Diagnostic Overlap between Arrhythmogenic Right Ventricular Cardiomyopathy and Myocarditis
Article Information
Tarun Dalia1*#, Farhad Sami2#, Arushi Dalia3, Archana Gautam4, Seyed Hamed Hosseini Dehkordi1, Mohamed El Khashab5
1Cardiovascular Disease Fellow, University of Kansas Medical Center, KS, USA
2Internal Medicine Resident, University of Kansas Medical Center, KS, USA
3Medical Student, class of 2022, Government Medical College, Amritsar, Punjab, India
4Nephrology Fellow, University of Kansas Medical Center, KS, USA
5Department of Cardiovascular Medicine, University of Kansas Medical Center, KS, USA
*Corresponding author: Tarun Dalia, Cardiovascular Disease Fellow, University of Kansas Medical Center, KS, USA.
# - Equally contributed to the manuscript.
Received: 02 December 2021; Accepted: 10 December 2021; Published: 16 December 2021
Citation: Dalia T, Sami F, Dalia A, Gautam A, Dehkordi SHH, Khashab ME. Diagnostic Overlap between Arrhythmogenic Right Ventricular Cardiomyopathy and Myocarditis. Cardiology and Cardiovascular Medicine 5 (2021): 708-714.
View / Download Pdf Share at FacebookAbstract
Background: Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D) is an inherited cardiomyopathy.
Case: 20 years old male had out of hospital cardiac arrest while playing basketball. Bystander CPR was started immediately, and ROSC was achieved after 2 shocks for Vfib. He was intubated and urgently taken to the local cardiac catherization lab. LHC showed normal coronary anatomy. Echo showed biventricular failure with LVEF of 5-10%. He was transferred to our hospital for advanced heart failure therapies. EKG showed normal sinus rhythm with T wave inversions in V1-V3 lead, normal QTc. Emergent RHC with endomyocardial biopsy was performed. Decision was made to proceed with VA-ECMO placement for cardiogenic shock. The biopsy showed inflammatory infiltrate and focal associated myocyte damage. He started showing signs of recovery on day 2 without needing immunosuppressive medications. He was diagnosed with myocarditis and discharged on day 7 with full LVEF recovery. One month later, he is asymptomatic. His echo shows dyskinetic and dilated RV with normal LV. Genetic testing showed PKP2 mutation. Hence, the diagnosis was modified to ARVC/D.
Conclusion: ARVD poses a diagnostic challenge, high index of clinical suspicion is required. Myocarditis can mimic ARVD. VA-ECMO can be utilized as bridge to recovery in these patients.
Keywords
Arrhythmogenic right ventricular cardiomyopathy (ARVC/D); Extracorporeal membrane Oxygenation (ECMO); Myocarditis; sudden cardiac death and PKP2 mutation
Article Details
Learning Objectives
- Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia can mimic conditions like Myocarditis, sarcoidosis and dilated cardiomyopathy. Hence can pose a diagnostic challenge. High index of clinical suspicion is required for diagnosis.
- Temporary mechanical support devices like VA-ECMO can be considered as bridge to recovery in these patients with cardiogenic shock.
1. Introduction
Sudden Cardiac Death (SCD) in a young, healthy individual is a tragic event. Annual incidence of sudden cardiac death in the United States (US) has been estimated to be 180 to 450,000 per year [1]. Coronary artery disease, channelopathies, myocarditis and structural heart disease are recognized as important causes of SCD. Early recognition of genetic causes of SCD can help prevent deaths in this young cohort. However, in absence of a family history of unexplained death, the diagnosis of an inherited cause of cardiomyopathy can be challenging at presentation and potentially missed. Arrhythmogenic Cardiomyopathy/Dysplasia (ARVC/D) is a heritable, genetic cause of cardiac myocyte dysfunction that predominantly affects the right ventricle. It is known to be a leading cause of SCD related to sport activities [2]. The phenotypic expression of the ARVC patient can range from asymptomatic individual to SCD caused by ventricular arrhythmias. The clinical presentation can sometime mimic myocarditis [3]. We present a case of a 20-year-old college student who suffered out-of-hospital cardiac arrest followed by successful by-stander cardiopulmonary resuscitation. The clinical picture was confounded by diagnostic overlap between myocarditis and ARVC.
