D-Neuron, Ligand Neuron of Trace Amine-Associated Receptor 1 (TAAR1): Key of Novel Non-D2 Receptor-Binding Antipsychotics
Article Information
Keiko Ikemoto*
Department of Neuropsychiatry, the University of Tokyo, Tokyo, Japan
*Corresponding author: Keiko Ikemoto, Department of Neuropsychiatry, the University of Tokyo, Tokyo, Japan.
Received: 21 December 2023; Accepted: 27 December 2023; Published: 09 January 2024
Citation: Keiko Ikemoto. D-Neuron, Ligand Neuron of Trace Amine-Associated Receptor 1 (TAAR1): Key of Novel Non-D2 Receptor-Binding Antipsychotics. Journal of Biotechnology and Biomedicine. 7 (2024): 15-20.
View / Download Pdf Share at FacebookAbstract
The latest psychopharmacological study showed effectiveness of a novel non-D2-receptor-binding drug, SEP-363856, for the treatment of schizophrenia. The compound is trace amine-associated receptor 1 (TAAR1) full agonist and also 5-hydroxytryptamin 1A (5-HT 1A) receptor partial agonist. I found the TAAR1 ligand neuron, D-neuron, in the striatum and nucleus accumbens (Acc), a neuroleptic acting site of human brains, though failed to find in the homologous area of monkey brains. To study human D-neuron functions, total of 154 post-mortem brains from Brain banks of Shiga University of Medical Science et al. and a modified immunohistochemical method using high qualified antibodies against monoamine-related substances, were used. The number of D-neurons in the caudate nucleus, putamen, and Acc was reduced in post-mortem brains with schizophrenia. The reduction was significant (p<0.05) in Acc. I proposed “D-cell hypothesis of schizophrenia”, that NSC dysfunction-based D-neuron reduction is cellular and molecular basis of mesolimbic dopamine (DA) hyperactivity, progressive pathophysiology and prospectiveness of TAAR1 medicinal chemistry, emphasizing importance of D-neuron.
Keywords
Dopamine; Trace amine; TAAR1; Schizophrenia; D-neuron
Article Details
Introduction
Schizophrenia is a mental illness, which afflicts approximately 1% of population, and manifests delusion, hallucination, disorganized thought, flattened affect, and impaired cognitive processes. The latest pharmacological research has demonstrated the effectiveness of a novel psychotropic agent, SEP-363956, with a unique, non-D2 receptor mechanism of action [1, 2]. The compound is trace amine-associated receptor 1 (TAAR1) full agonist, and also 5-hydroxytryptamin 1A (5-HT 1A) receptor partial agonist. In the present article, I show the histochemically visualized TAAR1 ligand neuron, D-neuron in a post-mortem brain specimen, and how the D-neuron relate to pathogenesis of schizophrenia, which is called “D-cell hypothesis of schizophrenia”. Dopamine (DA) dysfunction [3, 4], glutamate dysfunction [5,6], neurodevelopmental deficits [7,8], or neural stem cell (NSC) dysfunction [9,10], are well-known hypotheses for etiology of schizophrenia. DA dysfunction hypothesis suggested that mesolimbic DA hyperactivity caused positive symptoms such as paranoid-hallucinatory state of schizophrenia [3, 4]. It is also explained by the efficacy of DA D2 blockers for paranoid-hallucinatory state and also by hallucinogenic acts of DA stimulants including methamphetamine or amphetamine [3, 4]. Glutamate dysfunction theory was induced by the fact that intake of phencyclidine (PCP), an antagonist of N-methyl-D-aspartate (NMDA) receptor, produces equivalent to negative symptoms of schizophrenia, such as withdrawal or flattened affect, as well as positive symptoms [5,6]. The neurodevelopmental deficits hypothesis implicates that schizophrenia is the consequence of prenatal abnormalities resulting from the interaction of genetic and environmental factors [7, 8]. NSC dysfunction has also been shown to be a cause of schizophrenia [9, 10]. Although mesolimbic DA hyperactivity [3, 4] has been well documented in pathogenesis of schizophrenia, the molecular basis of this mechanism has not yet been detailed. In the present article, I show the rational of reduction of D-neurons [11] (trace amine (TA) neuron, type 1), ligand neurons of TA-associated receptor 1 (TAAR1 [12]), in the nucleus accumbens (Acc) in pathogenesis of mesolimbic DA hyperactivity of schizophrenia. The novel hypothesis, “D-cell hypothesis of schizophrenia”, is a pivotal theory to link NSC dysfunction hypothesis with DA hypothesis in etiology of schizophrenia.
