Determination of Meropenem, Ceftazidime and Piperacillin Levels in Serum and Meropenem in Cerebrospinal Fluid by Liquid Chromatography for Routine Quantification
Article Information
Stefan Günther*, 3, Andreas Reimer1, Horst Vogl1, Stephan Spenke2, Hanns-Christian Dinges3, Ann-Kristin Schubert3, Leopold HJ Eberhart3, Götz Geldner2
1Department of Pharmacy, Hospital Ludwigsburg, Ludwigsburg, Germany
2Clinic for Intensive Care, Emergency Medicine and Pain Therapy, Hospital Ludwigsburg, Ludwigsburg, Germany
3Department of Anaesthesiology & Intensive Care, Philipps University Marburg, Marburg, Germany
*Corresponding author: Stefan Günther. Department of Anaesthesiology & Intensive Care, Philipps University Marburg, Marburg, the Department of Pharmacy, Hospital Ludwigsburg, Ludwigsburg, Germany.
Received: 19 September 2022; Accepted: 27 September 2022; Published: 19 October 2022
Citation: Stefan Günther, Andreas Reimer, Horst Vogl, Stephan Spenke, Hanns-Christian Dinges, Ann-Kristin Schubert, Leopold HJ Eberhart, Götz Geldner. Determination of Meropenem, Ceftazidime and Piperacillin Levels in Serum and Meropenem in Cerebrospinal Fluid by Liquid Chromatography for Routine Quantification. Journal of Pharmacy and Pharmacology Research 6 (2022): 169-176
View / Download Pdf Share at FacebookAbstract
Objective: Therapeutic drug monitoring (TDM) of β-lactam antibiotics is a commonly used to prevent treatment failures in critically ill patients. A quick and simple high-performance liquid chromatography (HPLC) assay for the determination of meropenem, ceftazidime and piperacillin in human serum and meopenem in cerebrospinal fluid (CSF) was developed in this study.
Methods: The method used an Atlantis? T3 5.0µm stationary phase. The mobile phase A contained water (99.4% m/m) and formic acid (0.6% m/m) (pH 2.30). The mobile phase B contained acetonitrile (93.6% m/m), water (6% m/m) and formic acid (0.4% m/m). The method used gradient elution to determine meropenem, ceftazidime and piperacillin. UV absorbance detection at 309nm, 258nm, 235nm and 260nm respectively was used. For sample preparation, an internal standard was added, and acetonitrile/methanol was added for protein precipitation.
Results: The method was investigated for linearity, specificity, accuracy, and precision. Stability of the antibiotic substances and internal standard was assessed. The retention time of meropenem was 7.222min, the single run time was 23min. Meropenem was quantified from the lower limit of quantification (0.1mg/l in serum and CSF) to the upper limit of quantification (100mg/l in serum and 25mg/l in CSF). In routine analysis of meropenem samples, a high interindividual variability of serum and CSF levels was observed and the mean CSF/serum ratio was 0.129 ± 0.092. An external validation was passed for meropenem, ceftazidime and piperacillin using the presented protocol.
