Diabetic Mastopathy Coexisting with Appendix Neuroendocrine Tumor in A Type 1 Diabetic Woman: A Case Report
Article Information
Gabriele Iraci Sareri1, Dorica Cataldo2, Virginia Mancini3, Laura Nigi1*, Francesco Dotta1
1Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Siena, Italy
2Diabetes and Metabolic Diseases Unit, Azienda Ospedaliera Universitaria Senese, Siena, Italy
3Section of Pathology, Department of Medical Biotechnology, University of Siena, Siena, Italy
*Corresponding Author: Laura Nigi, MD, Ph.D., Department of Medical Sciences, Surgery and Neurosciences, University of Siena, Siena, Italy
Received: 25 June 2022; Accepted: 21 July 2022; Published: 01 August 2022
Citation: Gabriele Iraci Sareri, Dorica Cataldo, Virginia Mancini, Laura Nigi, Francesco Dotta. Diabetic Mastopathy Coexisting with Appendix Neuroendocrine Tumor in A Type 1 Diabetic Woman: A Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 554-557.
View / Download Pdf Share at FacebookAbstract
Diabetic mastopathy is a rare condition representing less than 1% of all benign breast lesions, occurring preferentially in pre-menopausal women affected by long-standing type 1 diabetes, especially in presence of microvascular diabetic complications. Its exact etiopathogenesis is still unclear, however an autoimmune background is highly hypothesized. In some cases, diabetic mastopathy strongly mimicks breast cancer. To date, no widely accepted diagnostic guidelines have been established for this pathological condition, however core-needle biopsy represents the gold standard for diagnosis. Surgical excision is the primary treatment option, taking into consideration the potential development of diabetic mastopathy into a breast cancer. We here describe for the first time the coexistence of diabetic mastopathy with a neuroendocrine tumor in a long-standing type 1 diabetic woman. This association apparently seems “incidental”, however it would be interesting to verify whether other cases will be reported in order to clarify the possible relationship between these two pathological conditions.
Keywords
Diabetic mastopathy; Neuroendocrine tumor; Type 1 diabetes; Diabetic complications
Diabetic mastopathy articles; Neuroendocrine tumor articles; Type 1 diabetes articles; Diabetic complications articles
Diabetic mastopathy articles Diabetic mastopathy Research articles Diabetic mastopathy review articles Diabetic mastopathy PubMed articles Diabetic mastopathy PubMed Central articles Diabetic mastopathy 2023 articles Diabetic mastopathy 2024 articles Diabetic mastopathy Scopus articles Diabetic mastopathy impact factor journals Diabetic mastopathy Scopus journals Diabetic mastopathy PubMed journals Diabetic mastopathy medical journals Diabetic mastopathy free journals Diabetic mastopathy best journals Diabetic mastopathy top journals Diabetic mastopathy free medical journals Diabetic mastopathy famous journals Diabetic mastopathy Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Neuroendocrine tumor articles Neuroendocrine tumor Research articles Neuroendocrine tumor review articles Neuroendocrine tumor PubMed articles Neuroendocrine tumor PubMed Central articles Neuroendocrine tumor 2023 articles Neuroendocrine tumor 2024 articles Neuroendocrine tumor Scopus articles Neuroendocrine tumor impact factor journals Neuroendocrine tumor Scopus journals Neuroendocrine tumor PubMed journals Neuroendocrine tumor medical journals Neuroendocrine tumor free journals Neuroendocrine tumor best journals Neuroendocrine tumor top journals Neuroendocrine tumor free medical journals Neuroendocrine tumor famous journals Neuroendocrine tumor Google Scholar indexed journals Neuroendocrine articles Neuroendocrine Research articles Neuroendocrine review articles Neuroendocrine PubMed articles Neuroendocrine PubMed Central articles Neuroendocrine 2023 articles Neuroendocrine 2024 articles Neuroendocrine Scopus articles Neuroendocrine impact factor journals Neuroendocrine Scopus journals Neuroendocrine PubMed journals Neuroendocrine medical journals Neuroendocrine free journals Neuroendocrine best journals Neuroendocrine top journals Neuroendocrine free medical journals Neuroendocrine famous journals Neuroendocrine Google Scholar indexed journals Chemotherapy articles Chemotherapy Research articles Chemotherapy review articles Chemotherapy PubMed articles Chemotherapy PubMed Central articles Chemotherapy 2023 articles Chemotherapy 2024 articles Chemotherapy Scopus articles Chemotherapy impact factor journals Chemotherapy Scopus journals Chemotherapy PubMed journals Chemotherapy medical journals Chemotherapy free journals Chemotherapy best journals Chemotherapy top journals Chemotherapy free medical journals Chemotherapy famous journals Chemotherapy Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Leukemia articles Leukemia Research articles Leukemia review articles Leukemia PubMed articles Leukemia PubMed Central articles Leukemia 2023 articles Leukemia 2024 articles Leukemia Scopus articles Leukemia impact factor journals Leukemia Scopus journals Leukemia PubMed journals Leukemia medical journals Leukemia free journals Leukemia best journals Leukemia top journals Leukemia free medical journals Leukemia famous journals Leukemia Google Scholar indexed journals Type 1 diabetes articles Type 1 diabetes Research articles Type 1 diabetes review articles Type 1 diabetes PubMed articles Type 1 diabetes PubMed Central articles Type 1 diabetes 2023 articles Type 1 diabetes 2024 articles Type 1 diabetes Scopus articles Type 1 diabetes impact factor journals Type 1 diabetes Scopus journals Type 1 diabetes PubMed journals Type 1 diabetes medical journals Type 1 diabetes free journals Type 1 diabetes best journals Type 1 diabetes top journals Type 1 diabetes free medical journals Type 1 diabetes famous journals Type 1 diabetes Google Scholar indexed journals Diabetic complications articles Diabetic complications Research articles Diabetic complications review articles Diabetic complications PubMed articles Diabetic complications PubMed Central articles Diabetic complications 2023 articles Diabetic complications 2024 articles Diabetic complications Scopus articles Diabetic complications impact factor journals Diabetic complications Scopus journals Diabetic complications PubMed journals Diabetic complications medical journals Diabetic complications free journals Diabetic complications best journals Diabetic complications top journals Diabetic complications free medical journals Diabetic complications famous journals Diabetic complications Google Scholar indexed journals
Article Details
1. Introduction
Diabetic mastopathy is a rare pathological condition which represents less than 1% of all benign breast lesions [1], first described in 1984 [2]. According to the literature, approximately 200 cases have been reported up to 2013 [3]. Diabetic mastopathy occurs mostly in pre-menopausal women (aged between 20 and 40 years) [3] affected by long-standing type 1 diabetes, especially in the presence of chronic microvascular diabetic complications (such as diabetic nephropathy, diabetic retinopathy, diabetic neuropathy)[4, 5]. In rare cases, diabetic mastopathy may occur in women with type 2 diabetes or without diabetes, as well as in men [3, 6, 7]. In the case of diabetic patients, the time window between the onset of diabetes and the detection of diabetic mastopathy is, on average, about 20 years, and the number and size of lesions seem to correlate with the progression of the underlying disease [6]. Moreover, diabetic mastopathy has been described in association with other autoimmune diseases (e.g. arthropathies or thyroid diseases such as Hashimoto’s thyroiditis), and in these cases it most frequently occurs with breast bilateral lesions [6].
Diabetic mastopathy is also known as ”lymphatic mastopathy“, ”fibrocystic mastopathy“, or ”fibrocystic breast degeneration"[8]. Its etiopathogenesis is still unclear, however an autoimmune background is highly hypothesized. In this regard, it has been shown that breast lesions contain infiltrating B-cell lymphocytes together with an increased HLA-DR expression by breast epithelial cells; these findings are common to other autoimmune diseases [1, 9]. It has been also hypothesized that fibroinflammatory changes, characteristic of diabetic mastopathy, could be partly related to hyperglycemia and the consequent glycosilation end-products (AGEs) storage within mammarian tissue [10]. AGEs would act as antigens inducing autoimmune B-lymphocyte proliferation and autoantibody production [11]; a release of cytokines would occur subsequently, causing the expansion of the extracellular matrix with increased collagen production and decreased collagen degradation [11].
Clinically diabetic mastopathy is characterized by the presence of breast nodules (bilateral in nearly 50% of cases) that are hard, painless, irregular and movable on palpation, involving all quadrants of the mammary gland (preferentially the upper lateral/medial part) with a size, on average, ranging from 0,5 to 3,7 cm [2].
Radiologically, a localized increased density or a heterogeneous parenchymal pattern is described [12]. In such cases diabetic mastopathy strongly mimicks breast cancer and it might not be possible to accurately differentiate these two conditions by imaging studies alone [2]. Core-needle biopsy represents the gold standard for diagnosis [13]: histologically a rich, mainly perivascular and peritubular B-lymphocytic infiltrate is observed, associated with foci of dense fibrosis and lobular atrophy [6]. To date, no widely accepted diagnostic guidelines of diabetic mastopathy have been established and over the years several authors have proposed different diagnostic criteria, as summarized by Chan et al (Table 1)[3, 11, 14, 15].
|
Logan et al. [14] |
Camuto et al. [15] |
Tomaszewski et al. [11] |
|
1. Long history of T1D, developing before menopause. |
1. Premenopausal women with long- standing T1D usually associated with microvascular complications. |
1. Lymphocytic lobulitis and ductitis with glandular atrophy. |
|
2. One or more rock-hard, irregular, easily movable, discrete painless, palpable breast mass or masses, often bilateral and occasionally solitary. |
2. A palpable breast mass that is hard, non-tender, and clinically suspicious for carcinoma. |
2. Lymphocytic ⁄ mononuclear perivascular inflammation- predominantly B cell. |
|
3. Radiographically dense glandular tissue. |
3. Mammographic studies show an increased density, but do not confirm the presence of a localized mass. Ultrasound scan also fails to identify a solid or cystic mass. |
3. Dense often keloid-like fibrosis. |
|
4. Strong acoustic shadowing of ultra sound waves |
4. Excisional or core biopsy shows dense keloidal fibrosis associated with a perivascular, periductal, or perilobular lymphocytic infiltrate. |
4. Endoscopic forceps biopsy |
|
5. Firm resistance to the back and forth motion of the needle for fine- needle aspiration. |
Table 1: Diabetic mastopathy diagnostic criteria proposed by different authors as summarized by Chan et al [3].
Although diabetic mastopathy lesions are benign and spontaneous regression can occur, surgical excision with an adequate normal breast tissue margin remains the primary treatment option, also taking into account that diabetic mastopathy could evolve into a breast cancer [6, 8, 16]. New lesions can also develop with a recurrence rate of 30% and education of patients in performing self-examination is crucial, as well as an annual mammography [17, 18].
2. Case Presentation
We present the case of a 41 years old woman, affected by type 1 diabetes since the age of 5 years (diabetes duration 36 years). The patient attends the Diabetes and Metabolic Diseases Unit, Azienda Ospedaliera Universitaria Senese and her diabetes treatment is based on a hybrid-closed loop insulin infusion system composed by an insulin pump associated with a continuous glucose sensor (Medtronic 780G system with smartguard associated with Guardian sensor). Over the years, the patient has always maintained a good glyco-metabolic control, without development of chronic diabetic complications, nor of other autoimmune diseases.
In april 2021 she was hospitalized for an acute appendicitis and underwent surgical appendectomy. The histological examination showed a well differentiated neuroendocrine neoplasia (NET G1). Colonscopy subsequently performed and Computed Tomography(CT)/Positron Emission Tomography(PET) examination did not show additional lesions. During the same month, as a routine examination, the patient underwent mammography, which showed “left breast: absence of evolutive focal lesions and suspicious microcalcifications; right breast: microcalcifications in the retroareolar site with benignity features, modest ductal ectasia and evidence of a 7 x 5 mm polymorphic cluster -classified as category 3 at BI-RADS system-, deserving of stereotaxis microhistological classification” After mammography, a core-needle biopsy was performed and the histological examination reported: "fibroadipose frustules with apocrine ductal hyperplasia with papillary architecture and slight cytological atypia. Ductal ectasies. Intraductal and stromal microcalcifications. Lesion with uncertain malignancy potential ". In view of these findings, it was decided to perform a right quadrantectomy surgery. The macroscopic appearance of the mammary gland viewed during surgery was suggestive of diabetic mastopathy, subsequently confirmed by the histological evaluation. In particular, periductal inflammatory infiltrate predominantly lymphocytic was described, in addition to the presence of intraductal papillomas and ductal hyperplasia. (Figure 1).
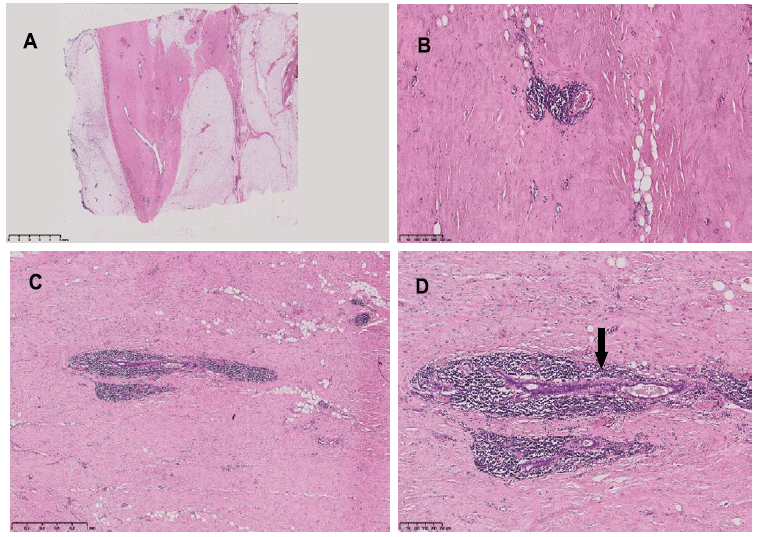
Figure 1: Breast histology. hematoxylin-eosin staining. A: sparse epithelium and glassy and dense stroma (OM 0,50x). B, C: Dense lymphocytic infiltrates surrounding blood vessels (B, OM 8x) and ducts (C, OM 3x). D: Duct with an atrophic epithelium and a thickned basement membrane-black arrow- (OM 8x).