D-neuron and TAAR1
The TA neuron in the rat central nervous system (CNS) was described by Jaeger et al. in 1983 [11]. Initially, they defined “the non-monoaminergic aromatic L-amino acid decarboxylase (AADC)-containing cell”, and called the “D-cell” [11]. “D” means decarboxylation. AADC is an equivalent enzyme to dopa decarboxylase (DDC). The D-cell contains AADC but not dopaminergic nor serotonergic [11]. Then, it is natural that the D-cell is thought to produce TAs [13, 14], such as β-phenylethylamine (PEA), tyramine and tryptamine. AADC is the rate-limiting enzyme for TA synthesis. However, it is confusing that these TAs are also “monoamines”, as each one has one amino residue. It would be better to use the nomenclature of “TA cells, type 1” for D-cells, and “TA neurons, type 1” for D-neurons. There are other types of TAs that are not synthesized by AADC. In the present article, I use the words, D-cell and D-neuron, signifying TA cell, type1 and TA neuron, type1, respectively.
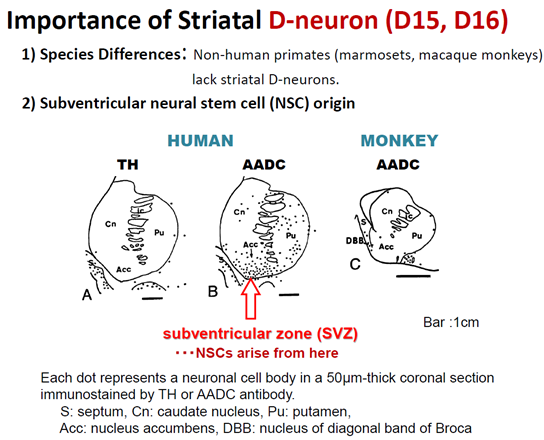
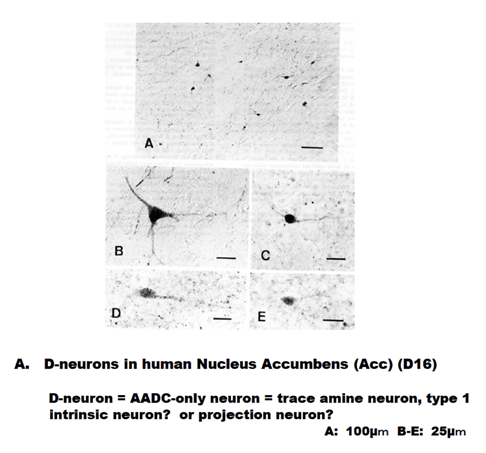
In the human Acc (Figure 1, Figure 2A), caudate nucleus (Cn) and putamen (Pu), there are D-neurons, though monkey homologous areas do not contain D-neurons (Figure 1). By using pathological and legal autopsy brains of patients with schizophrenia, I showed lack of D-neurons in the Acc (D16) of patients with schizophrenia (Figure 2B). Cloning of TA receptors in 2001 [15,16], elicited enormous efforts for exploring signal transduction of these G-protein coupled receptors whose genes are located on chromosome focus 6q23.1 [17]. The receptors have been shown to co-localize with DA or adrenaline transporters in monoamine neurons and to modulate the functions of monoamines [18, 19]. The TAAR1 having a large number of ligands, including, PEA, tyramine, 3-iodothyronamine, 3-methoxytyramine, normetanephrine, and psychostimulants, for example methamphetamine, 3,4-methylenedioxymethamphetamine (MDMA) and lysergic acid diethylamide (LSD) [15, 17, 20], has become a target receptor for exploring novel neuroleptics [21, 22].
