Development and Validation of a Prediction Model for Gestational Hypertension in Mezam Division
Article Information
Nkem Ernest NJUKANG1,5, 6,*, Thomas Obinchemti EGBE2, Nicolas TENDONGFOR7, Tah Aldof YOAH 1,9,10, Kah Emmanuel NJI 1,8, Martyn SAMA1, Fidelis Atabon AKO7, Joseph KAMGNO3,4
1Department of Public Health and Hygiene;
2Department of Obstetrics and Gynaecology, Faculty of Health Sciences, University of Buea, Cameroon;
3Faculty of Medicine and Biomedical Sciences/University of Yaounde I;
4Centre for Research on Filariasis and other Tropical Diseases (CRFilMT), Yaounde, Cameroon;
5Quality Assurance/Quality Management System Mentor, Global Health System Solutions (GHSS), Yaounde, Cameroon; 6Country Project Coordinator, Africa Field Epidemiology Network, Far North Region, Cameroon;
7Head of Department Public Health and Hygiene, University of Buea;
8African Capacity Building Foundation Harare, Zimbabwe;
9Department of School Health Promotion, Regional Delegation of Secondary education;
10Department of Public Health and Epidemiology, Biaka University Institute of Buea.
*Corresponding Author: Nkem Ernest NJUKANG, Department of Public Health and Hygiene, Quality Assurance/Quality Management System Mentor, Global Health System Solutions (GHSS), Yaounde, Cameroon.
Received: 12 November 2022; Accepted: 18 November 2022; Published: 30 November 2022
Citation:
Nkem Ernest NJUKANG, Thomas Obinchemti EGBE, Nicolas TENDONGFOR, Tah Aldof YOAH, Kah Emmanuel NJI, Martyn SAMA, Fidelis Atabon AKO, Joseph KAMGNO. Development and Validation of a Prediction Model for Gestational Hypertension in Mezam Division. Obstetrics and Gynecology Research 5 (2022): 288-295
View / Download Pdf Share at FacebookAbstract
Objective: Our study aimed to develop and validate a prediction model for identifying women at increased risk of developing gestational hypertension (GH) in Mezam division, Northwest Region (NWR) of Cameroon.
Method: A retrospective cohort design was employed. Data for a cohort of 1183 participants were randomly divided into derivation (n = 578) and validation (n = 585) datasets. Inclusion criterion was women without chronic hypertension. Primary outcome was Gestational hypertension. A questionnaire and data abstraction form were used for data collection. Chi square (χ2) test, independent sample t-test and multivariate logistic regression (to derive the prediction model) were used for data analysis. For each significant variable, a score was calculated by multiplying coefficient (β) by 100 and rounding to the nearest integer. Discrimination was estimated by used of the c-statistic.
Results: DBP, SBP, hypertension in previous pregnancy, stress and smoking (scores 10, 6, 210, 56 and 18, respectively) were predictors of incident GH. The model accuracy was assessed by the area under the receiver operating characteristic curve (AUC), with optimal cut-off value 936. With the derivation dataset, sensitivity, specificity and AUC of the model were 75.9%, 80.8% and 0.828 (95% CI 0.772–0.884) respectively. The model was validated by dividing the aggregated scores into three ranges (low, moderate and high) and their cumulative incidence calculated which were; 3.5%, 6.1% and 39.4%, respectively, in the derivation dataset and 4.7%, 6.2% and 30.2%, respectively, in the validation dataset. Calibration was good in both cohorts. The negative predictive value of women in the development cohort at high risk of GH was 92.0% compared to 94.0% in the validation cohort.
Conclusions: The prediction model revealed adequate performance after validation in an independent cohort a
Keywords
Prediction Model, Retrospective Cohort Design, Gestational Hypertension, Pregnant Women, Mezam Division
Prediction Model articles Prediction Model Research articles Prediction Model review articles Prediction Model PubMed articles Prediction Model PubMed Central articles Prediction Model 2023 articles Prediction Model 2024 articles Prediction Model Scopus articles Prediction Model impact factor journals Prediction Model Scopus journals Prediction Model PubMed journals Prediction Model medical journals Prediction Model free journals Prediction Model best journals Prediction Model top journals Prediction Model free medical journals Prediction Model famous journals Prediction Model Google Scholar indexed journals Retrospective Cohort Design articles Retrospective Cohort Design Research articles Retrospective Cohort Design review articles Retrospective Cohort Design PubMed articles Retrospective Cohort Design PubMed Central articles Retrospective Cohort Design 2023 articles Retrospective Cohort Design 2024 articles Retrospective Cohort Design Scopus articles Retrospective Cohort Design impact factor journals Retrospective Cohort Design Scopus journals Retrospective Cohort Design PubMed journals Retrospective Cohort Design medical journals Retrospective Cohort Design free journals Retrospective Cohort Design best journals Retrospective Cohort Design top journals Retrospective Cohort Design free medical journals Retrospective Cohort Design famous journals Retrospective Cohort Design Google Scholar indexed journals Gestational Hypertension articles Gestational Hypertension Research articles Gestational Hypertension review articles Gestational Hypertension PubMed articles Gestational Hypertension PubMed Central articles Gestational Hypertension 2023 articles Gestational Hypertension 2024 articles Gestational Hypertension Scopus articles Gestational Hypertension impact factor journals Gestational Hypertension Scopus journals Gestational Hypertension PubMed journals Gestational Hypertension medical journals Gestational Hypertension free journals Gestational Hypertension best journals Gestational Hypertension top journals Gestational Hypertension free medical journals