2. Case report
Twenty-year-old male with no prior medical history presented to our institution as a transfer from outside hospital after suffering out-of-hospital cardiac arrest while playing basketball. Patient underwent swift by-stander cardiopulmonary resuscitation and received two shocks via Automated External Defibrillator for ventricular fibrillation. Return of Spontaneous Circulation was achieved. Immediately thereafter, he was transferred to the local Emergency department. At presentation, patient had altered mentation which necessitated intubation for airway protection. Bedside echocardiogram performed showed biventricular failure with Estimated Left Ventricle (LV) ejection fraction of 10%. Patient was taken emergently to the Cardiac Catheterization Lab (CCL) for coronary angiogram which showed patent coronary arteries. An intra-aortic balloon pump was placed, and patient was transferred to our institution for further advanced heart failure therapies. Patient is a college student. He suffered an episode of syncope 1 week ago while in school after gym class with spontaneous recovery after a few minutes. It was reportedly attributed to hypoglycemia. No history of recent illicit drug use and SCD in family.
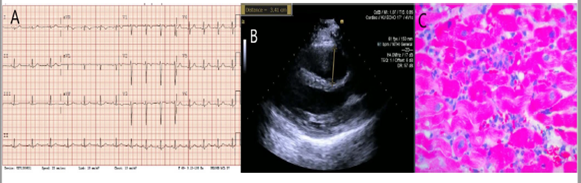
On presentation to our institute, patient was maintained on a ventilator. Physical exam showed normal jugular venous pressure, no lower extremity edema and lung crackles. Admission labs were remarkable for WBC 27K, troponin > 22.9, Creatine Kinase 3,018, ESR 2, CRP 1.47, AST 258, ALT 264, ALP 47. Urine drug screen was negative apart from benzodiazepine (likely from midazolam use during intubation). EKG showed T-wave inversions in lead V1-V3 (Figure 1A). QTc interval was prolonged at 496 ms. Stat echocardiogram was repeated which showed similar findings as above (Figure 1B). CCL was activated for urgent right heart catheterization and Endomyocardial Biopsy (EMB). Hemodynamic measurements revealed severe cardiogenic shock and bi-ventricular failure: RA pressure of 18 mmHg, RV 36/14 mmHg (16), PA 33/18 (25) mmHg, PCWP 20 mmHg, CO/CI (thermodilution) 2.75/1.35, CO/CI (Fick) 2.52/1.24. The multi-disciplinary cardiogenic shock conference was initiated to plan further course of action. Advanced heart failure cardiologist, Interventionalist, cardiothoracic surgeon, critical care physician and cardiology fellow participated in the conference. A consensus was reached to proceed with VA Extracorporeal Membrane Oxygenation (ECMO) as a temporary mechanical support. An expedited preliminary read of EMB demonstrated an inflammatory infiltrate predominantly including neutrophils and macrophages, with a few admixed T cells (Figure 1C). No giant cells were seen ruling out giant cell myocarditis. No micro-organisms were identified. The results suggested acute myocarditis. While on VA ECMO support, patient started showing signs of hemodynamic and myocardial recovery over the next 2 days without immunosuppressive medications. VA-ECMO was decannulated after 3 days. Patient was extubated the next day after that. Repeat echocardiography demonstrated EF of 70% with mild RV dilation and normal RV function.
Figure 1: A) EKG showed normal sinus rhythm with T wave inversions in V1-V3 lead, normal QTc. B) Parasternal long axis view of transthoracic echocardiogram showing dilated right ventricle outflow tract measuring 3.41 cm. C) Endomyocardial biopsy demonstrating an inflammatory infiltrate predominantly including neutrophils and macrophages, with a few admixed T cells and no giant cells seen.