Conclusion: The developed assay enable studying correlations between the applied dosage, serum concentration and CSF concentration of meropenem. Additionally, ceftazidime and piperacillin can be determined in human serum. Further studies with a higher number of samples can be performed to investigate
Keywords
Meropenem, Ceftazidime, Piperacillin, Therapeutic drug monitoring, HPLC, validation, human serum, cerebrospinal fluid
Article Details
1. Background
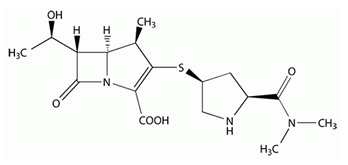
Meropenem ((4R,5S,6S)-3-[[(3S,5S)-5-[(Dimethylamino)carbonyl]-3-pyrrolidinyl]thio]-6-[(1R)-1-hydroxyethyl]-4-methyl-7-oxo-1-azabicyclo[3.2.0] hept-2-ene-2-carboxylic acid; figure 1[1]) is a carbapenem derivative with a broad spectrum of activity against gram-positive and gram-negative bacteria. It belongs to the β-lactam antibiotics and therefore penetrates the bacterial cell wall and inhibits the cell wall synthesis [2]. Due to its good stability against β-lactamases, there are only few resistances and it is used as an antibiotic of last resort on intensive care units (ICUs) [3, 4]. Meropenem is frequently recommended for the treatment of nosocomial infections like ventriculitis, which is a common complication when external ventricular drains (EVD) are used in therapy of acute subarachnoid hemorrhage, intraventricular bleedings or other acute intracranial pathologies [5]. Taking into account the emerging rise of antimicrobial resistance and the few new antimicrobials available for clinical uses, the dose optimization strategy for existing drug therapies becomes increasingly important to achieve the maximum therapeutic efficacy [3, 6, 7] Therapeutic drug monitoring (TDM) of β-lactam antibiotics is a frequently used tool to optimize the treatment with several antibiotics. Especially on ICUs TDM allows accurate dosing in critically ill patients that have altered pharmacokinetics due to various stages of organ failure and are therefore prone to over- and under dosing [8-10]. However, the main challenge for critical care physicians remains achieving, maintaining and controlling appropriate antibiotic concentrations in target tissues. In cases of ventriculitis , the blood-CSF-barrier limits the penetration of meropenem to its target tissue [11]. Recent analysis indicate highly variable penetration of meropenem into CSF in ventriculitis patients [12, 13]. Furthermore, some data show that traditional dosing of meropenem (3 x 2g as intermittent infusion) cannot achieve CSF concentrations above the minimum inhibitory concentration (MIC) [13]. To prevent therapy failure continuous infusion of β-lactam antibiotics is suggested for maintaining concentrations over the dosing interval [14]. Moreover, standard doses of meropenem (maximum 6g/24h [15]) could be insufficient, leading to dosage regimes higher than standard [16]. Using median initial doses of 8.8g/24h and TDM-guided dose optimization ensured sufficient CSF concentrations in all patients within 48h [16]. To satisfy the need of intensive care units, we describe a simple method to determinate meropenem in human serum and cerebrospinal fluid. The aim of this study is to demonstrate the development, validation and routine use of internal standard high-performance liquid chromatography assay for meropenem in human serum and cerebrospinal fluid. Additionally this method is able to determine ceftazidime and piperacillin in human serum.
2. Materials and Methods
2.1 Antibacterial agents and other substances
We used meropenem powder for solution for injection/infusion, commercially available from Dr. Friedrich Eberth Arzneimittel (Ursensollen, Germany), piperacillin/tazobactam powder for solution for injection/infusion, commercially available from Fresenius Kabi Deutschland (Bad Homburg, Germany) and ceftazidime powder for solution for injection/infusion, commercially available from Dr. Friedrich Eberth Arzneimittel (Ursensollen, Germany). Also cefotaxime powder for solution for injection/infusion, commercially available from Fresenius Kabi Deutschland (Bad Homburg, Germany), cefazolin powder for solution for injection/infusion, commercially available from MIP Pharma GmbH (Blieskastel, Germany) and porcine serum from bio&sell GmbH (Feucht, Germany). Patient serum and patient cerebrospinal fluid were received from ICUs for TDM.
2.2 Solvents
We purchased formic acid, sodium hydroxide, methanol (HPLC grade) and acetonitrile (HPLC grade) from Th. Geyer GmbH & Co. KG (Renningen, Germany). Purified water was purchased from Fresenius Kabi Deutschland GmbH (Bad Homburg, Germany).
2.3 High-performance liquid chromatography (HPLC)
We used a high-performance liquid chromatography system by Shimadzu that contains a temperate autosampler, column oven and UV-Vis detector. Labsolution (Shimadzu, Germany) software was used to control the chromatographic system of the double internal standard based method. The stationary phase was AtlantisÒ T3 5µm, 15cm x 4,6mm Column (Waters Corpotation, Milford, MA, USA).
The mobile phase A contained water (99.4% m/m), formic acid (0.6% m/m) and was adjusted to pH 2.30 by the addition of 1M sodium hydroxide. The mobile phase B contained acetonitrile (93.6% m/m), water (6% m/m) and formic acid (0.4% m/m). We used a gradient elution method consisting of mobile Phase A and mobile phase B as seen in table 1.
