the hypothesis of the pathogenic role of this mutation. This testifies to the fragility of the mitochondria, that are unable of meeting the cellular energy needs in aerobiosis as has already been described in senescent cells [27]. It can be speculated that cells with high ATP production requirements may be particularly vulnerable at the MFN2 deficit [28]. It is unclear whether the described mutation on the MFN2 gene causes an alteration of mitochondrial fusion resulting in ATP deficiency or whether it has a direct effect on bioenergetics [29] resulting in mitochondrial fragmentation. The reduction in membrane potential found appears to be secondary to dysfunctional mitochondrial coupling [30]. The scientific literature confirms that mitochondria with hyperpolarized membrane potential stagnating in the neuronal soma without ever reaching more crucial regions such as the growth cone or dendritic endings, as they are dysfunctional [31]. They will be later eliminated by autophagy [29].
What is surprising is that the above-mentioned changes are found exclusively in the proband and not in the mother carrying the same genetic mutation. Genetic analysis did not reveal any substantial differences between the mother's genome and that of the proband except that the proband is the carrier of an additional mutation, the same VUS as the father in the PMP22 gene. Myelin, like all glial cells, has been shown to supply lactate and glucose as an energy substrate for mitochondria to ensure axonal integrity [32]. We could speculate that the VUS found on PMP22 gene may generate only partially functional PMP22 protein. As PMP22 is involved in maintaining myelin structure, this could alter communication with other internodal proteins and thus reduce the supply of energy substrate for the mitochondria. We can therefore speculate that we are facing a double trouble and that the presence of VUS on PMP22 may promote the mitochondria dysfunction caused by the MFN2 mutation. Mutations in CASPR or in NF155 have already been shown to be responsible for the accumulation of mitochondria with abnormal morphology near the nodes [33],[34]. The pathogenetic hypothesis is therefore that impairment of myelin composition may facilitate alterations in the transport of already dysfunctional mitochondria. The absence of aggregation of PMP22 in fibroblasts is probably due to the fact that VUS does not cause an excess of the protein like duplication but, rather, changes the configuration of the protein making it less functional. This hypothesis should be corroborated using other in vivo models.
Conclusion
In conclusion, our data support the pathogenetic role of the c.702G>A MFN2 mutation and indicate that a more complex gene regulatory mechanism is involved in determining the severity of the clinical phenotype. Analysis of mitochondrial function from fibroblast cultures proves to be a useful biomarker in the era of next-generation sequencing.
Acknowledgments
Authors would like to thank the patients for their participation in this study. The study was supported by an Association Française contre les Myopathies (AFM) grant (#23207 to S.B.) and Agence Nationale pour la Recherche (ANR) grant (Labex ICST). We thank Sophie Pagnotta from CCMA (electron microscopy platform) for performing electron microscopy experiments.
Statements and Declarations
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
References
- Barreto LCLS, Oliveira FS, Nunes PS et Epidemiologic Study of Charcot-Marie-Tooth Disease: A Systematic Review. Neuroepidemiology 46 (2016): 157-165.
- El-Abassi R, England JD, Carter Charcot-Marie- Tooth disease: an overview of genotypes, phenotypes, and clinical management strategies. PM R 6 (2014): 342-355.
- Saporta ASD, Sottile SL, Miller LJ, et Charcot-Marie- Tooth disease subtypes and genetic testing strategies. Ann Neurol 69 (2011): 22-33.
- Reilly MM. Sorting out the inherited neuropathies. Pract Neurol 7 (2007): 93-105.
- Morena J, Gupta A, Hoyle Charcot-Marie-Tooth: From Molecules to Therapy. Int J Mol Sci 20 (2019): E3419.
- Li J, Parker B, Martyn C, et al. The PMP22 gene and its related Mol Neurobiol 47 (2013): 673-698.
- Feely SME, Laura M, Siskind CE et al. MFN2 mutations cause severe phenotypes in most patients with CMT2A. Neurology 76 (2011): 1690-1696.
- Züchner MFN2 Hereditary Motor and Sensory Neuropathy. In: Adam MP, Everman DB, Mirzaa GM, et al., editors. GeneReviews®. Seattle (WA): University of Washington, Seattle, (1993).
- Pipis M, Feely SME, Polke JM, et al. Natural history of Charcot-Marie-Tooth disease type 2A: a large (2013;) international multicentre study. Brain 143 (2020): 3589-
- Chung KW, Kim SB, Park KD, et al. Early onset severe and late-onset mild Charcot-Marie-Tooth disease with mitofusin 2 (MFN2) mutations. Brain 129 (2006): 2103-2118.