One year later, the patient is in apparent good health. Follow-up mammography was negative for pathological lesions. Follow-up oncologic evaluation (radiological examinations and neuroendocrine markers) was not indicative for disease recurrence.
3. Discussion
Based on our knowledge the case we described, namely diabetic mastopathy in conjunction with a NET in a woman with type 1 diabetes, has never been described previously and appears as an “incidental” association. In our patient, diabetic mastopathy occurred more than 20 years after diabetes diagnosis and presented as a hard painless breast nodule, of comparable size to nodules usually described in diabetic mastopathy. Moreover, in our patient diabetic mastopathy was not associated to other autoimmune diseases or even to chronic diabetic complications (in particular microvascular complications). Undoubtedly it will be important for our patient to perform a breast self-examination periodically, an annual mammography, a periodic screening for chronic diabetic complications and autoimmune diseases (and obviously, oncologic follow-up).
4. Conclusion
In conclusion, it would be interesting to verify if other cases of association between diabetic mastopathy and neuroendocrine tumors will be described in the future to hypothesize a possible association between these two pathological conditions.
Conflict of Interest
None of the authors have any conflict of interest to report.
References
- Lammie GA, Bobrow LG, Staunton MD, et al. Sclerosing lymphocytic lobulitis of the breast--evidence for an autoimmune pathogenesis. Histopathology 19 (1991): 13-20.
- Xiao XC, Shi JS, Hua W. Diabetic mastopathy in an elderly woman misdiagnosed as breast cancer: A case report and review of the literature. World J. Clin. Cases 9 (2021): 3458-3465.
- Chan CL, Ho RS, Shek TW, Kwong A. 2013. Diabetic mastopathy. Breast J. 19: 533-538.
- Soler NG, Khardori R. Fibrous disease of the breast, thyroiditis, and cheiroarthropathy in type I diabetes melliitus. Lancet. 1 (1984): 193-195.
- Thorncroft K, Forsyth L, Desmond S, et al. The diagnosis and management of diabetic mastopathy. Breast J. 13 (2007): 607-613.
- Guzik P, Geca T, Topolewski P, et al. Diabetic Mastopathy. Review of Diagnostic Methods and Therapeutic Options. Int J Environ Res Public Health. 19 (2021): 448.
- Weinstein SP, Conant EF, Orel SG, et al. Diabetic mastopathy in men: Imaging findings in two patients. Radiology 219 (2001): 797-799.
- Agochukwu NB, Wong L. Diabetic Mastopathy: A Systematic Review of Surgical Management of a Rare Breast Disease. Ann. Plast. Surg. 78 (2017): 471-475.
- Schwartz IS, Strauchen JA. Lymphocytic mastopathy. An autoimmune disease of the breast? Am. J. Clin. Pathol. 93 (1990): 725-730.
- Rajasundaram S, Varadharajan V, Deepa C, et al. Diabetic mastopathy-An uncommon presentation of a common disease. Breast J. 26 (2020): 1409-1411.
- Tomaszewski JE, Brooks JS, Hicks D, et al. Diabetic mastopathy: A distinctive clinicopathologic entity. Hum. Pathol. (1992): 780-786.
- Moschetta M, Telegrafo M, Triggiani V, et al. Diabetic mastopathy: A diagnostic challenge in breast sonography. J. Clin. Ultrasound. 43 (2015): 113-117.
- Andrews-Tang D, Diamond AB, Rogers L, et al. Diabetic Mastopathy: Adjunctive Use of Ultrasound and Utility of Core Biopsy in Diagnosis. Breast J. 6 (2000): 183-188.
- Logan WW, Hoffman NY. Diabetic fibrous breast disease. Radiology 172 (1989): 667-670.
- Camuto PM, Zetrenne E, Ponn T. Diabetic mastopathy: A report of 5 cases and a review of the literature. Arch. Surg. 135 (2000): 1190-1193.
- Baratelli GM, Riva C. Diabetic fibrous mastopathy: Sonographic-pathologic correlation. J. Clin. Ultrasound. 33 (2005): 34-37.
- Ely KA, Tse G, Simpson JF, et al. Diabetic mastopathy. A clinicopathologic review. Am. J. Clin. Pathol. 113 (2000): 541-545.
- Sankaye S, Kachewar S. Diabetic mastopathy. Australas Med. J. 5 (2012): 296-299.