The pump flow rate was 1.0ml/min. UV absorbance detection was used at 309nm (meropenem), 235nm (piperacillin), 258nm (ceftazidime), 260nm (cefotaxime) and 270nm (cefazolin). The column oven temperature was set to 20°C in routine. The method was running for 23min, the median retention times were 7.222min for meropenem, 17.541min for piperacillin, 6.704min for ceftazidime, 9.861min for cefotaxime and 12.105min for cefazolin at 20°C.
Table 1: Gradient time program for HPLC
|
Time (min) |
Solvent B concentration (%) |
|
0.01 |
10 |
|
1 |
10 |
|
11 |
35 |
|
15 |
35 |
|
16 |
10 |
|
23 |
10 |
2.4 Reference standards
To determine the content of the commercially available powders for solution for injection/infusion we used chemical reference substances (CRS). Meropenem trihydrate CRS (content 86.9%), piperacillin CRS (content 95.2%), ceftazidime CRS (content 85.5%), cefotaxime acid CRS (content 90.6%) and cefazolin (content 99.2%) were purchased from Sigma-Aldrich Chemie GmbH (Tauf kirchen, Germany).
2.5 Sample preparation
We prepared samples by mixing 250µl patient serum or CSF with 50µl internal standard (cefotaxime 125mg/l and cefazolin 125mg/l) and 500µl acetonitrile/methanol (1:1) for precipitation. The samples were mixed for 10s and centrifuged at 10 000 RPM for 10min. 200µl of the supernatant were diluted with 460µl water and 50µl of this mixture was injected.
3. Results
3.1 Selectivity
Selectivity of the analytical method was proven using six individual sources of the appropriate blank matrix (human serum), which were individually analyzed and evaluated for interference. No relevant interference was detected but to prevent interference with the internal standard we decided to use a mixture of two internal standards. If there is an interference with cefotaxime we can use cefazolin to analyze the patient sample. Interference may occur in patients who received cefotaxime or cefazolin in earlier therapy regimes.
3.2 Carry-over
To prevent carry-over we injected blank samples after high concentration samples [17]. There was no carry-over detected in the blank samples.
3.3 Lower limit of quantification
The lower limit of quantification is defined as the lowest concentration of analyte in a sample, which can reliably be quantified, with an acceptable accuracy and precision. LLOQ is aimed to be at least 5 times the signal of a blank sample [17]. For this analytical method, the LLOQ for meropenem is 0.1mg/l in serum and CSF, 0.2mg/l for ceftazidime in serum and 10mg/l for piperacillin in serum.
3.4 Calibration curve
For time-dependent drugs, the main parameter associated with therapeutic success is the percentage of time that the levels of antibiotics at the infection site exceed the minimum inhibitory concentration (%ƒ T > MIC) of the pathogen [18]. Due to the clinically sensible breakpoint against the pathogenic Pseudomonas spp. at 2 mg/l [19] we defined the target concentration in CSF > 2mg/l. For meropenem levels in serum we defined target concentrations of 8 – 16mg/l (100% ƒT > 4x MIC - 100% ƒT > 8x MIC). Ceftazidime serum target concentrations 32 – 48mg/l (100% ƒT > 4x MIC - 100% ƒT > 6x MIC) and piperacillin serum target concentrations 64 – 96mg/l (100% ƒ T > 4x MIC - 100% ƒT > 6x MIC) due to their MIC breakpoints against Pseudomonas spp [19].
According to the target concentration range a minimum of six calibration concentration levels were used for each method [17]. The LLOQ is defined being the lowest calibration standard and the highest calibration standard defines the upper limit of quantification (ULOQ) as seen in table 2 [17]. LLOQ is 0.1mg/l for meropenem in serum and liquor, the ULOQ is 100mg/l in serum and 25mg/l in CSF. LLOQ is 0.2mg/l for ceftazidime in serum; the ULOQ is 75mg/l in serum. LLOQ is 10mg/l for piperacillin in serum; the ULOQ is 200mg/l in serum.
Table: 2 Antibiotic concentration levels used for the calibration curves. Every calibration sample contains 25mg/l internal standard (cefotaxime and cefazolin).
|
Conc. Level |
Meropenem serum |
Meropenem CSF |
Ceftazidime serum |
Piperacillin serum |
|
#1 |
0.1 mg/l |
0.1 mg/l |
0.2 mg/l |
10 mg/l |
|
#2 |
0.2 mg/l |
0.2 mg/l |
0.5 mg/l |
25 mg/l |
|
#3 |
0.5 mg/l |
0.5 mg/l |
1 mg/l |
50 mg/l |
|
#4 |
1 mg/l |
1 mg/l |
2.5 mg/l |
100 mg/l |
|
#5 |
5 mg/l |
2.5 mg/l |
5 mg/l |
160 mg/l |
|
#6 |
12.5 mg/l |
5 mg/l |
10 mg/l |
200 mg/l |
|
#7 |
25 mg/l |
10 mg/l |
20 mg/l |
not used |
|
#8 |
50 mg/l |
25 mg/l |
30 mg/l |
not used |
|
#9 |
100 mg/l |
not used |
50 mg/l |
not used |
|
#10 |
not used |
not used |
75 mg/l |
not used |
For the calibration standards, we used porcine serum and residual material of human CSF. To prepare the calibration standards we spiked 200µl matrix with 50µl antibiotic solution (target concentration level x5 mg/l). The following steps were performed analog the sample preparation. All calibration curves analysis used freshly spiked samples. The correlation between mean area ratio and concentration ratio was strong for all calibration curves (R2 >0.9999).
3.5 Accuracy
The accuracy describes the closeness of the determined value obtained by the method to the nominal concentration of the analyte. Accuracy was assessed on samples spiked with known amounts of the analyte. These samples were spiked independently from the calibration standards and were analyzed against the calibration curve. For the validation of the accuracy, we analyzed LLOQ, low, medium and high concentration samples. The mean concentration within a value of 15% from the nominal values is commonly considered acceptable, except for the LLOQ, which is acceptable within 20% of the nominal value [17]. The accuracy was demonstrated with all mean concentrations between 88.56% and 100.82% of the nominal value.
3.6 Precision
The precision of the analytical method describes the closeness of repeated individual measures of analyte in the same sample. Precision can be expressed as the relative standard deviation (RSD). Precision of the analytical method should be demonstrated for the LLOQ, low, medium and high sample concentrations. The RSD value should not exceed 15% for the low, medium and high concentration samples, except for the LLOQ, which should not exceed 20% [17]. Precision was demonstrated for every antibiotic substance with all RSD values ranging between 0.64% and 12.95%.
3.7 Stability
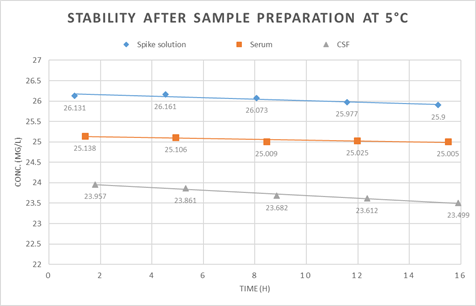
The low stability of meropenem in aqueous solutions or biological fluids is often reported in literature [20-22]. Even transport between clinic and laboratory is difficult due to the limited stability [21]. To detect stability, we analyzed the degradation of meropenem, ceftazidime and piperacillin under relevant conditions. Therefore, we evaluated the stability of meropenem in spiked porcine serum and CSF after sample preparation at 5°C. This simulates the conditions in our autosampler and no relevant degradation was detected over 15h as seen in figure 2. Additionally we analyzed the stability of meropenem, ceftazidime and piperacillin in biological matrix.
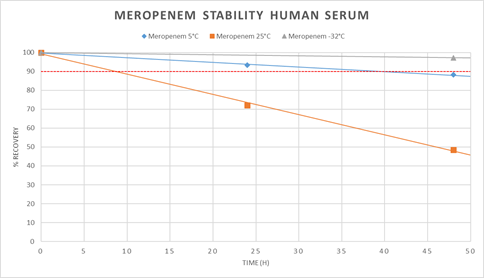
We spiked human serum with a mixture of all three antibiotics and measured the concentrations at the beginning, after 24h and 48h. One sample was stored at 5°C, one in the freezer at -32°C and one at ambient temperature 25°C. The concentrations at 25°C decreased very fast compared to the samples at 5°C and -32°C as shown for meropenem (figure 3). The same degradation progress was detected for ceftazidime and piperacillin. At 25°C the value 90% of start concentration was passed within the first 24h and at 5°C after 48h. At -32°C the value 90% of start concentration was not passed within 48h. Consequently, we concluded to freeze the collected patient samples and analyze them within 24h after collection.