Gestational Hypertension famous journals Gestational Hypertension Google Scholar indexed journals Pregnant Women articles Pregnant Women Research articles Pregnant Women review articles Pregnant Women PubMed articles Pregnant Women PubMed Central articles Pregnant Women 2023 articles Pregnant Women 2024 articles Pregnant Women Scopus articles Pregnant Women impact factor journals Pregnant Women Scopus journals Pregnant Women PubMed journals Pregnant Women medical journals Pregnant Women free journals Pregnant Women best journals Pregnant Women top journals Pregnant Women free medical journals Pregnant Women famous journals Pregnant Women Google Scholar indexed journals Mezam Division articles Mezam Division Research articles Mezam Division review articles Mezam Division PubMed articles Mezam Division PubMed Central articles Mezam Division 2023 articles Mezam Division 2024 articles Mezam Division Scopus articles Mezam Division impact factor journals Mezam Division Scopus journals Mezam Division PubMed journals Mezam Division medical journals Mezam Division free journals Mezam Division best journals Mezam Division top journals Mezam Division free medical journals Mezam Division famous journals Mezam Division Google Scholar indexed journals Hypertensive disorders of pregnancy articles Hypertensive disorders of pregnancy Research articles Hypertensive disorders of pregnancy review articles Hypertensive disorders of pregnancy PubMed articles Hypertensive disorders of pregnancy PubMed Central articles Hypertensive disorders of pregnancy 2023 articles Hypertensive disorders of pregnancy 2024 articles Hypertensive disorders of pregnancy Scopus articles Hypertensive disorders of pregnancy impact factor journals Hypertensive disorders of pregnancy Scopus journals Hypertensive disorders of pregnancy PubMed journals Hypertensive disorders of pregnancy medical journals Hypertensive disorders of pregnancy free journals Hypertensive disorders of pregnancy best journals Hypertensive disorders of pregnancy top journals Hypertensive disorders of pregnancy free medical journals Hypertensive disorders of pregnancy famous journals Hypertensive disorders of pregnancy Google Scholar indexed journals preeclampsia articles preeclampsia Research articles preeclampsia review articles preeclampsia PubMed articles preeclampsia PubMed Central articles preeclampsia 2023 articles preeclampsia 2024 articles preeclampsia Scopus articles preeclampsia impact factor journals preeclampsia Scopus journals preeclampsia PubMed journals preeclampsia medical journals preeclampsia free journals preeclampsia best journals preeclampsia top journals preeclampsia free medical journals preeclampsia famous journals preeclampsia Google Scholar indexed journals eclampsia articles eclampsia Research articles eclampsia review articles eclampsia PubMed articles eclampsia PubMed Central articles eclampsia 2023 articles eclampsia 2024 articles eclampsia Scopus articles eclampsia impact factor journals eclampsia Scopus journals eclampsia PubMed journals eclampsia medical journals eclampsia free journals eclampsia best journals eclampsia top journals eclampsia free medical journals eclampsia famous journals eclampsia Google Scholar indexed journals maternal deaths articles maternal deaths Research articles maternal deaths review articles maternal deaths PubMed articles maternal deaths PubMed Central articles maternal deaths 2023 articles maternal deaths 2024 articles maternal deaths Scopus articles maternal deaths impact factor journals maternal deaths Scopus journals maternal deaths PubMed journals maternal deaths medical journals maternal deaths free journals maternal deaths best journals maternal deaths top journals maternal deaths free medical journals maternal deaths famous journals maternal deaths Google Scholar indexed journals BP in pregnancy articles BP in pregnancy Research articles BP in pregnancy review articles BP in pregnancy PubMed articles BP in pregnancy PubMed Central articles BP in pregnancy 2023 articles BP in pregnancy 2024 articles BP in pregnancy Scopus articles BP in pregnancy impact factor journals BP in pregnancy Scopus journals BP in pregnancy PubMed journals BP in pregnancy medical journals BP in pregnancy free journals BP in pregnancy best journals BP in pregnancy top journals BP in pregnancy free medical journals BP in pregnancy famous journals BP in pregnancy Google Scholar indexed journals
Article Details
1.0. INTRODUCTION
Hypertensive disorders of pregnancy (HDP), which include, gestational hypertension (GH), preeclampsia and eclampsia are the third leading cause of maternal deaths globally [1, 2], with majority of the death occurring in low- and middle-income countries (LMICs). It is a condition in which the expectant mother present with raised blood pressure (BP) during pregnancy as defined by the American College of Obstetricians and Gynecologists (ACOG) in 1986 and approved by the WHO [3, 4]. HDP incorporates a spectrum of conditions, including preexisting HTN, gestational HTN, preeclampsia/eclampsia and superimposed pre-eclampsia [5]. A 2006 systematic review of the causes of maternal mortality by the WHO revealed that HDP are accountable for 2-43% of maternal deaths across countries and 9.1% of pregnancy-related deaths in Africa [6]. In sub-Saharan Africa, HDP remain a major call for concern owing to their increasing incidence, gravity and associated complications [7]. Not only have developing countries faced this problem, but developed countries too. A study done by Colin (2012) in UK, found HDP complicating up to 15% of pregnancies and a quarter of all antenatal admissions. Another study by Chang et al. [8] in the United States established that pregnancy-induced HTN was the major reason for 15.7% of maternal deaths.
