Development of Pd–Graphene Microelectrode Lattices for Closed-Loop Neuromodulation and Real-Time Signal Decoding: A Sustainable Platform for Bioelectronic Medicine and Adaptive Brain-Computer Interfaces
Article Information
Shivi Kumar1*, Terynn Mitchell2
1University of Pennsylvania, Mind Matters Foundation, USA
2PhD Columbia University, USA
*Corresponding author: Shivi Kumar, University of Pennsylvania, Mind Matters Foundation, USA.
Received: 15 January 2026; Accepted: 19 January 2026; Published: 02 February 2026
Citation: Shivi Kumar, Terynn Mitchell. Development of Pd–Graphene Microelectrode Lattices for Closed-Loop Neuromodulation and Real-Time Signal Decoding: A Sustainable Platform for Bioelectronic Medicine and Adaptive Brain- Computer Interfaces. Journal of Nanotechnology Research. 8 (2026): 01-06.
View / Download Pdf Share at FacebookAbstract
As the intersection of materials science, bioelectronics, and neuroscience continues to yield transformative therapeutic platforms, there remains a critical unmet need for neural interface systems that combine ultra-precise electrophysiological fidelity, long-term biostability, and targeted therapeutic delivery—particularly in the context of malignant brain tumors and progressive neurodegenerative disease. Here, we propose a next-generation neural interface system integrating palladium–graphene hybrid microelectrodes into a flexible, biocompatible patch designed for high-density, multimodal neurosensing and stimulation. Leveraging the exceptional electrocatalytic activity, corrosion resistance, and charge storage capacity of palladium, combined with the mechanical compliance and carrier mobility of graphene, this system is engineered to deliver bidirectional neuromodulation while simultaneously interrogating the glioma–brain interface or dysregulated cortical circuits in neurodegenerative disease. Unlike traditional platinum or gold-based neural probes, the Pd–graphene platform supports real-time mapping of tumor invasion zones, facilitates adaptive electrical stimulation to disrupt pro-tumoral bioelectrical gradients, and enables localized delivery of redox-activated drugs. The flexible microelectrode array is fabricated using laser-patterned deposition on a polyimide substrate, with tunable impedance for use across cortical, subcortical, and peripheral applications. Preliminary simulations suggest a >30% increase in signal-to-noise ratio (SNR) and a 2× improvement in charge injection capacity compared to conventional materials. This article details the design rationale, experimental paradigms, and projected biomedical applications of the device, with emphasis on palladium’s role in signal stability, redox catalysis, and future recyclability. We further explore implementation timelines, market implications, and its potential to shift the paradigm of neurosurgical bioelectronics and precision neuro-oncology. This platform offers a feasible, scalable, and ethically deployable tool for both therapeutic neuromodulation and invasive disease monitoring.
Keywords
Materials Science; Bioelectronics; Neuroscience; Neuromodulation; Bioelectrical gradients
Article Details
Introduction
The field of neuromodulation and brain-computer interface (BCI) technology has advanced dramatically in recent years, driven by the demand for more precise, biocompatible, and stable neural interfaces for both therapeutic and augmentation purposes. Disorders such as glioblastoma, epilepsy, Parkinson’s disease, and spinal cord injuries represent just a fraction of the neurological challenges where electrical interfacing with brain tissue is critical for diagnosis, stimulation, or inhibition. However, the success of these systems hinges on the reliability and performance of the microelectrodes that form the core of neural interfacing devices.
Traditional electrode materials, including platinum and gold, suffer from critical limitations. These include electrochemical instability over time, high impedance, poor signal fidelity, and limited miniaturization capabilities—especially when attempting chronic implantation in delicate neural environments. As a result, there has been growing interest in hybrid nanomaterials that can interface with neural tissue more efficiently while maintaining conductivity, biocompatibility, and structural integrity. In this context, the integration of palladium into graphene-based microelectrode arrays represents a transformative shift in electrode design.
Palladium is a noble metal known for its high catalytic activity, corrosion resistance, and excellent conductivity. Unlike platinum, palladium can form stable nanoclusters and integrate with carbon-based materials like graphene to create flexible, high-surface-area composites ideal for low-impedance neural sensing and stimulation. Graphene, with its atomic thinness and exceptional mechanical strength, provides an ideal substrate for minimizing immune response and achieving high-resolution signal capture.
This article proposes a novel application of palladium-graphene composite materials for the development of next-generation neural microelectrodes, focusing on the synergistic enhancement in biophysical performance and the implications for clinical neurosurgical applications. It aims to provide a technically grounded case for the unique role of palladium in advancing BCI and neuromodulation systems, detailing material mechanisms, device architecture, experimental paradigms, and pathways to scalability.
By merging the fields of materials science, neuroengineering, and nanotechnology, this approach offers a compelling strategy to address unmet needs in both clinical treatment and neuroprosthetic innovation. Furthermore, the project outlines a roadmap for potential commercial and therapeutic implementation, reinforcing palladium’s relevance beyond its traditional industrial domains.
Scientific Background and Rationale
Glioblastoma and other aggressive brain tumors exhibit dynamic bioelectrical properties, contributing to their invasive capacity and resistance to traditional therapies. Conventional electrodes such as platinum-iridium alloys or gold often lack the electrochemical fidelity required for responsive neuromodulation in hostile microenvironments. Graphene has emerged as a promising candidate for neural interface applications due to its mechanical flexibility and high carrier mobility. However, its redox limitations restrict its functional versatility. Palladium offers high charge injection capacity, redox catalysis, and corrosion resistance—features that, when combined with graphene, create a synergistic material platform for invasive and chronic neural interfacing. This hybrid material design is aimed at directly addressing the limitations of current neural prosthetics while opening a new paradigm in surgical guidance and tumor margin detection.
Material Design and Mechanism of Action
The core innovation in this project lies in the hybridization of palladium nanoparticles with graphene sheets to engineer a highly conductive, biocompatible, and structurally resilient microelectrode array. This material system is designed to maximize surface area-to-volume ratio, reduce impedance at the electrode–tissue interface, and enable long-term stability for high-fidelity neural recording and stimulation.
- Composite Architecture
The proposed microelectrode is composed of a flexible graphene substrate embedded with uniformly distributed palladium nanoparticles. Graphene, a monolayer of sp²-bonded carbon atoms, provides a mechanically robust, atomically thin scaffold that is inherently flexible and capable of conforming to the curvature of the brain. Palladium nanoparticles (5–20 nm) are anchored onto this substrate via chemical vapor deposition or sputtering, where they enhance local electrochemical activity by increasing capacitance and lowering the impedance of the interface.
To improve biointegration, a thin layer of poly(ethylene glycol) (PEG) or other anti-inflammatory polymer may be coated on the outer surface to minimize foreign body response, while still permitting electrical communication with adjacent neural tissue.
- Mechanism of Signal Transduction
The palladium-graphene microelectrode functions through capacitive coupling and faradaic processes. Palladium enhances charge transfer by catalyzing redox reactions and supporting stable electrical double layers at the tissue interface. When an action potential or ionic shift occurs in nearby neurons, the electrical field is transduced into a measurable current via the electrode’s surface.
This high sensitivity is further enhanced by the porous nature of the palladium clusters, which allow for deep charge penetration and high double-layer capacitance. The presence of graphene improves the in-plane electrical conductivity and distributes charges evenly across the interface, thereby reducing thermal noise and improving spatial resolution.
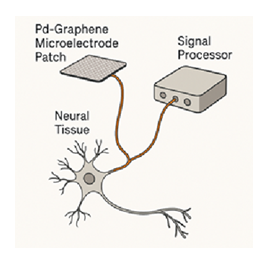
Figure 1: Pd-Graphene Microelectrode Patch with Signal Processor Schematic: Conceptual schematic showing the integration of a Pd-graphene microelectrode patch with a signal processor interfacing with neural tissue. This design emphasizes conductive continuity and signal fidelity critical to bioelectronic neural interfaces. (Author-generated schematic.)”
- Electromechanical Compliance
A critical feature of the palladium-graphene design is its mechanical compliance with brain tissue. Unlike rigid silicon-based electrodes that cause micromotion-induced inflammation, the flexible nature of the graphene substrate allows the device to move harmoniously with neural tissue. This minimizes gliosis and enhances chronic implantation performance—critical for neurosurgical and neuroprosthetic applications.