- Choi B-O, Nakhro K, Park HJ et al. A cohort study of MFN2 mutations and phenotypic spectrums in Charcot- Marie-Tooth disease 2A patients. Clin Genet 87 (2015): 594-598.
- Lee M, Park C-H, Chung H-K et Cerebral white matter abnormalities in patients with charcot-marie-tooth disease. Ann Neurol 81 (2017): 147-151.
- Brockmann K, Dreha-Kulaczewski S, Dechent P, et al. Cerebral involvement in axonal Charcot-Marie-Tooth neuropathy caused by mitofusin2 J Neurol 255 (2008): 1049-1058.
- Muglia M, Zappia M, Timmerman V, et Clinical and genetic study of a large Charcot-Marie-Tooth type 2A family from southern Italy. Neurology 56 (2001): 100-103.
- Chung KW, Suh BC, Cho SY, et al. Early-onset Charcot- Marie-Tooth patients with mitofusin 2 mutations and brain J Neurol Neurosurg Psychiatry 81 (2010): 1203-1206.
- Lawson VH, Graham BV, Flanigan KM. Clinical and electrophysiologic features of CMT2A with mutations in the mitofusin 2 gene. Neurology 65 (2005): 197-204.
- Lin H-P, Ho KWD, Jerath Late onset CMT2A in a Family with an MFN2 Variant: c.2222T>G (p.Leu741Trp). J Neuromuscul Dis 6 (2019): 259-261.
- Cartoni R, Martinou J-C. Role of mitofusin 2 mutations in the physiopathology of Charcot-Marie-Tooth disease type Exp Neurol 218 (2009): 268-273.
- Fenton AR, Jongens TA, Holzbaur ELF. Mitochondrial dynamics: Shaping and remodeling an organelle Curr Opin Cell Biol 68 (2021): 28-36.
- Yapa NMB, Lisnyak V, Reljic B, et al. Mitochondrial dynamics in health and disease. FEBS Lett 595 (2021): 1184-1204.
- Vital A, Latour P, Sole G, et al. A French family with Charcot-Marie-Tooth disease related to simultaneous heterozygous MFN2 and GDAP1 Neuromuscul Disord 22 (2012): 735-741.
- Meggouh F, de Visser M, Arts WFM, et al. Early onset neuropathy in a compound form of Charcot-Marie-Tooth Ann Neurol 57 (2005): 589-591.
- Cassereau J, Casasnovas C, Gueguen N, et Simultaneous MFN2 and GDAP1 mutations cause major mitochondrial defects in a patient with CMT. Neurology 76 (2011): 1524-1526.
- Aguer C, Gambarotta D, Mailloux RJ, et al. Galactose enhances oxidative metabolism and reveals mitochondrial dysfunction in human primary muscle cells. PLoS One 6 (2011): e28536.
- Amiott EA, Lott P, Soto J, et Mitochondrial fusion and function in Charcot-Marie-Tooth type 2A patient fibroblasts with mitofusin 2 mutations. Exp Neurol 211 (2008): 115-127.
- Beresewicz M, Charzewski L, Krzysko KA, et Molecular modelling of mitofusin 2 for a prediction for Charcot-Marie-Tooth 2A clinical severity. Sci Rep 8 (2018): 16900.
- Hutter E, Renner K, Pfister G, et al. Senescence-associated changes in respiration and oxidative phosphorylation in primary human fibroblasts. Biochem J 380 (2004): 919-928.
- Hyun D-H, Lee J. A New Insight into an Alternative Therapeutic Approach to Restore Redox Homeostasis and Functional Mitochondria in Neurodegenerative Diseases. Antioxidants (Basel) 11 (2021):7
- Loiseau D, Chevrollier A, Verny C, et al. Mitochondrial coupling defect in Charcot-Marie-Tooth type 2A disease. Ann Neurol 61 (2007): 315-323.
- Twig G, Elorza A, Molina AJA, et Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27 (2008): 433-446.
- Baloh RH, Schmidt RE, Pestronk A, et al. Altered axonal mitochondrial transport in the pathogenesis of Charcot- Marie-Tooth disease from mitofusin 2 J Neurosci 27 (2007): 422-430.
- Nave K-A. Myelination and the trophic support of long Nat Rev Neurosci 11 (2010): 275-283.
- Sun X, Takagishi Y, Okabe E, et A novel Caspr mutation causes the shambling mouse phenotype by disrupting axoglial interactions of myelinated nerves. J Neuropathol Exp Neurol 68 (2009): 1207-1218.
- Pillai AM, Thaxton C, Pribisko AL, et al. Spatiotemporal ablation of myelinating glia-specific neurofascin (Nfasc NF155) in mice reveals gradual loss of paranodal axoglial junctions and concomitant disorganization of axonal J Neurosci Res 87 (2009): 1773-1793.