Some studies have estimated the prevalence of HDP to be higher, 15% in Harare Zimbabwe, 17% in Nigeria and 17.5% in the Far North Region of Cameroon [9]. A study by Egbe et al., [10] in the Northwest Region of Cameroon reported a 14.5% death due to HDP. Antenatal screening for HTN and proteinuria followed by close monitoring and treatment of pre-eclampsia reduced eclampsia related maternal mortality by 48-68% [11, 12]. Therefore, availability of skilled health personnel with knowledge and skills in managing hypertension is vital for prevention of hypertensive related complications [13, 14]. The major causes of HDPs are not fully known [14, 15], however accurate prediction of pregnant women at increased risk of HDP could lead to better antenatal care (ANC) and a reduction of complications from the condition. Clinical prediction models predict the probability of individuals having certain health conditions or obtaining defined health outcomes. They combine two or more variables from patient data to predict clinical outcome and prior to application in clinical practice are externally validated [16-18].
The major approaches to predicting the occurrences of GH comprise the use of maternal clinical characteristics, uterine artery Doppler and biomarkers [19, 20]. Although a good number of prediction models for HDP, mainly preeclampsia and eclampsia have been developed in high-income countries, they may not be suitable for LMICs because of differences in the availability and the cost of diagnostic tools [21].
The aim of this study was to develop and externally validate a contextually appropriate and low-cost clinical prediction model for GH based on maternal characteristics obtained at ANC visit for use in primary care settings in Cameroon and potentially other LMIC.
2.0. METHODS
2.1. Study area/settings: The study was conducted in Mezam Division, NWR of Cameroon. The division is made up of five health districts of which three (Bamenda, Tubah and Santa) were selected for the study. A total of ten facilities were purposefully selected for the study: Bamenda district (Regional hospital, Mulang HC, CMA Nkwen, Azire IHC, St. Blaise and St. Mary Hospital), Santa district (Santa DH and Akum IHC) and Tubah district (Tubah DH and CMA Bambili).
2.2. Study design, population, period and eligibility: The prediction model was developed from a cross-sectional retrospective cohort of 1165 pregnant women attending ANC services in ten primary and secondary care facilities in Mezam division. The study went on for four months i.e. March to June 2018. Eligibility criterion was pregnant women without chronic hypertension and ≥36 weeks of gestation.
2.3. Sample size and sampling technique: The sample size for the cross-sectional study was calculated by using a single population proportion sample size calculation formula by considering the following assumptions: P=8.2% [22]. The precision (d) was 8.2%/5. By considering 10% none response rate, the final sample size became 1210.
n= Z P (1-P)/d2 [23]
n = Number of participants for the study, Z= 1.96 for 95% confidence interval.
P= 8.2% (Mboudou et al., 2009), d = 8.2/5%, q= 1- p.
n= (1.96)2×0.082× (1-0.082)/ (0.0164)2 = 1,075.2 ≈ 1,076. Considering 10% of 1,075=107.6
Therefore 1,076 + 107.6 = 1184. However, the final sample size reached was 1210.
A consecutive sampling method was used after potential participants had given written informed consent/ascent. Ethical approval for the study was granted by the Ethical Review Board Faculty of Health Science, University of Buea.
2.4. Data collection: Data was collected by face to face interview using pretested structured questionnaire and checklist. The checklist was used to extract information from the pregnant woman’s ANC card. The midwives were given standardized training on data collection. Predictors/variable for the study were selected based on a review of the literature on variables known to be associated with GH [24-26]. Information on the following predictors: maternal age, educational status, stress, smoking, alcohol intake, fruits & vegetable intake, family history of hypertension, hypertension in previous pregnancies and family twin/triplets etc. were obtained by interview while BP (mercury sphygmomanometer), height (stadiometer), weight (balance) and urine protein (2+or more on urine dipstick) were obtained from ANC cards.
2.5. Outcome: The outcome, GH, was defined as a systolic BP ≥140 mm Hg or more and or a diastolic BP ≥90 mm Hg or more on at least two separate occasions and after 20 weeks of gestation (3, 4).
2.6. Data analysis: The mean and SD of continuous predictors were calculated for hypertensive (GH) and non-hypertensive cases. Means were compared using the independent t-test; percentages for categorical data were assessed by χ2 test. Predictors that were related to GH by a predetermined p value of 0.20 or less were considered for multivariable logistic regression model. After running the multivariate regression, significant variables were assigned scores based on the regression coefficient (β) i.e. for each variable, the coefficient was multiplied by 100 and rounded up to the nearest integer. The scores were used to generate the model.