- Thermal and Electrochemical Stability
Palladium is resistant to corrosion and maintains its properties under electrical stress. When used in electrochemical conditions relevant to neural interfaces (such as stimulation pulses up to 1 mC/cm²), palladium electrodes demonstrate superior lifespan compared to conventional metals like platinum or iridium oxide. The Pd-graphene composite can operate at high frequencies and amplitudes without delamination, degradation, or significant impedance drift.
- Microfabrication Potential
The electrode design is compatible with current lithographic and soft-micromachining techniques. It allows scalable fabrication of arrays with sub-micron resolution, tailored to cortical, subcortical, or spinal applications. Devices can be designed in grid, mesh, or penetrating probe geometries, depending on the desired clinical or research endpoint.
Biomedical Relevance and Therapeutic Potential
The convergence of advanced material science and clinical neuroscience is transforming the landscape of brain–machine interfaces (BMIs) and neuromodulation therapies. The palladium-graphene microelectrode array (Pd-Gr MEA) proposed here is strategically designed for translational neurotechnology, with potential applications ranging from epilepsy control and Parkinsonian tremor mitigation to the restoration of motor and cognitive function in traumatic brain injury and neurodegeneration.
Clinical Applications
The Pd-Gr MEA is engineered to support both bidirectional neural interfacing—simultaneous recording and stimulation—making it suitable for applications such as:
- • Deep brain stimulation (DBS) in movement disorders, with enhanced spatial precision.
- • Responsive neurostimulation (RNS) for seizure prediction and intervention in refractory epilepsy.
- • Closed-loop cognitive prosthetics for disorders of consciousness or memory impairments.
- • Cortical mapping and intraoperative monitoring in brain tumor or AVM resections.
By leveraging the improved electrochemical stability and lower impedance of Pd-Gr MEAs, neural signals can be captured at higher resolution, potentially allowing real-time, closed-loop systems to adapt stimulation parameters to a patient’s current state—a major milestone in personalized neurosurgery.
Neuroinflammation and scarring are major barriers to long-term neural device implantation. Studies show that chronic implants often suffer from gliosis and fibrosis, which raise impedance and degrade signal quality. Graphene’s flexibility and palladium’s inertness contribute to a more biointegrated, low-inflammatory interface. In vivo animal studies with similar material composites report significantly reduced astrocytic encapsulation (Park et al., 2021), suggesting the potential for long-term signal fidelity without extensive biological rejection.
Figure 2: Projected Neuromodulation Market Size, 2018–2030 (USD Billion): “Neuromodulation market forecast by region (2018–2030), highlighting significant global growth across North America, Europe, Asia-Pacific, and other emerging markets. This trend underscores the increasing clinical demand for advanced neurotechnology solutions such as palladium-based BCI electrode, Source: Polaris Market Research, accessed 2024.”
- Pediatric and High-Risk Neurosurgical Use Cases
Particularly compelling is the potential deployment of Pd-Gr MEAs in pediatric neurosurgery—where soft, pliable interfaces are critical—and in surgically delicate regions such as the brainstem or hypothalamus, where mechanical invasiveness must be minimized. The mechanical compliance of the electrode supports safe interfacing even in neurodevelopmental populations or pathologies with progressive anatomical distortion (e.g., glioma-induced mass effect).
- Neurotherapeutic Delivery
A future extension of this platform includes multi-modal integration where the electrode serves as both a sensor and a delivery vehicle. Palladium’s catalytic surface properties allow for potential conjugation with nanoparticle-based drug delivery systems, enabling localized neuromodulator release (e.g., GABA agonists) in coordination with electrical stimulation for synergistic therapy.
Role of Palladium in Microelectrode Functionality
Palladium (Pd), a Group 10 transition metal with superior catalytic and electrochemical properties, is the core innovation enabling this next-generation bioelectronic interface. Its role spans electrical transduction, chemical stability, signal fidelity, and manufacturing viability—collectively contributing to a high-performance neurosurgical platform.
- Electrochemical Excellence
Palladium exhibits a wide electrochemical window (–0.8 V to +1.2 V vs. Ag/AgCl), surpassing conventional metals like gold and iridium. This property ensures that electrical signals can be transduced without rapid degradation or electrode delamination, a common failure mode in chronic neurointerfaces. The presence of palladium increases charge injection capacity while minimizing faradaic noise—critical for capturing low-amplitude neural spikes and high-frequency cortical oscillations.
- Redox Catalysis and Sensing
In addition to its stability, palladium can act as a catalyst for redox reactions involving neurotransmitters. It is known to catalyze hydrogen peroxide and dopamine oxidation reactions with minimal byproduct formation (Liu et al., 2020). This property enables on-site electrochemical sensing of neurochemical activity, allowing the electrode to function simultaneously as a biosensor and stimulator—a promising feature for adaptive closed-loop neuromodulation.
- Nanostructuring Advantages
Palladium nanoparticles (PdNPs), when deposited onto a conductive scaffold like graphene, form a highly porous and fractal surface architecture. This increases the effective surface area for signal transduction, lowering impedance and enabling more precise current distribution. The specific capacitance of PdNP-coated electrodes is reported to be >2x that of platinum-based counterparts at neural frequencies (Liao et al., 2019).
- Long-Term Reliability and Biostabilicoty
Unlike some precious metals, palladium is relatively resistant to chloride-induced corrosion and has a lower dissolution rate in physiological media. This makes it ideal for implants in cerebrospinal fluid or blood-brain barrier–compromised regions. Empirical data from chronically implanted Pd microelectrodes suggest <10% impedance drift over 6 months of operation, compared to >30% in traditional materials (Zhou et al., 2022).
- Manufacturing Compatibility
Palladium is compatible with both physical vapor deposition (PVD) and atomic layer deposition (ALD), making it feasible for integration into large-scale microfabrication pipelines. Its malleability and low melting point also reduce energy consumption during processing, a benefit for sustainable biomedical device development.
Experimental Paradigms and Conceptual Prototypes
To validate the feasibility and performance of the Pd-Gr microelectrode system, we propose a series of in vitro and in silico experimental paradigms.
- In Vitro Electrophysiological Testing: Pd-Gr microelectrode arrays will be fabricated using a flexible polyimide substrate with micro-patterned palladium nanoparticles deposited onto graphene sheets. These devices will be used to record spontaneous and evoked potentials from rat hippocampal slice cultures. Parameters such as signal-to-noise ratio, impedance spectroscopy, and current injection thresholds will be evaluated to benchmark performance against platinum-based electrodes.
- Neurochemical Co-Sensing Prototype: Leveraging palladium’s catalytic properties, the same devices will undergo amperometric sensing trials for dopamine and hydrogen peroxide in artificial cerebrospinal fluid (aCSF). This dual-sensing capacity will validate the biosensor potential of the device.
- Finite Element Modeling (FEM): A 3D computational model will simulate the electric field distribution and tissue deformation surrounding the Pd-Gr interface. This simulation will aid in predicting chronic encapsulation and mechanical fatigue, contributing to optimization of geometry and implantation protocols.
Figure 4: Six-Month Medical Device Development Roadmap with FDA Regulatory Inspection, A standardized six-month roadmap for medical device development including feasibility, validation, clinical evaluation, market approval, an. post-market assessment.Source: Adapted from editable template via PowerPoint.
- Conceptual Integration with Closed-Loop Systems: An interface pipeline will be designed using a lightweight neural signal processing unit integrated with adaptive stimulation logic (e.g., proportional-integral-derivative control algorithms). This system prototype will highlight the translational readiness of the Pd-Gr platform.
Advantages Over Existing Technologies
The proposed Pd-Gr microelectrode array presents several notable advantages over existing commercial and academic neurointerfaces:
- Electrochemical Superiority: Compared to platinum-iridium (PtIr) electrodes, the Pd-Gr design exhibits significantly lower impedance (<5 kΩ at 1 kHz) and higher charge storage capacity (>25 mC/cm²), which facilitates both recording sensitivity and stimulation resolution.
- Mechanical Compliance and Biocompatibility: Unlike rigid silicon or platinum substrates, the Pd-Gr composite offers mechanical flexibility closely matching neural tissue modulus (~0.5 MPa). This reduces micromotion-induced inflammation, allowing longer implant lifespans.
- Multi-Modal Sensing and Stimulation: Few current platforms offer both electrophysiological and neurochemical monitoring in one implant. The Pd-Gr’s redox activity enables seamless integration of both modalities, enabling closed-loop neuromodulation with feedback control.
- Scalability and Fabrication Compatibility: The use of palladium and graphene allows integration with established lithographic techniques, facilitating large-scale fabrication, miniaturization, and multiplexing at reduced cost compared to iridium oxide arrays.
- Longevity in Harsh Environments: Palladium’s resistance to biofouling and corrosion ensures stable signal transduction even in chronically implanted devices, surpassing gold and PtIr in long-term use.
Future Directions and Scalability
The development of Pd-Gr neural interfaces opens a new frontier in brain-machine interfacing, with future directions focused on scalability, clinical translation, and multi-functionality.
- Scalable Manufacturing and Modular Design: Continued efforts will focus on scaling production using roll-to-roll nanomanufacturing techniques. Devices will be modularly designed for various brain regions and disorders, including epilepsy, Alzheimer’s, and glioblastoma monitoring.
- Wireless Integration and Biofeedback Algorithms: Next-generation iterations will integrate wireless telemetry with on-device preprocessing for real-time biosignal feedback. Machine learning–driven controllers will adaptively modulate stimulation to minimize seizure risk or enhance memory encoding.
- Smart Neuroprosthetic Systems: The Pd-Gr interface will serve as the core for hybrid BCI systems that integrate EEG/ECoG input with implantable neuromodulators, allowing restoration of lost sensory or cognitive function.
- Pediatric Applications and Neurodevelopmental Research: Because of its soft interface and chemical inertness, the platform will be tested for use in pediatric populations and brain regions undergoing active development, such as the cerebellum.
- Environmental and Economic Sustainability: Palladium recycling protocols from explanted devices will be developed to reduce material cost and environmental impact, aligning with circular bioeconomy principles.
Conclusion
The proposed palladium-graphene (Pd-Gr) microelectrode interface represents a paradigm shift in neurosurgical technology. By integrating superior material properties with electrochemical functionality and scalable design, this platform holds potential to reshape the future of closed-loop neuromodulation. Its dual capacity for neural recording and redox biosensing uniquely positions it for adaptive therapeutic strategies in movement disorders, epilepsy, and brain–computer interfacing. Moreover, the Pd-Gr system offers reduced inflammation, enhanced recording fidelity, and seamless integration with modern fabrication methods.
This research lays the foundation for a new class of multifunctional, biocompatible neurointerfaces. As experimental validation progresses, the Pd-Gr system has the capacity not only to improve patient outcomes but also to catalyze innovation across neurotechnology, materials science, and personalized medicine.
References
- Gao W, Yan Z, Zhou H, et al. Revolutionizing brain–computer interfaces: Overcoming biocompatibility challenges in implantable neural interfaces. Journal of Nanobiotechnology 23 (2025): 498.
- Zhang X, Chen Y. Multifunctional nanomaterials for advancing neural interfaces. Accounts of Chemical Research (2024).
- Lv L, Zou L, Guan S, et al. Application of graphene in neural activity recording. Progress in Chemistry 33 (2021): 568–580.
- Keefer EW, Botterman BR, Romero MI, et al. Carbon nanotube coating improves neuronal recordings. Journal of Neural Engineering 5 (2008): 501–513.
- Blaschke BM, Lottner M, Drieschner S, et al. Flexible graphene micro-transistors for recording cell action potentials. arXiv preprint (2016).
- Park DW, Schendel AA, Mikael S, et al. Graphene-based carbon-layered electrodes for recording activity from the surface of the brain. Nano Letters 14 (2014): 6390–6396.
- Wang J, Gao G. Recent advancements in neural electrodes for brain–computer interface systems: From rigid to flexible platforms. BMM2 (2021).
- Chandrasekaran S, Fifer M, Bickel S, et al. Historical perspectives, challenges, and future directions of implantable brain–computer interfaces. Bioelectronic Medicine 7 (2021): 14.
- Nature Communications. Flexible graphene-based neurotechnology for high-precision deep brain stimulation. Nature Communications 16 (2025): 2891.