3.8 Quality control samples
We performed quality control samples to show our system und methods work as we expect on days with analysis of unknown samples. Therefore, high and low concentration samples were prepared out of antibiotic (meropenem + ceftazidime + piperacillin) and internal standard (cefotaxime + cefazolin) stock solution with porcine serum. The low concentration sample was spiked with 4mg/l meropenem, 16mg/l ceftazidime and 34mg/l piperacillin. The high concentration sample was spiked with 16mg/l meropenem, 65mg/l ceftazidime and 137mg/l piperacillin. We defined the acceptable concentration range of the measured antibiotics with ±7% and the acceptable area range of internal standard with ±7.5% due to the recommendation of the EMA guideline on bioanalytical method validation17. They recommend ranges of ±15% but we decided to define closer limits with ±7% and ±7.5%.
3.9 External validation
To verify the performance of the method an external validation assay was passed. This assay was offered by INSTAND (Gesellschaft zur Förderung der Qualitätssicherung in medizinischen Laboratorien e.V., Düsseldorf). The achieved certificate is valid for 12 months and proves that two samples with unknown concentration of meropenem, ceftazidime and piperacillin were analyzed correctly within acceptable limits. The results are shown in table 3.
Table 3: Results of the external validation by INSTAND
|
Substance |
Sample |
Unit |
Measured conc. |
Target conc. |
Lower limit |
Upper limit |
Deviation |
Result +/- |
|
Ceftazidime |
1 |
mg/l |
0 |
0 |
0 |
2.50 |
+ |
|
|
2 |
mg/l |
3.80 |
4.08 |
2.86 |
5.30 |
-6.9% |
+ |
|
|
Meropenem |
1 |
mg/l |
58.9 |
63.2 |
44.2 |
82.2 |
-6.8% |
+ |
|
2 |
mg/l |
46.1 |
48.3 |
33.8 |
62.8 |
-4.6% |
+ |
|
|
Piperacillin |
1 |
mg/l |
14.6 |
17.4 |
12.2 |
22.6 |
-16.1% |
+ |
|
2 |
mg/l |
120 |
132 |
92.4 |
172 |
-9.1% |
+ |
3.10 Routine analysis
The method we described here is routinely used in our laboratory to determine meropenem levels in human sera and CSF. Within the setting described above, we measured 64 pairs of simultaneous collected human serum and CSF samples from critically ill patients on intensive care units. The serum levels of meropenem ranged between 5.4mg/l up to 49.3mg/l (mean 18.6mg/l ± 7.6mg/l, median 16.4mg/l). The CSF levels of meropenem ranged between 0.3mg/l up to 17.9mg/l (mean 2.6mg/l ± 2.6mg/l, median 1.9mg/l). For our measurements, the mean CSF/serum ratio was 0.129 ± 0.092. Patient characteristics and dosage regimes are collected in table 4.
Table 4: Patient characteristics and dosage regimes
|
No. of patients |
30 |
|
Gender (No. M/no. F) |
19/11 |
|
Mean age (yr) |
53.0 |
|
Mean body weight (kg) |
80.3 |
|
No. of serum/CSF pair |
64 |
|
Mean serum conc. (mg/l) |
18.7 ± 7.6 |
|
Mean CSF conc. (mg/l) |
2.6 ± 2.6 |
|
Mean CSF/serum ratio |
0.129 ± 0.092 |
|
Dose continuous 8000mg/24h |
14 |
|
Dose continuous 6000mg/24h |
46 |
|
Dose continuous 4000mg/24h |
2 |
|
Dose continuous 3000mg/24h |
1 |
|
Dose continuous 2000mg/24h |
1 |
|
Mean dose continuous /24h (mg) |
6.3 ± 1.1 |
|
Indication ventriculitis |
14 |
|
Indication meningitis |
2 |
|
Indication subarachnoid hemorrhage |
1 |
|
Indication brainstem abscess |
2 |
|
Indication shunt infection |
3 |
|
Indication unknown |
8 |
4. Discussion
The developed assay is reproducible, accurate, precise, and linear across the range of the calibration curves. The preparation of our samples is quick and simple. The HPLC assay time of 23min is acceptable for the processing of samples for routine TDM.