The risk score of each participant was further calculated. The total score for each woman was related to her risk of developing GH. The individual risk score plus their hypertensive status were used to generate a receiver operating characteristic curve (ROC) and the area under the curve (AUC). A table of sensitivity and 1-specificity (False Positive Rate (FPR)) was equally generated and from it, specificity was calculated. Sensitivity and specificity scores were summed together and the sum with the highest score was used to extrapolate the sensitivity, specificity and optimal cut off value for the model.
The predictive power of the risk-score model was evaluated to identify the risk of developing GH in the derivation and validation datasets. The aggregated scores were divided into three ranges i.e. low, moderate and high risk of GH. For scores of <1260, 1260–1460 and >1461, respectively and the observed cumulative incidence of GH was compared with predicted risk by chi-square test for trend. The model’s accuracy was assessed by the area under the receiver operating characteristic curve (AUC) based on the sum of scores.
The performance of the model in the development and validation cohort was assessed by discrimination and calibration. Discrimination is the ability of the model to distinguish between women who develop GH and those who do not and was assessed using the AUC. The AUC ranges from 0.5 (no discrimination) to 1.0 (perfect discrimination). Data analysis was performed by use of SPSS software (V.21.0, IBM SPSS Statistics, and Chicago, Illinois, USA) and Epi Info 7.
3.0. RESULTS
3.1. Comparing baseline characteristics of pregnant women with/without GH in the derivation and validation cohort.
3.1.1. Derivation cohort
Women with and without GH differed with respect to age (28.2 (SD 5.9) years vs 26.5 (SD: 5.2) years, p=0.007). There was no difference in mean MET/min/week between women who developed GH and those without GH (769.1 (SD 702.1) vs 827.0 (SD (695.8), p=0.492). There was no difference in mean vegetable/fruits consumption between women who developed GH and those without GH (3.4 (SD 1.1) vs 3.6 (SD (1.2), p=0.380). However, there was a difference in the mean BMI of women with and without GH (27.8 kg/m2 (SD 4.9) vs 26.8 kg/m2 (SD 4.1), p<0.046). The mean systolic BP differed between women who developed GH and those who did not (123 mm Hg (SD 16.3) vs 109 mm Hg (SD 10.8), p<0.001), as did mean diastolic BP (75.3 mm Hg (SD 10.1) vs 66.3 mm Hg (SD 7.9), p<0.001). The mean age difference between pregnancy among women with and without GH differed significantly (4.1 years (SD 2.0) vs 3.5 years (SD 1.9), p=0.041). About 21.9% of women with GH had mothers who had GH compared to 13.1% of women without GH though not significant (p<0.161). Furthermore 50% of women with GH had a history of GH in a previous pregnancy compared to 12.1% of women without GH (p<0.001). Fifty percent of the women who smoke had GH compared to 13.4% of the non-smokers (p=0.033). About 16.2% of women with GH were stressed during the pregnancy compared to 13.1% who were not stressed (p=0.078). For alcohol intake, 16.5% of the women with GH were drinkers compared to 12.6% who were not (p=0.263). The proportion of women with and without GH did not differ in salt consumption (13.6% vs 13.6%) (p=0.997). There was no significant difference between those with and without GH for marital status, education and occupation. However, there was an almost significant difference for parity, gravidity and mode of delivery between those with and without GH (Table 1).
3.1.2. Validation cohort
Women with and without GH did not differ with respect to age (27.5 (SD 5.9) years vs 26.9 (SD: 5.4) years, p=0.378). There was no difference in mean MET/min/week between women who developed GH and those without GH (760 (SD 660) vs 890 (SD (734.1), p=0.136). There was no difference in mean vegetable/fruits consumption between women who developed GH and those without GH (3.4 (SD 1.2) vs 3.5 (SD (1.2), p=0.521). More so, there was no difference in the mean BMI of women with and without GH (27.6 kg/m2 (SD 4.8) vs 27.1 kg/m2 (SD 4.2), p<0.327). The mean systolic BP differed between women who developed GH and those who did not (120.7 mm Hg (SD 17.3) vs 109.6 mm Hg (SD 11.2), p<0.001), as did mean diastolic BP (74.8 mm Hg (SD 9.5) vs 67.2 mm Hg (SD 7.7), p<0.001). The mean age difference between pregnancy among women with and without GH differed significantly (4.4 years (SD 2.8) vs 3.6 years (SD 2.1), p=0.018). About 14.2% of women with GH had mothers with family HTN in pregnancy compared to 12.2% of women without GH (p<0.420). Furthermore 50% of women with GH had a history of GH in a previous pregnancy compared to 12.1% of women without GH (p<0.001). Sixty-six percent of the women who smoke had GH compared to 12.7% of the non-smokers (p<0.001). About 14.2% of women with GH were stressed during the pregnancy compared to 12.2% who were not stressed (p=0.451). For alcohol intake, 20% of the women with GH were drinkers compared to 11.5% who were not (p=0.012). The proportion of women with and without GH did not significantly differ in salt consumption (15.5% vs 12.8%) (p=0.506). There was no significant difference between those with and without GH for marital status, education, occupation, parity and gravidity. However, there was significant difference for mode of delivery for women with and without GH (Table 1).
|
Derivation dataset |
Validation dataset |
|||||
|
Predictors |
GH (Yes)/79 |
GH (No)/501 |
p-value |
GH (yes)/77 |
GH (No)/508 |
p-value |
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
Mean (SD) |
|||
|
Age (years) |
28.2(5.9) |
26.5(5.2) |
0.007 |
27.5(5.9) |
26.9(5.4) |
0.378 |
|
MET/min/week |
769(702.1) |
872(695.8) |
0.492 |
760(660) |
890(734.1) |
0.136 |
|
F_V serving/day |
3.4(1.1) |
3.6(1.2) |
0.38 |
3.2(1.2) |
3.5(1.2) |
0.521 |
|
Systolic BP |
123(16.3) |
109(10.8) |
0 |
120.7(17.3) |
109.6(11.2) |
0.001 |
|
Diastolic BP |
75.3(10.1) |
66.3(7.9) |
0 |
74.8(9.5) |
67.2(7.7) |
0.001 |
|
Body Mass Index |
27.8(4.9) |
26.8(4.1) |
0.046 |
27.6(4.8) |
27.1(4.2) |
0.327 |
|
Pregnancy Gap (years) |
4.1(2.0) |
3.5(1.9) |
0.041 |
4.4(2.8) |
3.6(2.1) |
0.008 |
|
Proportion/% |
Proportion/% |
|
Proportion/% |
Proportion/% |
|
|
|
Alcohol intake |
22(16.5) |
57(12.6) |
0.263 |
25(20.0) |
56(11.5) |
0.012 |
|
Salt intake |
9(13.6) |
70(13.6) |
0.997 |
13(15.5) |
68(12.8) |
0.506 |
|
HTN in Previous Pregnancy |
12(50.0) |
67(12.1) |
<0.001 |
14(38.9) |
67(11.7) |
<0.001 |
|
Twin/Triplet |
6(38.8) |
73(13.0) |
0.013 |
5(25.0) |
76(12.8) |
0.113 |
|
Smoking |
2(50.0) |
57(13.4) |
0.033 |
41(66.7) |
77(12.7) |
<0.001 |
|
Stress |
45(16.2) |
34(13.1) |
0.078 |
43(14.2) |
38(12.2) |
0.451 |
|
Family History of HTN |
7(21.9) |
73(13.1) |
0.161 |
8(17.0) |
73(12.9) |
0.42 |
|
Marital Status |
|
|
0.552 |
|
|
0.361 |
|
Married |
59(12.6) |
390(86.9) |
|
61(75.3) |
425(79.7) |
|
|
Single/Separated |
20(15.3) |
111(84.7) |
|
20(24.7) |
108(20.3) |
|
|
Educational Status |
|
|
0.49 |
|
|
0.283 |
|
None/Primary |
14(17.1) |
84(18.3) |
|
13(16.0) |
97(18.2) |
|
|
Secondary |
31(39.2) |
167(32.1) |
|
34(42.0) |
185(34.7) |
|
|
High School |
17(26.6) |
130(25.9) |
|
12(14.8) |
124(23.3) |
|
|
Tertiary |
13(16.5) |
120(24.0) |
|
22(27.2) |
127(23.8) |
|
|
Occupational Status |
|
|
0.85 |
|
|
0.969 |
|
HW/Peasants |
15(17.1) |
82(18.1) |
|
15(18.5) |
97(17.8) |
|
|
Student |
19(24.1) |
142(28.3) |
|
22(27.2) |
135(28.3) |
|
|
Petit trading |
27(34.2) |
170(33.9) |
|
27(33.3) |
180(33.6) |
|
|
Employed/Business |
18(22.8) |
107(21.4) |
|
17(21.0) |
123(23.1) |
|
|
Parity |
|
|
0.068 |
|
|
0.784 |
|
Nulliparous |
30(13.9) |
186(37.1) |
|
31(33.3) |
190(17.8) |
|
|
Singleton |
13(8.6) |
139(27.1) |
|
19(23.5) |
144(27.0) |
|
|
Multiparous |
36(17.0) |
176(35.1) |
|
31(38.3) |
199(37.3) |
|
|
Gravidity |
|
|
0.076 |
|
|
0.289 |
|
1 |
28(35.4) |
159(31.7) |
|
30(37.0) |
166(31.1) |
|
|
2-6 |
46(58.2) |
331(66.1) |
|
49(60.5) |
350(65.1) |
|
|
≥7 |
5(6.3) |
176(35.1 |
|
2(2.5) |
17(3.2) |
|
|
Mode of Delivery |
|
|
0.058 |
|
|
0.004 |
|
Normal |
33(60.0) |
248(77.0) |
|
36(70.6) |
243(69.4) |
|
|
Induced |
7(14.0) |
45(14.0) |
|
3(5.9) |
70(20.0) |
|
|
Caesarean section |
10(20.0) |
29(9.0) |
|
12(23.5) |
37(10.6) |
|
HTN= Hypertension; MET= Metabolic Equivalent Task; SD= Standard Deviation; F_V= Fruits & Vegetable; GH= Gestational Hypertension
Table 1: Comparing baseline characteristics of pregnant women with/without GH in the derivation and validation datasets
3.2. Generation of the prediction model
From the Multivariate Regression analysis, the following variables; DBP, SBP, stress, smoking and HTN in previous pregnancy reached statistical significance and were retained in the derivation dataset. The variables were assigned a score based on the regression coefficient (β) i.e. multiplying the coefficient by 100 and rounding up to the nearest integer (Table 2).
|
Risk factors |
Mean (SD)/ |
Β |
OR (95% CI) |
P-value |
Scores (β x 100) |
|
Proportion (%) |
|||||
|
DBP |
75.3(10.1) |
0.098 |
1.10 (1.06-1.15) |
<0.001 |
10 |
|
SBP |
123(16.3) |
0.061 |
1.06 (1.04-1.09) |
<0.001 |
6 |
|
HTN in previous pregnancy |
75.3(10.1) |
2.097 |
8.14 (2.99-22.14) |
<0.001 |
210 |
|
Stress |
45(16.2) |
0.564 |
1.76 (1.1-3.12) |
0.054 |
56 |
|
Smoking |
2(50.0) |
1.84 |
6.29 (2.61-58.46) |
0.006 |
18 |
OR, Odd Ratio; 95% CI, 95% confidence interval.
This resulted to the risk score equation below;
Risk score = 10xDBP + 6xSBP + 210xHTN in previous pregnancy + 56xStress + 18xSmoking.
From the above equation, the total risk score for each participant was calculated.
Table 2: Risk factors of Gestational Hypertension in the Derivation model
3.3. Evaluation of the model’s predictive performance
The risk scores of each participant alongside the hypertensive status were used to generate a receiver operating characteristic curve (ROC) and the area under the curve (AUC). We equally had a table of sensitivity and 1-specificity (False Positive Rate (FPR)) data. From the FPR, specificity was calculated. The sensitivity and specificity scores were summed together for each participant and the sum with the highest score was used to extrapolate the sensitivity, specificity and optimal cut off value for the model. Thus, the Sensitivity, Specificity, Optimal cut-off value for the risk-score model and AUC/C-statistic were 75.9%, 80.8%, 1443 and 0.828 (95% CI 0.772–0.884) respectively with the derivation dataset. Using a contingency table, the positive predictive value (PPV) and negative predictive value (NPV) for the development model was calculated and were 37.8% and 96.1% respectively. Similarly, the sensitivity, specificity, PPV and NPV for the validation data set were 95.2%, 89.7%, 24.7% and 99.8% respectively.
|
|
Sensitivity |
Specificity |
PPV |
NPV |
|
Development Data |
75.90% |
80.80% |
37.80% |
91.60% |
|
Validation Data |
95.20% |
89.70% |
24.70% |
99.80% |
Table 3: Sensitivity, Specificity, Positive and Negative Predictive Value for the Model
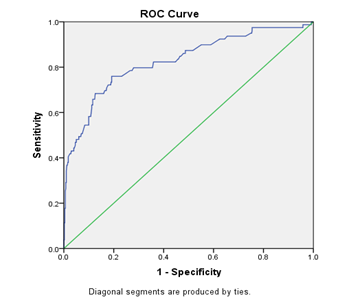
Figure 1: ROC curve for the derivation data set.
3.4. Validation of the Model
To validate the model, we applied this scoring method to the validation dataset. The aggregated scores were divided into 3 ranges i.e. low, moderate and high risk of GH (Table 4). For scores of <1260, 1260–1460 and >1461, the cumulative incidence of GH was 3.5%, 6.1% and 39.4%, respectively, in the derivation dataset and 4.7%, 6.2% and 30.2%, respectively, in the validation dataset. The observed incidence increased with increasing risk score or estimated probability in the 2 data sets (both Ptrend <0.001). The sensitivity, specificity, PPV and NPV for the low, moderate and high-risk ranges were determined as shown in (Table 4) below.
|
Development Data Set |
|||||||
|
Score range |
Pre-HTN |
HTN |
Incidence* |
Sensitivity |
Specificity |
PPV |
NPV |
|
<1260 |
138 |
5 |
3.50% |
66.70% |
98.60% |
50.00% |
99.30% |
|
1260-1460 |
277 |
18 |
6.10% |
75% |
96.50% |
37.50% |
99.40% |
|
>1460 |
86 |
56 |
39.40% |
80.00% |
78.80% |
50.00% |
91.90% |
|
Validation Data Set |
|||||||
|
<1260 |
123 |
6 |
4.70% |
40.00% |
98.40% |
47.10% |
97.60% |
|
1260-1460 |
278 |
18 |
6.20% |
25.00% |
96.50% |
16.70% |
97.90% |
|
>1460 |
132 |
57 |
30.20% |
80.60% |
79.70% |
43.90% |
95.50% |
Non-HTN= Non-hypertensive; HTN= Hypertensive; *P for trend < 0.001
Table 4: Estimated probability and observed incidence of GH in the derivation and validation datasets.
4.0. DISCUSSION
We developed and externally validated a simple prediction model for GH in two different cohorts of pregnant women attending ANC services in the same settings which is in line with the general recommendation that before being applied in clinical practice, prediction models should be externally validated. The AUC of the model in the development cohort was 0.828 (95% CI 0.772–0.884) and was in line with results of other studies [17,27]. In the Netherlands, Nijdam et al (2010) [28] derived a prediction model for identifying nulliparous women who developed hypertension before 36 weeks of gestation using systolic BP, diastolic BP and weight. The AUC of the original model of 0.78 (95% CI 0.75 to 0.82) reduced to 0.75 (95% CI 0.68 to 0.81) after external validation. The small decrease in AUC in our study implies that the model predicts well based on data routinely collected as part of ANC and can be applied to the pregnant women in the study setting.
Majority of prediction models for HDPs, such as the SCOPE model [21] have been focused on preeclampsia and eclampsia which are severer forms of the disorder. Nevertheless, milder forms such as GH are also associated with poor pregnancy outcomes. Considering that GH can be well managed to prevent progression to preeclampsia and eclampsia, a model that identifies women at risk of the condition is useful.
Limitation of this study was the application of clinical characteristics only and not including some biomarkers as well as uterine artery Doppler (use to measure blood flow between mother and baby) in the model. This is because of the non-routine use of these parameters during ANC in Mezam division. Both approaches are expensive and the equipment for analysing these biomarkers is generally not available in many primary health facilities. Nonetheless, future research could assess the added value of these biomarkers as a recent systematic review for first trimester prediction of preeclampsia showed that a combination of uterine artery Doppler, maternal characteristics and two or more biomarkers yielded detection rates of 38–100% [29]. The best rates were reported for the combination of Inhibin A, PLGF, PAPP-A, uterine artery Doppler and maternal characteristics (Kuc et al., 2014). The difficulty of predicting GH using only maternal clinical characteristics has been pointed out [30]; however, the feasibility of applying these models in low-resource settings currently remains limited due to constraints in the availability of diagnostic equipment and the high cost of the tests which are beyond the means of most people who require them. Thus, despite the increased predictive value of adding biomarkers to the predictive model; the need to derive reasonably accurate prediction models that use variables, which are routinely easy to obtain for low-resource settings is important.
In the development cohort, 142 (24.5%) women were classified as being at high risk of developing GH. Fifty-six of them eventually developed GH giving a PPV of 27.2% and NPV of 92%. In the validation cohort, 189 (30.8%) women were classified as being at high risk of GH and 56 of them developed the condition given an incidence of 30.2%. The PPV was 33.3% and the NPV 94%. Classifying women into different risk categories allows for closer monitoring of pregnant women at high risk. This will include more frequent ANC visits or referral for specialist care. Given that the addition of biomarkers in the screening of women could enhance the identification of those at high risk of GH, future research should explore the added value of biomarkers in the early identification of pregnant women at increased risk of HDPs in LMICs.
Such studies should be accompanied by comparative cost-effectiveness of the routine data only predictive models and the models that combine routine data and biomarkers to provide essential health technology assessment information for future decision-making. In the interim however, despite the fact that the modest PPV in the development and validation cohorts show the limitation and difficulty of predicting GH using only demographic and clinical characteristics the model has the potential of identifying pregnant women at increased risk of GH for subsequent care and monitoring. Its further validation and use are worth serious consideration in low-resource settings.
5.0. Conclusion
We developed and validated a prediction model for GH at ANC visit using maternal data retrospectively collected in a LMIC setting. Our results are easily converted into a simple user friendly clinical decision-making support tool for use in ANC clinics in low resource settings that enables frontline providers of maternal health services to use a score chart to quickly categorise women into different risk levels. The strength of this model is the use of a few maternal clinical variables already routinely obtained by caregiver’s during routine ANC. Such a simple predictive model to aid frontline providers of maternal care to estimate the probability of GH later on in the pregnancy and take relevant precautions is potentially lifesaving. Obtaining the information does not involve expensive procedures such as uterine artery Doppler [30]. The application of the model at the ANC should aid in the early detection of women at risk of GH and contribute to efforts to provide clinical decision-making support to improve maternal health outcomes. We would recommend its validation in other low-income settings as well as implementation research to inform implementation, monitoring and evaluation at scale in Cameroon.
References
- J M Maternal death due to hypertensive disorders in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2008; 22(3): 559-567.
- Ye C, Ruan Y, Zou L, et al., The 2011 Survey on Hypertensive Disorders of Pregnancy (HDP) in China: Prevalence, Risk Factors, Complications, Pregnancy and Perinatal Outcomes. 2014; 9(6).
- Mc Caw BAM, MacGillivray I, Hawkins N, et al., International variation in the incidence of hypertension in pregnancy among primiparae: the Jamaican experience. West Indian Medical Journal. 1997; 46(2): 29-30.
- Cunnigham F G, Leveno K, Bloom Sea. Williams Obstetrics. Edition r, editor. Medical Publishing Division 2010.
- Osungbade K O, Ige O K. Public health perspectives to pre-eclampsia in developing countries: implication for health system strengthening. Journal of Pregnancy 2011.