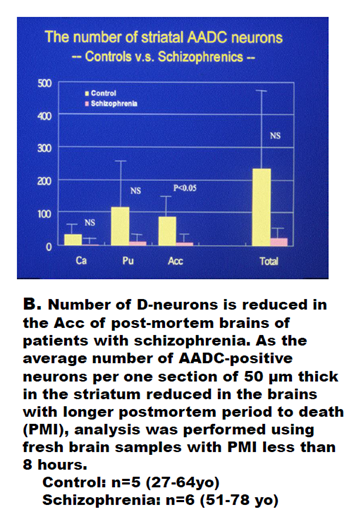
Figure 2B: Lack of striatal D-neuron in nucleus accumbens (Acc) of schizophrenia. The number of AADC-immunostained neurons (= D-neuron) [36] is reduced in the striatum of schizophrenia. In the Acc, the reduction of D-neurons is significant (P<0.05). To detect the D-neuron lacking in Acc of patients with schizophrenia, total of 154 post-mortem brains from Brain banks of Shiga University of Medical Science et al. (1997-2003) were examined. (By approval of the Ethical Committee of National Minami Hanamaki Hospital).
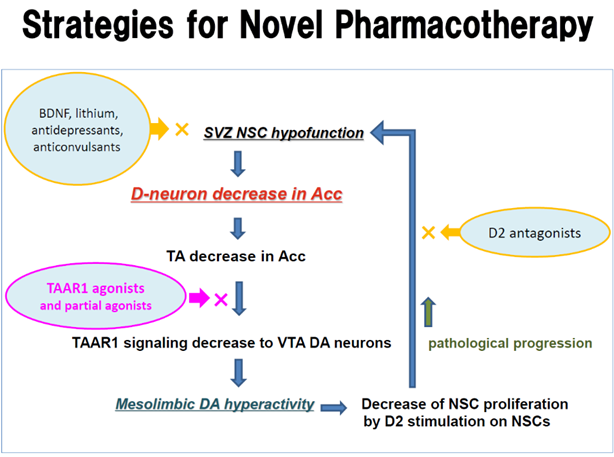
TAAR1 knockout mice showed schizophrenia-like behaviors with a deficit in prepulse inhibition [23, 24]. TAAR1 knockout mice showed greater locomotor response to amphetamine and released more DA (and noradrenaline) in response to amphetamine than wild type mice [23]. It has been shown that TAAR1 has a thermoregulatory function [24]. Interestingly, TAAR1 is the only human receptor that has been shown to bind endogenous TAs [25]. As is the important fact, TAAR1 stimulation increase of DA neurons in the midbrain ventral tegmental area (VTA) reduced firing frequency of VTA DA neurons [21-25]. This demonstrated critical role of TAAR1 stimulation decrease for mesolimbic DA hyperactivity in schizophrenia. A new theory, “D-cell hypothesis”, explains pathophysiology of mesolimbic DA hyperactivity of schizophrenia (Figure 3). In brains of patients with schizophrenia, dysfunction of NSC in the subventricular zone of lateral ventricle (SVZ) causes D-neuron decrease in the striatum and Acc [10, 26]. This induces TA decrease in these nuclei. Lateral ventricle enlargement seen in schizophrenia brain imaging [27, 28] is also due to NSC dysfunction [9, 10]. TAAR1 stimulation decrease in DA terminals of VTA DA neurons, caused by TA decrease, increases firing frequency of VTA DA neurons [22, 23]. This increases DA release and DA turnover in the Acc, resulting in mesolimbic DA hyperactivity (Fig. 3). D2 stimulation of NSC in the striatum is shown to inhibit forebrain NSC proliferation [26, 29]. Striato-accumbal DA hyperactivity may accelerate D-neuron decrease, which accelerates hyperactivity of mesolimbic DA system. D2 blocking agents in pharmacotherapy of schizophrenia block inhibition to forebrain NSC proliferations [29], and may also form TAAR1 ligands, such as 3-methoxytyramine and normetanephrine [30]. This is consistent with clinical evidences that initial pharmacotherapy using D2 antagonists is critical for preventing progressive pathognomonic procedures of schizophrenia [31].
