De Novo versus Relapse of Glomerulonephritis Post COVID-19 Vaccination: Case Series in a Single Center in Saudi Arabia
Article Information
Abdullah Alhwiesh1, Lamees Alayoobi1, Mahmoud Ibrahim2, Mohamed Nasreldin1, Mahmood Alnokeety1, Hend Aljenaidi1, Wael Mostafa3, Mohammed Alyousef3, Ameera Alnemer1, Sana Alsolami4, Khalid Sharofna1, Kaltham Alfalah1, Zahraa Almarri1, Kareemah Alquraish1
1Nephrology Division, Department of Internal Medicine, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Saudi Arabia
2Pulmonary Division, Department of Internal Medicine, King Fahd Hospital of the University, Imam Abdulrahman Bin Faisal University, Saudi Arabia
3Pathology Department at King Fahad Hospital of the University, Saudi Arabia
4Pathology Department at Dammam regional lab, Saudi Arabia
*Corresponding Author: Lamees Alayoobi, Nephrology fellow at King Fahad Hospital of the University, Imam Abdulrahman Bin Faisal University, Saudi Arabia.
Received: 08 November 2021; Accepted: 22 December 2021; Published: 30 December 2021
Citation: Alhwiesh A, Alayoobi L, Ibrahim M, Nasreldin M, Alnokeety M, Aljenaidi H, Mostafa W, Alyousef M, Alnemer A, Alsolami S, Sharofna K, Alfalah K, Almarri Z, Alquraish K. De Novo Versus Relapse of Glomerulonephritis Post COVID- 19 Vaccination: Case Series in A Single Center in Saudi Arabia. Archives of Nephrology and Urology 4 (2021): 135-142.
View / Download Pdf Share at FacebookAbstract
The development of de novo or relapse of an already existing glomerular disease has been reported in association with some COVID-19 vaccinations, specifically the mRNA vaccines. Although reported cases are sparse and the number of reported cases are considered low in comparison to the total number of administered vaccine doses worldwide, the healthcare providers as well as the patients should be aware about such a serious complication in order to provide prompt and proper management. Here we report 3 cases of glomerular diseases in relation to COVID-19 vaccination. One case of IgA nephropathy, a case of IgA vacuities and nephropathy and a case of a relapse of a minimal change disease.
Keywords
COVID-19 vaccine associated glomerular disease (CVAGD); IgA nephropathy (IgAN); IgA vasculitis (IgAV); Henoch-Schonlein purpura (HSP); Minimal change disease (MCD)
COVID-19 vaccine associated glomerular disease (CVAGD) articles; IgA nephropathy (IgAN) articles; IgA vasculitis (IgAV) articles; Henoch-Schonlein purpura (HSP) articles; Minimal change disease (MCD) articles
COVID-19 vaccine associated glomerular disease articles COVID-19 vaccine associated glomerular disease Research articles COVID-19 vaccine associated glomerular disease review articles COVID-19 vaccine associated glomerular disease PubMed articles COVID-19 vaccine associated glomerular disease PubMed Central articles COVID-19 vaccine associated glomerular disease 2023 articles COVID-19 vaccine associated glomerular disease 2024 articles COVID-19 vaccine associated glomerular disease Scopus articles COVID-19 vaccine associated glomerular disease impact factor journals COVID-19 vaccine associated glomerular disease Scopus journals COVID-19 vaccine associated glomerular disease PubMed journals COVID-19 vaccine associated glomerular disease medical journals COVID-19 vaccine associated glomerular disease free journals COVID-19 vaccine associated glomerular disease best journals COVID-19 vaccine associated glomerular disease top journals COVID-19 vaccine associated glomerular disease free medical journals COVID-19 vaccine associated glomerular disease famous journals COVID-19 vaccine associated glomerular disease Google Scholar indexed journals IgA nephropathy articles IgA nephropathy Research articles IgA nephropathy review articles IgA nephropathy PubMed articles IgA nephropathy PubMed Central articles IgA nephropathy 2023 articles IgA nephropathy 2024 articles IgA nephropathy Scopus articles IgA nephropathy impact factor journals IgA nephropathy