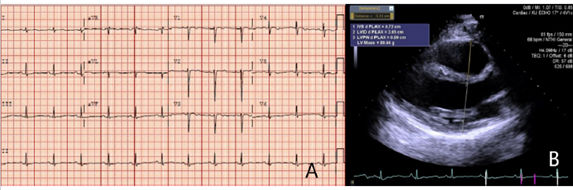
Electrophysiologists (EP) was consulted to review the case for an ICD evaluation. They recommended discharging the patient on wearable cardiac defibrillator and outpatient follow up. Patient was eventually discharged 7 days later on metoprolol succinate 25 mg daily. Cardiac MRI was performed which showed normal left ventricle and right ventricle function, mild RV dilation and no evidence of inflammation, or delayed gadolinium enhancement. ECG on follow-up showed normal sinus rhythm with T wave inversions in lead V3-V5 (Figure 2A) and echocardiography on follow-up revealed full EF recovery but right ventricular outflow tract remained mildly dilated at 3.31 cm on para sternal long axis view (Figure 2B). Patient also underwent genetic testing which revealed PKP2 mutation pointing towards diagnosis of ARVD. Patient’s presumed initial diagnosis of fulminant myocarditis corrected to ARVC. As ARVC diagnosis was confirmed, patient subsequently underwent subcutaneous ICD placement for secondary prevention of SCD three months post discharge.
3. Discussion
ARVC is a heritable disorder of cardiac myocytes that causes pathological loss of myocytes accompanied by fibrofatty replacement of myocardium of right ventricle, but bi-ventricular involvement is not uncommon. Although, the exact prevalence of ARVC in general population is unknown, it is thought to be 1:1000 to 5000 [4]. ARVC increases the risk of SCD particularly in young population and athletes and due to undiagnosed cases, its prevalence may be under-estimated by 30% [5]. The clinical course of our patient and step-wise diagnostic approach undertaken offers educational value to clinicians in diagnosing this uncommon but potentially lethal condition.
ARVC is a form of heterogenous genetic cardiomyopathy which has been linked to mutations in genes coding for desmosomes between cardiac myocytes. It is hypothesized that due to mutations affecting desmosomes, cardiac myocytes lose their inter-cellular connections which eventually culminates in cell death. Several genetic mutations have been linked to ARVC including JUP, PKP2, DSP, DSG2, etc. [6] PKP2 mutation which is relevant to our case was identified in 27% of the patients in a study conducted by Gerull et al. [7].
The clinical manifestation of ARVC patients may range from asymptomatic patients with underlying structural heart disease to patients presenting with SCD, end stage heart failure. Our patient had reported episode of syncope after a gym class at school. This could have been caused by underlying arrhythmia. Genetic testing in ARVC is warranted if there is sufficient clinical suspicion for ARVC. In today’s practice, utilization of genetic testing for cardiomyopathy can vary among clinicians. Genetic mutation is included as one of the diagnostic criteria for ARVC [8]. Our patient was initially labelled with a presumed diagnosis of acute fulminant myocarditis, but latter workup revealed ARVC based on fulfillment of 2 major criteria- EKG and genetic testing [9]. (Table 1) summarizes the revised task force criteria for diagnosis of ARVC fulfilled by our patient.