Previous studies described large interindividual variability in the concentrations of meropenem in plasma and CSF13. A reduced distribution into CSF has been documented for β-lactams, especially carbapenems, due to their hydrophilic nature [23, 24]. Additionally results were published on whether meropenem plasma concentrations simply used as a surrogate parameter of CSF concentrations may lead to under dosing [25]. These results are in line with Blassmann et al. who is reporting a median CSF/plasma penetration of 9% in 21 neurocritical care patients with ventriculitis [13]. We measured serum and CSF levels at the same time during continuous infusion of meropenem; this gave us also the chance to calculate the CSF/serum ratio and for our 64 pairs of serum and CSF levels the mean ratio was 0.129 ± 0.092. Compared to the non β-lactam antibiotic linezolid with a reported CSF/serum ratio of 0.71 ± 0.16 [26], meropenem has a very poor CNS penetration. Our data from routine analysis with a mean CSF/serum ratio of 12.9% support the data from Blassmann et al. and suggest a high interindividual variability of serum levels, CSF levels and the CSF/serum ratio due to large standard deviations. If these analysis are compared with reported CSF penetration of between 21 and 39% in patients with bacterial meningitis [27, 28], it is suspected that dosing regimens for meropenem in patients with meningitis cannot be extrapolated to patients with ventriculitis.
Our data contains two patients with proven or suspected meningitis in which the measured CSF/serum ratio are higher than the mean CSF/serum ratio (13.7% and 23.3% compared with mean 12.9%). It is likely, that drug penetration in inflamed meninges is greater than in patients with non-inflamed. Consequently, in critically ill patients with CNS infections, the standard dosing regimen of meropenem with 6g daily does not predictively achieve optimal plasma and CSF concentrations in all patients. Results like these push the need for TDM of meropenem in plasma and in CSF to avoid either the risk of dose-dependent toxicity or that of treatment failure. The development of meropenem-induced toxicity is significantly affected in patients with a high serum meropenem concentration. The threshold concentrations for which there is 50% risk of developing a neurotoxicity event is described with meropenem cmin = 64.2mg/l and a nephrotoxicity event with cmin = 44.45mg/l [29].
Furthermore, optimized dosing strategies like administration of higher than standard dosages or administration by continuous infusion should be taken into consideration. Continuous infusion has been demonstrated to improve PK/PD target attainment in various further studies of time-dependent antibiotics [30-32]. Recommended daily doses for meropenem are 6g in adults [5]. High initial meropenem doses (median 8.8g/24h by continuous infusion) together with dose adjustments according to TDM ensured sufficient CSF concentrations in all patients according to Tiede et al [16] Consistent evidence is now available showing that therapeutic drug monitoring and guided individual dose optimization of meropenem is justified and feasible in clinical practice to reduce underexposure, improve tolerability and possibly response to therapy [16].
We have demonstrated meropenem, ceftazidime and piperacillin to be stable in human serum up to 48h in frozen condition at -32°C. This is important because it was shown that meropenem was unstable when stored at temperatures above 4°C [20-22]. Furthermore, meropenem, ceftazidime and piperacillin were stable after treatment with acetonitrile/methanol. Accordingly, the prepared samples can be assayed under storage conditions of 5°C within 24h period and no relevant loss of meropenem, ceftazidime or piperacillin was detected.
5. Conclusion
In the present study, we developed a simple method for the quantification of meropenem in human serum and CSF. The developed method could be easily and quickly performed and enabled the quantification of meropenem in patient samples for routine TDM. In the future, this method can be used to evaluate the serum and CSF concentrations of meropenem in critically ill patients. Consequently, meropenem dosage regimes should be tailored to individual patients. This is essential because our data suggests that there is a high variability in serum concentrations, CSF concentrations and CSF/serum ratios.
Furthermore, the developed method creates the chance to study CSF penetration of meropenem because the simplest way to study the entry of drugs into the CNS is to measure drug concentrations in the CSF during a continuous drug infusion33. Additionally, this method enables to quantify ceftazidime and piperacillin concentrations in human serum. Our investigation was limited due to the lack of information about clinical outcomes of the patients and the lack of microbiological analysis. In conclusion, our results are in line with other studies that showed a high variability of serum and CSF levels of meropenem, and future studies can be performed using the method described above.
Ethics approval
Ethics Committee of “Ärztekammer Baden-Württemberg” in Stuttgart, Germany (authorization number: F-2020-057).
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests
None declared.
Contributors
SG and HV: development and validation of analytical assays. SG, SS, GG, HV and AR: collection of routine data. SG, AS and HD: analysis and interpretation of data. SG, HV, AR, SS, LE, GG, AS and HD: revision for intellectual content and approval of the final version.
Acknowledgments
None
References
- Meropenem sodium Carbonate. Biomol GmbH - Life Science Shop. Accessed March 8 (2022).
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: Past, Present, and Future. Antimicrob Agents Chemother 55 (2011): 4943-4960.
- Streit F, Perl T, Schulze MH, Binder L. Personalised beta-lactam therapy: basic principles and practical approach. LaboratoriumsMedizin 40 (2016): 385-397.
- Gonçalves-Pereira J, Póvoa P. Antibiotics in critically ill patients: a systematic review of the pharmacokinetics of β-lactams. Crit Care 15 (2011): R206.
- Tunkel AR, Hasbun R, Bhimraj A, et al. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis. Clin Infect Dis Off Publ Infect Dis Soc Am 64 (2017): e34-e65.
- Villegas MV, Briceno DF, Ruiz SJ, Furtado GH, Nicolau DP. Assessing the pharma codynamic profile of intravenous antibiotics against prevalent Gram-negative organisms collected in Colombia. Braz J Infect Dis 15 (2011): 413-419.
- Pascale R, Giannella M, Bartoletti M, Viale P, Pea F. Use of meropenem in treating carbapenem-resistant Enterobacteriaceae infections. Expert Rev Anti Infect Ther 17 (2019): 819-827.
- McKenzie C. Antibiotic dosing in critical illness. J Antimicrob Chemother 66 (2011): ii25-ii31.
- Mabilat C, Gros MF, Nicolau D, et al. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur J Clin Microbiol Infect Dis 39 (2020): 791-797.
- Pea F, Della Siega P, Cojutti P, et al. Might real-time pharmacokinetic/pharmacodynamic optimisation of high-dose continuous-infusion meropenem improve clinical cure in infections caused by KPC-producing Klebsiella pneumoniae? Int J Antimicrob Agents 49 (2017): 255-258.
- Kumta N, Roberts JA, Lipman J, Wong WT, Joynt GM, Cotta MO. A Systematic Review of Studies Reporting Antibiotic Pharmacokinetic Data in the Cerebrospinal Fluid of Critically Ill Patients with Uninflamed Meninges. Antimicrob Agents Chemother 65 (2020): e01998-20.
- Mader MMD, Czorlich P, König C, et al. Intrathecal penetration of meropenem and vancomycin administered by continuous infusion in patients suffering from ventriculitis—a retrospective analysis. Acta Neurochir (Wien) 160 (2018): 2099-2105.
- Blassmann U, Roehr AC, Frey OR, et al. Cerebrospinal fluid penetration of meropenem in neurocritical care patients with proven or suspected ventriculitis: a prospective observational study. Crit Care 20 (2016): 343.
- Abdul-Aziz MH, Alffenaar JWC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a Position Paper. Intensive Care Med 46 (2020): 1127-1153.
- GmbH RLS. Meronem® 500 mg und 1000 mg - PatientenInfo-Service. Accessed March 8 (2022).
- Tiede C, Chiriac U, Dubinski D, et al. Cerebrospinal Fluid Concentrations of Meropenem and Vancomycin in Ventriculitis Patients Obtained by TDM-Guided Continuous Infusion. Antibiotics 10 (2021): 1421.