- Kahn K S, Wojdyla D, Say L, et al., WHO analysis of causes of maternal death: a systematic review. Lancet 2006; 367(9516):1066-1074.
- Zenebe W, Segni H, Woldie M. Hypertension disorders of pregnancy in Jimma University Specialized Hospital. Ethopian Journal of Health Sciences. 2011; 21(3):1-10.
- Chang J, Elam-Evans L D, Berg C J, et al. Pregnancy-related mortality surveillance--United States, 1991--1999. MMWR Surveill Summ. 2003; 52(2): 1-8.
- Tebeu P M, Ngassa P, Kouam L, et al., Maternal mortality in Maroua Provincial Hospital, Cameroon (2003-2005). West Indian Medical Journal. 2007; 56(6): 502-507.
- Egbe T O, Therence N D, Gregory EH-E, et al., Determinants of Maternal Mortality in Mezam Division in the North West Region of Cameroon: A Community-based Case Control Study. International journal of tropical disease and health. 2016; 15(2): 1-15.
- Bhutta Z-A, Ali S, Cousens S, et al. Alma-Ata: Rebirth and Revision 6 Interventions to address maternal, newborn and child survival: What difference can integrated primary health care strategies make? Lancet 2008; 372(9642): 927-989.
- Afusat O B. Prevalence and Associated Factors of Anxiety and Depression Among Pregnant Women. Open Access J Neurol Neurosurg. 2018; 9(2): 1-10.
- L D Pre-eclampsia and the hypertensive disorders of pregnancy. British Medical Bulletin 2003; 67: 161-176.
- Mayega R W, Makumbi F, Rutebemberwa E, et al., Modifiable socio-behavioural factors associated with overweight and hypertension among persons aged 35 to 60 years in eastern Uganda. PLoS one 2012; 7(3): 231-238.
- Ahmed N U, Rahman M, Islam MD, et al., Socio-demographic clinical characteristics and status of hypertension control among rural hypertensive patients. Faridpur Medical College Journal. 2011; 6(1): 5-9.
- Altman D G, Vergouwe Y, Royston Pea. Prognosis and prognostic research: validating a prognostic model. Biomed Journal 2009; 38(b605).
- Moons KG, Kengne A P, Grobbee DEea. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012; 98(1): 691-698.
- Moons K G, Royston P, Vergouwe Yea. Prognosis and prognostic research: what, why, and how? Biomed Journal 2009; 338(b375).
- Akolekar R, Syngelaki A, Sarquis Rea. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11 to 13 weeks. Prenatal Diagnosis 2011; 21(2): 66-74.
- Poon L C, Nicolaides K H. First-trimester maternal factors and biomarker screening for preeclampsia. Prenatal Diagnosis 2014; 34(6): 618-627.
- Kenny L C, Black M A, Poston Lea. Early pregnancy prediction of preeclampsia in nulliparous women, combining clinical risk and biomarkers the screening for pregnancy endpoints (SCOPE) international cohort study. Hypertension. 2014; 64(1): 644–652.
- MBOUDOU E T, FOUMANE P, BELLEY PRISO E, et al. HYPERTENSION AU COURS DE LA GROSSESSE: Aspects cliniques et épidémiologiques a l’Hôpital Gynéco-Obstétrique et Pédiatrique de Yaounde, Cameroun. Clinics in Mother and Child Health 2009; 6(2): 1087-1093.
- Naing L, Winn T, Rusli B. Practical issues in calculating the sample size for prevalence studies. Arch Orofac Sci. 2006; 1(1): 9-14.
- O'Brien T E, Ray J G, Chan W S. Maternal body mass index and the risk of pre-eclamsia: a systematic overview. . Epidemiology 2009; 14(1): 368-374.
- Tebeu P M, Foumane P, Fosso G, et al., Risk Factors for Hypertensive Disorders in Pregnancy: A Report from the Maroua Regional Hospital, Cameroon. J Reprod Infertil 2011; 12(3): 227-234.
- Mbouemboue O P, Diallo C, TMarcel T, et al. A Study on Factors Related to Hypertensive Disorders in Pregnancy in Ngaoundere (Adamawa Region,Cameroon). Clinical Medicine Research. 2016; 5(2): 6-12.
- Hukkelhoven C W, Steyerberg E W, Habbema J D: Predicting outcome after traumatic brain injury: development and validation of a prognostic score based on admission characteristics. Journal of Neurotrauma 2005, 22: 1025-1039.
- Nijdam M E, Janssen K J, Moons KGea: Prediction model for hypertension in pregnancy in nulliparous women using information obtained at the first antenatal visit. . Journal of Hypertension 2010, 28(1): 119-126.
- Kuc S, Wortelboer E J, van Rijn Bea: Evaluation of 7 serum biomarkers and uterine artery Doppler ultrasound for first- trimester prediction of preeclampsia: a systematic review. Obstetric and Gynecologic Survey 2011, 66(4): 225-239.
- Onwudiwe N, Yu CKH, Poon LCYea: Prediction of pre-eclampsia by a combination of maternal history, uterine artery Doppler and mean arterial pressure. . Ultrasound Obstet Gynecol 2008, 32(7): 877-883.