|
Global or regional dysfunction and structural alterations |
|
Major Criteria |
|
By 2D echo: |
|
Regional RV akinesia, dyskinesia, or aneurysm |
|
and 1 of the following (end diastole): |
|
PLAX RVOT ≥32 mm (corrected for body size [PLAX/BSA] ≥19 mm/m2) |
|
PSAX RVOT ≥36 mm (corrected for body size [PSAX/BSA] ≥21 mm/m2) |
|
or fractional area change ≤33 percent |
|
Repolarization abnormalities |
|
Major Criteria |
|
Inverted T waves in right precordial leads (V1, V2, and V3) or beyond in individuals >14 years of age (in the absence of complete right bundle-branch block QRS ≥120 ms) |
|
Minor Criteria |
|
Inverted T waves in leads V1 and V2 in individuals >14 years of age (in the absence of complete right bundle-branch block) or in V4, V5, or V6 |
|
Inverted T waves in leads V1, V2, V3, and V4 in individuals >14 years of age in the presence of complete right bundle-branch block |
|
Family History |
|
Major Criteria |
|
Identification of a pathogenic mutation categorized as associated or probably associated with ARVC/D in the patient under evaluation |
|
Diagnostic terminology for ARVD/C used by 2010 Revised Task Force: |
|
Definite diagnosis: 2 Major, OR 1 Major and 2 Minor criteria, OR 4 Minor from different categories |
|
Borderline diagnosis: 1 Major and 1 Minor, OR 3 Minor criteria from different categories |
|
Possible diagnosis: 1 Major, OR 2 Minor criteria from different categories |
Table 1: International Task Force Criteria for diagnosing ARVC fulfilled by our patient.
The main histopathological feature in ARVC is replacement of myocytes by fibrofatty tissue [8]. However, myocyte death is preceded by inflammation which eventually leads to cell necrosis [10]. The tissue evaluation in our patient did not reveal fibrofatty replacement but had evidence of severe inflammation without evidence of any microorganisms, which had been shown in prior studies [11,12]. This was likely early histopathological manifestation of ARVC and can mimic myocarditis. Hence, it is important that a myocarditis like presentation causing SCD in a young athlete should not preclude further investigation into ARVD as a potential cause of SCD.
The International Task Force comprising of experts from Europe and US recommend avoiding exercise to reduce risk of ventricular arrhythmias. Beta blocker therapy is also recommended to reduce ventricular arrhythmias. In addition, ICD placement is recommended for patients who have cardiac arrest or are at high risk of SCD. Catheter ablation should be considered for incessant/refractory VT triggering ICD shocks which is refractory to antiarrhythmic therapy [13]. In addition, all family members of the patient should undergo genetic testing to identify pathological gene mutations.
References
- Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation 121 (2010): 246-215.
- Corrado D, Basso C, Pavei A, et al. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. Jama 296 (2006): 1593-1601.
- Scheel PJ, Murray B, Tichnell C, et al. Arrhythmogenic Right Ventricular Cardiomyopathy Presenting as Clinical Myocarditis in Women. Am J Cardiol 145 (2021): 128-134.
- Peters S, Trümmel M, Meyners W. Prevalence of right ventricular dysplasia-cardiomyopathy in a non-referral hospital. Int J Cardiol 97 (2004): 499-501.
- Groeneweg JA, Bhonsale A, James CA, et al. Clinical Presentation, Long-Term Follow-Up, and Outcomes of 1001 Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patients and Family Members. Circ Cardiovasc Genet 8 (2015): 437-446.
- Corrado D, Basso C, Judge DP. Arrhythmogenic Cardiomyopathy. Circ Res 121 (2017): 784-802.
- Gerull B, Heuser A, Wichter T, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular cardiomyopathy. Nat Genet 36 (2004): 1162-1164.
- Gandjbakhch E, Redheuil A, Pousset F, et al. Clinical Diagnosis, Imaging, and Genetics of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: JACC State-of-the-Art Review. J Am Coll Cardiol 72 (2018): 784-804.
- Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation 121 (2010): 1533-1541.
- Thiene G, Corrado D, Nava A, et al. Right ventricular cardiomyopathy: is there evidence of an inflammatory aetiology?. Eur Heart J 12 (1991): 22-25.
- Lopez-Ayala JM, Pastor-Quirante F, Gonzalez-Carrillo J, et al. Genetics of myocarditis in arrhythmogenic right ventricular dysplasia. Heart Rhythm 12 (2015): 766-773.
- Asimaki A, Saffitz JE. The role of endomyocardial biopsy in ARVC: looking beyond histology in search of new diagnostic markers. J Cardiovasc Electrophysiol 22 (2011): 111-117.
- Corrado D, Wichter T, Link MS, et al. Treatment of Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia: An International Task Force Consensus Statement. Circulation 132 (2015): 441-453.