- European Medicines Agency. Guideline on bioanalytical method validation. Published online (2015).
- Steffens NA, Zimmermann ES, Nichelle SM, Brucker N. Meropenem use and therapeutic drug monitoring in clinical practice: a literature review. J Clin Pharm Ther n/a (n/a).
- EUCAST: Clinical breakpoints and dosing of antibiotics. Accessed July 12 (2021).
- Kipper K, Anier K, Leito I, Karjagin J, Oselin K, Herodes K. Rapid Determination of Meropenem in Biological Fluids by LC: Comparison of Various Methods for Sample Preparation and Investigation of Meropenem Stability. Chromatographia 70 (2009): 1423.
- Martens-Lobenhoffer J, Monastyrski D, Tröger U, Bode-Böger SM. Stability of meropenem in plasma versus dried blood spots (DBS). J Pharm Biomed Anal 170 (2019): 279-284.
- Jamieson C, Allwood MC, Stonkute D, Wallace A, Wilkinson AS, Hills T. Investigation of meropenem stability after reconstitution: the influence of buffering and challenges to meet the NHS Yellow Cover Document compliance for continuous infusions in an outpatient setting. Eur J Hosp Pharm 27 (2020): e53-e57.
- Di Paolo A, Gori G, Tascini C, Danesi R, Del Tacca M. Clinical pharmacokinetics of antibacterials in cerebrospinal fluid. Clin Pharmacokinet 52 (2013): 511-542.
- Nau R, Lassek C, Kinzig-Schippers M, Thiel A, Prange HW, Sörgel F. Disposition and Elimination of Meropenem in Cerebrospinal Fluid of Hydrocephalic Patients with External Ventriculostomy. Antimicrob Agents Chemother 42 (1998): 2012-2016.
- Lonsdale DO, Udy AA, Roberts JA, Lipman J. Antibacterial therapeutic drug monitoring in cerebrospinal fluid: difficulty in achieving adequate drug concentrations: Case report. J Neurosurg 118 (2013): 297-301.
- Günther S, Reimer A, Vogl H, et al. Therapeutic drug monitoring of linezolid: HPLC-based assays for routine quantification of linezolid in human serum and cerebrospinal fluid. Eur J Hosp Pharm Sci Pract. Published online January 6 (2022): ejhpharm-2021-003036.
- Dagan R, Velghe L, Rodda JL, Klugman KP. Penetration of meropenem into the cerebrospinal fluid of patients with inflamed meninges. J Antimicrob Chemother 34 (1994): 175-179.
- Chou YW, Yang YH, Chen JH, Kuo CC, Chen SH. Quantification of meropenem in plasma and cerebrospinal fluid by micellar electrokinetic capillary chromatography and application in bacterial meningitis patients. J Chromatogr B 856 (2007): 294-301.
- Imani S, Buscher H, Marriott D, Gentili S, Sandaradura I. Too much of a good thing: a retrospective study of β-lactam concentration–toxicity relationships. J Antimicrob Chemother 72 (2017): 2891-2897.
- Minichmayr IK, Schaeftlein A, Kuti JL, Zeitlinger M, Kloft C. Clinical Determinants of Target Non-Attainment of Linezolid in Plasma and Interstitial Space Fluid: A Pooled Population Pharmacokinetic Analysis with Focus on Critically Ill Patients. Clin Pharmacokinet. 2017;56(6):617-633.
- De Waele JJ, Lipman J, Akova M, et al. Risk factors for target non-attainment during empirical treatment with β-lactam antibiotics in critically ill patients. Intensive Care Med 40 (2014): 1340-1351.
- Roberts JA, Roberts MS, Robertson TA, Dalley AJ, Lipman J. Piperacillin penetration into tissue of critically ill patients with sepsis—Bolus versus continuous administration? Crit Care Med 37 (2009): 926-933.
- Nau R, Sörgel F, Eiffert H. Penetration of Drugs through the Blood-Cerebrospinal Fluid/Blood-Brain Barrier for Treatment of Central Nervous System Infections. Clin Microbiol Rev 23 (2010): 858-883.