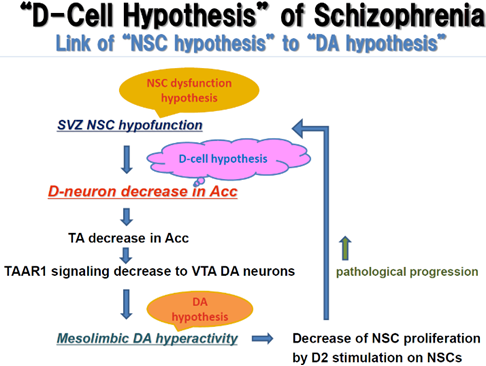
Figure 3: Scheme of “D-cell hypothesis of schizophrenia”. In a schizophrenia brain, dysfunction of neural stem cells (NSC) in the subventricular zone (SVZ) of lateral ventricle causes D-neuron decrease in the striatum and nucleus accumbens (Acc). This induces TA decrease in these nuclei and TAAR1 stimulation decrease into DA terminals of VTA DA neurons, causing firing frequency increase in VTA DA neurons [22, 23]. This increases DA release and DA turnover in the Acc, being the molecular basis of mesolimbic DA hyperactivity of schizophrenia. Striatal DA hyperactivity causes excessive D2 stimulation of NSC in the striatum and inhibits forebrain NSC proliferation [26, 29], which accelerates D-neuron decrease and accelerates mesolimbic DA hyperactivity. The rational is that lack of striato-accumbal D-neuron, due to SVZ NSC dysfunction, is pivotal in explaining mesolimbic DA hyperactivity of schizophrenia.
D-cell hypothesis not only links DA hypothesis with NSC dysfunction hypothesis, but also explains the mechanisms of disease progression of schizophrenia (Figure 3, Figure 4). To inhibit the cycle of pathological progression, intervention indicated by × in figure 4 is effective. Early studies have shown formation of some TAAR1 ligands by administration of D2 antagonists including haloperidol and chlorpromazine [30]. In animal studies, effectiveness of TAAR1 ligands for schizophrenia-like symptoms of schizophrenia model animals has been shown [22]. Recent clinical trial studies have shown the efficacy of a novel agent, SEP-363856, TAAR1 full agonist and 5HT 1A receptor partial agonist for treatment of schizophrenia [1, 2]. Efficacy of TAAR1 full agonist to schizophrenia seems to be equivalent to that of DA agonist for treatment of Parkinson’s disease which lacks DA neurons in the midbrain substantia nigra.
TAAR1 agonists and partial agonists
D2 antagonists
Neurotrophic substances
Disease progression is inhibited by neurotrophic substances, for example, brain-derived neurotrophic factor (BDNF), lithium, anticonvulsants, or antidepressants. Neurotrophic effects of these substances activate NSC functions, and inhibit striato-accumbal D-neuron decrease. Stress, aging, and alcohol intake suppresses NSC functions, which also causes a psychotic state.
D2 antagonists
Duration of untreated psychosis is a predictor of long-term outcome of schizophrenia. Importance of early intervention for first episode schizophrenia by using D2 antagonist has been emphasized [31]. Chronic D2 blocker administration has preventive effect for recurrence of psychoses. D2 antagonists block disease progression (Figure 4). D2 antagonists may have dual actions for inhibiting this cycle of disease progression by also forming some TAAR1 ligands (for example 3-methoxytyramine or normetanephrine) [30] which increase TAAR1 stimulation (Figure 4).
Neurotrophic substances
Disease progression is inhibited by neurotrophic substances (Figure 4), for example, brain-derived neurotrophic factor (BDNF), lithium, anticonvulsants, or antidepressants. Neurotrophic effects of these substances activate NSC functions [32], and inhibit striato-accumbal D-neuron decrease. Stress, aging, and alcohol intake suppresses NSC functions, which causes vulnerability to a psychotic state. Although it has not yet been detailed which type of TA in the human central nervous system is related to psychiatric symptoms, clinical and/or pharmacological observations enables us to determine a pivotal type of TA. Early in 1994, Sabelli and Mosnaim proposed “Phenylethylamine hypothesis of affective behavior”, indicating involvement of PEA in animal behaviours [33]. PEA, having similar chemical structure of amphetamine, is the most probable TA which affects on psychiatric symptoms. One of the initial clinical symptoms frequently observed in first episode schizophrenia is disturbance of sleep-wake-rhythm, that is, insomnia and daytime hypersomnia. As PEA is the specific substrate for monoamine oxidase, type B (MAOB), MAOB knockout mice contained elevated level of PEA in the striatum by 8-10 times of that of controls [34]. Clinically, MAOB inhibitor, selegiline ameliorates daytime sleepiness of narcolepsy or other neuropsychiatric diseases, which is explained by PEA increase via inhibition of PEA degradation. Subventricular NSC dysfunction leads to D-neuron decrease and consequent TA decrease in Acc and striatum of schizophrenia [11].
From an aspect of food intake, PEA is included in chocolate. High incidence of chocolate habit of Novel Prizewinners, that is, having chocolate more than twice a week, has been reported [35]. PEA seems to be closely related to higher mental functions. Too much chocolate intake of children is generally restricted, possibly aimed at preventing D-neuron down regulation. Based on recent progress in medicinal chemistry, there are various TAAR1 ligands. The 1st chemical compound proceeded to phase III clinical trial was SEP-363856 [1, 2], of which successful results have recent been shown. SEP-363856, TAAR1 full agonist, did not show extrapyramidal symptoms, implicating an improved antipsychotic agent [1, 2]. “D-cell hypothesis” proposed by a postmortem brain study of schizophrenia shows D-cell-involved etiological dynamism in also wide spectrum of psychotic state in neurological as well as psychiatric illnesses. NSC functions affect not only on D-neuron activity, but also clinical course and prognoses of neuropsychiatric illnesses (Figure 3, Figure 4). NSC protection as well as NSC activation is supposed to be critical for prevention and improving prognoses of neuropsychiatric illnesses.
Conclusion
The D-neuron, i.e., the TA neuron, type 1, is a clue for neuropsychiatric research, and key to TAAR1 medicinal chemistry. The rational is that D-cell hypothesis of schizophrenia is a pivotal theory to link NSC dysfunction hypothesis with DA hypothesis, which explains molecular basis of mesolimbic DA hyperactivity of schizophrenia. Effectiveness of SEP-363856 in the treatment of schizophrenia verified “D-cell hypothesis of schizophrenia”. Further exploration of NSC- and D-neuron-mediated signal transduction of normal and/or disease state(s) is crucial for future direction of neuropsychiatric research.
Acknowledgement
There is no COI (conflict of interest). The present study was supported by Grants-in-Aids for Scientific Research from Japan Society for the Promotion of Science (C1-10680713, C1-12680740, C-22591265), Research Resource Network (RRN), and Sumitomo Pharmaceutical Corporation, and by INSERM U52, CNRS ERS 5645, Claude Bernard University (France), Shiga University of Medical Science (Japan), Fujita Health University (Japan), Clinical Research Institute of National Minami Hanamaki Hospital (Japan), Fukushima Medical University (Japan ) and Iwaki City in Japan. The post-mortem brain study was admitted by the Ethical Committee of National Minami Hanamaki Hospital (Japan). The author is grateful for Professor Michal Jouvet, and Dr. Kunio Kitahama (INSERM U52, CNRS ERS 5645, Department of Experimental Medicine, Claude Bernard University, France).
References
- Dedic N, et al. SEP-363856, a Novel Psychotropic Agent with a Unique, Non-D2 Receptor Mechanism of Action. J Pharmacol Exp Ther 371 (2019): 1-14.
- Koblan KS, et al. A Non-D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N Engl J Med 382 (2020): 1497-1506.
- Hokfelt T, Ljungdahl A, Fuxe K, et al. Dopamine nerve terminals in the rat limbic cortex: aspects of the dopamine hypothesis of schizophrenia. Science 184 (1974): 177-179.
- Toru M, Nishikawa T, Mataga N, et al. Dopamine metabolism increases in post-mortem schizophrenic basal ganglia. J Neural Transm 54 (1982): 181-191.
- Watis L, Chen SH, Chua HC, et al. Glutamatergic abnormalities of the thalamus in schizophrenia: a systematic review. J Neural Transm 115 (2008): 493-511.
- Olbrich HM, et al. Frontolimbic glutamate alterations in first episode schizophrenia: evidence from a magnetic resonance spectroscopy study. World J Biol Psychiatry 9 (2008): 59-63.
- Christison GW, Casanova MF, Weinberger DR, et al. A quantitative investigation of hippocampal pyramidal cell size, shape, and variability of orientation in schizophrenia. Arch Gen Psychiatry 46 (1989): 1027-1032.
- McGlashan TH, Hoffman RE. Schizophrenia as a disorder of developmentally reduced synaptic connectivity. Arch Gen Psychiatry 57 (2000): 637-648.
- Duan X, et al. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130 (2007): 1146-1158.
- Reif A, et al. Neural stem cell proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry 11 (2006): 514-522.
- Jaeger CB, et al. Some neurons of the rat central nervous system contain aromatic-L-amino-acid decarboxylase but not monoamines. Science 219 (1983): 1233-1235.
- Pei Y, et al. Trace amines and the trace amine-associated receptor 1: Pharmacology, neurochemistry, and clinical implications. Frontiers in Neuroscience 10 (2016): 148.
- Boulton AA. Amines and theories in psychiatry. Lancet 2 (1974): 52-53.
- Boulton AA, Juorio AV. The tyramine; are they involved in the psychoses? Biol Psychiatry 14 (1979): 413-419.
- Bunzow JR, et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol Pharmacol 60 (2001): 1181-1188.
- Borowsky B, et al. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc Natl Acad Sci USA 98 (2001): 8966-8971.
- Miller GM. The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity. J Neurochem 116 (2011): 164-176.
- Xie Z, Miller GM. Trace amine-associated receptor 1 as a monoaminergic modulator in brain. Biochem Pharmacol 78 (2009): 1095-1004.
- Lindemann L, et al. Trace amine-associated receptor 1 modulates dopaminergic activity. J Pharmacol Exp Ther 324 (2008): 948-956.
- Zucchi R, Chiellini G, Scanlan TS, et al. Trace amine-associated receptors and their ligands. Br J Pharmacol 149 (2006): 967-978.
- Bradaia A, et al. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc Natl Acad Sci USA 106 (2009): 20081-20086.
- Revel FG, et al. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol Psychiatry 18 (2013): 543-556.
- Wolinsky TD, et al. The Trace Amine 1 receptor knockout mouse: an animal model with relevance to schizophrenia. Genes. Brain Behav 6 (2007): 628-639.
- Panas HN, et al. Normal thermoregulatory responses to 3-iodothyronamine, trace amines and amphetamine-like psychostimulants in trace amine associated receptor 1 knockout mice. J Neurosci Res 8 (2010): 1962-1969.
- Lam VM, et al. In-vivo pharmacology of Trace-Amine Associated Receptor 1. Eur. J. Pharmacol 763 (2015): 136-142.
- Sanai N, et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427 (2004): 740-744.
- Degreef G, et al. Volumes of ventricular system subdivisions measured from magnetic resonance images in first-episode schizophrenic patients. Arch Gen Psychiatry 49 (1992): 531-537.
- Horga G, et al. Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur Arch Psychiatry Clin Neurosci 261 (2011): 467-476.
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci 25 (2005): 5815-5823.
- Carlsson A, Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acra Pharmacol Toxicol (Copenh) 20 (1963): 140-144.
- Penttilä M, Jääskeläinen E, Hirvonen N, et al. Duration of untreated psychosis as predictor of long-term outcome in schizophrenia: systematic review and meta-analysis. Br. J. Psychiatry 205 (2014): 88-94.
- Fernandes BS, et al. Peripheral brain-derived neurotrophic factor in schizophrenia and the role of antipsychotics: meta-analysis and implications. Mol. Psychiatry 20 (2015): 1108-1119.
- Sabelli HC, Mosnaim AD. Phenylethylamine hypothesis of affective behavior. Am. J. Psychiatry 131 (1974): 695-699.
- Grimsby J, et al. Increased stress response and beta-phenylethylamine in MAOB-deficient mice. Nat. Genet 17 (1997): 206-210.
- Golomb BA. Chocolate habits of Nobel prizewinners. Nature 499 (2013): 409.
- Ikemoto K, et al. Quantitative analysis of tyrosine hydroxylase-, aromatic L-amino acid decarboxylase- or phenylethanolamine-N-methyltransferase-immunoreactive neurons in the human medullary C1 region. Acta Histochem. Cytochem 33 (2000): 259-265.