Scopus journals IgA nephropathy PubMed journals IgA nephropathy medical journals IgA nephropathy free journals IgA nephropathy best journals IgA nephropathy top journals IgA nephropathy free medical journals IgA nephropathy famous journals IgA nephropathy Google Scholar indexed journals IgA vasculitis articles IgA vasculitis Research articles IgA vasculitis review articles IgA vasculitis PubMed articles IgA vasculitis PubMed Central articles IgA vasculitis 2023 articles IgA vasculitis 2024 articles IgA vasculitis Scopus articles IgA vasculitis impact factor journals IgA vasculitis Scopus journals IgA vasculitis PubMed journals IgA vasculitis medical journals IgA vasculitis free journals IgA vasculitis best journals IgA vasculitis top journals IgA vasculitis free medical journals IgA vasculitis famous journals IgA vasculitis Google Scholar indexed journals Henoch-Schonlein purpura articles Henoch-Schonlein purpura Research articles Henoch-Schonlein purpura review articles Henoch-Schonlein purpura PubMed articles Henoch-Schonlein purpura PubMed Central articles Henoch-Schonlein purpura 2023 articles Henoch-Schonlein purpura 2024 articles Henoch-Schonlein purpura Scopus articles Henoch-Schonlein purpura impact factor journals Henoch-Schonlein purpura Scopus journals Henoch-Schonlein purpura PubMed journals Henoch-Schonlein purpura medical journals Henoch-Schonlein purpura free journals Henoch-Schonlein purpura best journals Henoch-Schonlein purpura top journals Henoch-Schonlein purpura free medical journals Henoch-Schonlein purpura famous journals Henoch-Schonlein purpura Google Scholar indexed journals Minimal change disease articles Minimal change disease Research articles Minimal change disease review articles Minimal change disease PubMed articles Minimal change disease PubMed Central articles Minimal change disease 2023 articles Minimal change disease 2024 articles Minimal change disease Scopus articles Minimal change disease impact factor journals Minimal change disease Scopus journals Minimal change disease PubMed journals Minimal change disease medical journals Minimal change disease free journals Minimal change disease best journals Minimal change disease top journals Minimal change disease free medical journals Minimal change disease famous journals Minimal change disease Google Scholar indexed journals Severe Acute Respiratory Syndrome-Corona Virus-2 articles Severe Acute Respiratory Syndrome-Corona Virus-2 Research articles Severe Acute Respiratory Syndrome-Corona Virus-2 review articles Severe Acute Respiratory Syndrome-Corona Virus-2 PubMed articles Severe Acute Respiratory Syndrome-Corona Virus-2 PubMed Central articles Severe Acute Respiratory Syndrome-Corona Virus-2 2023 articles Severe Acute Respiratory Syndrome-Corona Virus-2 2024 articles Severe Acute Respiratory Syndrome-Corona Virus-2 Scopus articles Severe Acute Respiratory Syndrome-Corona Virus-2 impact factor journals Severe Acute Respiratory Syndrome-Corona Virus-2 Scopus journals Severe Acute Respiratory Syndrome-Corona Virus-2 PubMed journals Severe Acute Respiratory Syndrome-Corona Virus-2 medical journals Severe Acute Respiratory Syndrome-Corona Virus-2 free journals Severe Acute Respiratory Syndrome-Corona Virus-2 best journals Severe Acute Respiratory Syndrome-Corona Virus-2 top journals Severe Acute Respiratory Syndrome-Corona Virus-2 free medical journals Severe Acute Respiratory Syndrome-Corona Virus-2 famous journals Severe Acute Respiratory Syndrome-Corona Virus-2 Google Scholar indexed journalsArticle Details
1. Introduction
The emergence and rapid transmission of the Severe Acute Respiratory Syndrome-Corona Virus-2 (SARS-CoV-2) caused by the novel coronavirus COVID-19, has led the World Health Organization (WHO) to declare COVID-19 disease a pandemic on 11th of March 2020. In order to contain the emerging COVID-19 pandemic, seven vaccinations were granted an emergency use authorization, and in August 23rd the U.S. