Differentiating Malignant Hyperthermia, Neuroleptic Malignant Syndrome, and Serotonin Syndrome: A Clinical Review and Diagnostic Algorithm
Article Information
Brian Gabriel*
4th Year Osteopathic Medical Student, Ohio University Heritage College of Osteopathic Medicine, Athens, Ohio, USA
*Corresponding Author: Brian Gabriel, 4th Year Osteopathic Medical Student, Ohio University Heritage College of Osteopathic Medicine, Athens, Ohio, USA.
Received: 17 August 2025; Accepted: 25 August 2025; Published: 03 September 2025
Citation: Brian Gabriel. Differentiating Malignant Hyperthermia, Neuroleptic Malignant Syndrome, and Serotonin Syndrome: A Clinical Review and Diagnostic Algorithm. Anesthesia and Critical Care 7 (2025): 64-67.
View / Download Pdf Share at FacebookAbstract
This review summarizes the clinical presentation, distinguishing clinical features, and underlying pathophysiology of malignant hyperthermia (MH), neuroleptic malignant syndrome (NMS), and serotonin syndrome (SS). MH results from exposure to inhalational anesthetics or succinylcholine and is due to uncontrolled calcium release in skeletal muscle cells. Hypercarbia and masseter spasms occur early, followed by hyperthermia and rigor-mortis–like rigidity. NMS results from dopamine receptor blockade in the central nervous system. Associated features include altered mental status, dysautonomia, and lead-pipe rigidity, typically developing over several days. SS is produced by medications that prevent serotonin degradation and usually presents abruptly. Characteristic features of SS include clonus and gastrointestinal symptoms.
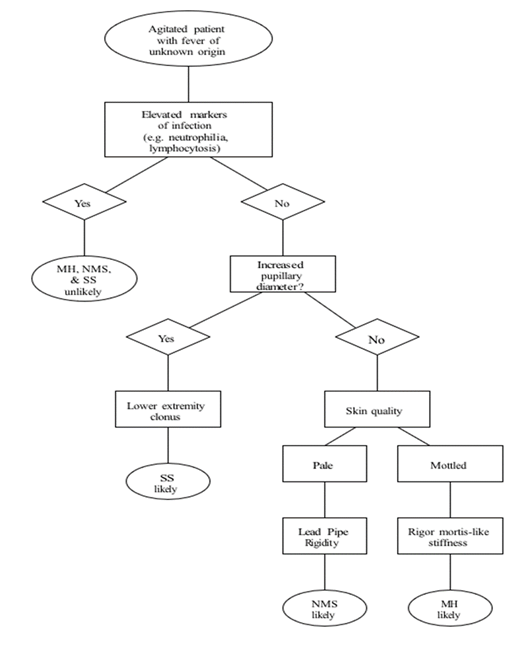
MH, NMS, and SS share overlapping clinical features, and no definitive diagnostic tests exist for these conditions. A thorough medication history is the most important diagnostic tool, but patients often present with confusion or altered mental status that limit their ability to communicate. When history is unavailable, diagnosis may be aided by recognizing characteristic features associated with each condition. A novel diagnostic algorithm is proposed that combines key findings to assist clinicians in distinguishing MH, NMS, and SS. Improved recognition and earlier diagnosis will help guide treatment and improve patient outcomes.
Keywords
Malignant hyperthermia; Neuroleptic malignant syndrome; Serotonin syndrome; Differential diagnosis; Diagnostic algorithm
Malignant hyperthermia articles; Neuroleptic malignant syndrome articles; Serotonin syndrome articles; Differential diagnosis articles; Diagnostic algorithm articles.
Article Details
1. Introduction
Muscle rigidity and hyperthermia rarely occur together, but when they do, the presentation is life-threatening. Determining the underlying cause is difficult and often requires an extensive differential diagnosis. Three potential etiologies—malignant hyperthermia (MH), neuroleptic malignant syndrome (NMS), and serotonin syndrome (SS)—frequently present with overlapping signs and symptoms. The similarities between these conditions, combined with the lack of definitive diagnostic testing, make diagnosis challenging. A comprehensive medication history is the most critical component of establishing the correct diagnosis, although this is not always feasible. When history is unobtainable, physical and laboratory findings may help guide diagnosis.
2. Malignant Hyperthermia
MH is a hypermetabolic crisis resulting from a defect in skeletal muscle calcium channels (ryanodine receptor), most often triggered by exposure to inhalational anesthetics or succinylcholine. Rare cases have been reported in association with heat stroke and stress [1]. Patients may tolerate general anesthesia uneventfully but develop MH upon later exposures [1]. Although the receptor defect is heritable, family history has not proven to be a reliable predictor of susceptibility [2].
Symptom onset usually occurs within one hour after anesthesia but can appear up to one hour into the perioperative period [2,3]. The most salient findings are muscle rigidity and hyperthermia, although they may not be the first symptoms to appear and may be absent in some cases [2]. Rigidity is often described as “board-like” and resembles rigor mortis. The rigidity itself can exacerbate hyperthermia by preventing muscle relaxation. Core temperature monitoring is essential when MH is suspected, as temperatures can rise by 1–2°C every five minutes, reaching 46°C or higher [4-6].
Masseter spasms are often the earliest sign, especially when inhalational anesthetics are administered with succinylcholine, and they rarely occur in NMS or SS [2,3]. Hypercarbia should raise suspicion for MH in both operative and postoperative settings. An end-tidal CO2 >55 mmHg during controlled ventilation or >60 mmHg during spontaneous ventilation is highly suggestive. Similarly, arterial blood gases may show PaCO2 >60 mmHg (controlled) or >65 mmHg (spontaneous), along with pH <7.25 [7].
Treatment for MH includes prompt administration of dantrolene to reduce muscle rigidity, along with supportive measures for hyperthermia and metabolic derangements.
3. Neuroleptic Malignant Syndrome
NMS results from dopamine receptor antagonism, most commonly due to antipsychotic medications (e.g., aripiprazole, haloperidol, risperidone) or antiemetic dopamine antagonists (e.g., metoclopramide, promethazine). Rapid dose escalation, switching to a higher-potency agent, or overdose may precipitate NMS.
The hallmark features of NMS are altered mental status, generalized “lead-pipe” rigidity, hyperthermia, and autonomic instability [8]. Symptoms typically appear in sequence: mental status changes first, followed by rigidity, then hyperthermia, and finally autonomic dysfunction [5,9,10]. The progression is slow compared with MH or SS, with symptom onset usually over 1–3 days (commonly around two days).
Mental status changes may include mutism, stupor, disorientation, or coma [5,11,12]. Rigidity is generalized, and hyperthermia occurs in approximately 40% of cases, with temperatures often exceeding 40°C [5,9]. These elevations can rapidly progress to multiorgan failure if untreated. Diaphoresis, pallor, labile hypertension, and orthostatic hypotension are also seen [9,10], features generally not associated with MH or SS.
Laboratory findings may help support diagnosis. Creatine kinase (CK) is often markedly elevated; DSM-5 criteria include a CK ≥4× the upper limit of normal [14]. Levels >600 IU/L are common, and >1000 IU/L is highly suggestive [9,11]. Serum iron concentrations are often low (mean ~5.71 μmol/L) and may provide additional diagnostic utility [9].
Treatment requires immediate discontinuation of dopamine antagonists and supportive care. Bromocriptine, a dopamine agonist, is first-line pharmacotherapy. In severe cases, it may be combined with dantrolene, although the added benefit is unclear.
4. Serotonin Syndrome
SS is defined by the triad of altered mental status, autonomic instability, and neuromuscular hyperactivity due to toxic levels of serotonin in the central nervous system. Presentations range from mild to severe. Common causative agents include SSRIs (sertraline, fluoxetine, escitalopram), SNRIs (duloxetine, venlafaxine), MAOIs (phenelzine, linezolid), and serotonin-releasing agents (e.g., amphetamines, MDMA). High-risk cases often involve concurrent use of multiple serotonergic agents, such as an SSRI and MAOI [15].
Symptoms typically develop within 24 hours of exposure and progress rapidly [5,16]. Mental status changes include anxiety, agitation, delirium, and pressured speech, which are generally milder than those seen in NMS [17]. Many patients have psychiatric comorbidities, making these symptoms more difficult to interpret. Gastrointestinal symptoms—including nausea, vomiting, diarrhea, and hyperactive bowel sounds—are more common in SS than in NMS [5,16].
Autonomic findings include tachycardia, hypertension, diaphoresis, sialorrhea, dilated pupils, and hyperthermia. Hyperthermia in SS is usually less severe than in MH or NMS, but fatal cases may exceed 41.6°C [9,16]. Hypotension occurs more frequently than hypertension [16].
Neuromuscular abnormalities are the most diagnostic features. Myoclonus occurs in nearly half of cases, but clonus (inducible, spontaneous, or ocular) and hyperreflexia are the most distinguishing findings, particularly in the lower extremities [5,15,18]. These features are not present in MH or NMS.
Treatment consists of discontinuing serotonergic agents and providing supportive care to control hyperthermia and autonomic dysfunction. Cyproheptadine is the drug of choice, particularly in mild to moderate cases, although its role in severe cases is less clear.
5. Conclusion
MH, NMS, and SS share many clinical similarities, making differentiation challenging. A thorough medication history remains the strongest tool for identifying the offending agent, but when this is unobtainable, physical findings and laboratory results can be invaluable. The proposed diagnostic algorithm aims to help clinicians distinguish among these syndromes in real time, facilitating earlier recognition and treatment. Early diagnosis and intervention are essential for improving patient outcomes (Figure 1).
References
- Prakash S, Rathore C, Rana K, et al. Fatal serotonin syndrome: a systematic review of 56 cases in the literature. Clinical toxicology (Philadelphia, Pa.) 59 (2021): 89-100.
- Larach MG, Gronert GA, Allen GC, et al. Clinical presentation, treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesthesia and Analgesia 110 (2010): 498-507.
- Visoiu M, Young MC, Wieland K, et al. Anesthetic drugs and onset of malignant hyperthermia. Anesthesia and Analgesia 118 (2014): 388-396.
- Rosenberg H, Pollock N, Schiemann A, et al. Malignant hyperthermia: a review. Orphanet Journal of Rare Diseases 10 (2015): 93.
- Boyer EW, Shannon M. The serotonin syndrome. The New England Journal of Medicine 352 (2005): 1112-1120.
- Ali SZ, Taguchi A, Rosenberg H. Malignant hyperthermia. Best practice & research. Clinical Anaesthesiology 17 (2003): 519-533.
- Larach MG, Localio AR, Allen GC, et al. A clinical grading scale to predict malignant hyperthermia susceptibility. Anesthesiology 80 (1994): 771-779.
- Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. The Journal of Nervous and Mental Disease 182 (1994): 168-173.
- Oruch R, Pryme IF, Engelsen BA, et al. Neuroleptic malignant syndrome: an easily overlooked neurologic emergency. Neuropsychiatric Disease and Treatment 13 (2017): 161-175.
- Berman BD. Neuroleptic malignant syndrome: a review for neurohospitalists. The Neurohospitalist 1 (2011): 41-47.
- Tse L, Barr AM, Scarapicchia V, et al. Neuroleptic Malignant Syndrome: A Review from a Clinically Oriented Perspective. Current Neuropharmacology 13 (2015): 395-406.
- Caroff SN, Mann SC. Neuroleptic malignant syndrome. The Medical clinics of North America 77 (1993): 185-202.
- Guzé BH, Baxter LR, Jr. Current concepts. Neuroleptic malignant syndrome. The New England Journal of Medicine 313 (1985): 163-166.
- Battle DE. Diagnostic and Statistical Manual of Mental Disorders (DSM). CoDAS 25 (2013): 191-192.
- Isbister GK, Buckley NA, Whyte IM. Serotonin toxicity: a practical approach to diagnosis and treatment. The Medical Journal of Australia 187 (2007): 361-365.
- Prakash S, Rathore C, Rana K, et al. Fatal serotonin syndrome: a systematic review of 56 cases in the literature. Clinical toxicology (Philadelphia, Pa.) 59 (2021): 89-100.
- Keck PE, Arnold LM. The serotonin syndrome. Psychiatric Annals 30 (2000): 333-343.
- Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM: Monthly Journal of the Association of Physicians 96 (2003): 635-642.
- Wang RZ, Vashistha V, Kaur S, et al. Serotonin syndrome: Preventing, recognizing, and treating it. Cleveland Clinic Journal of Medicine 83 (2016): 810-817.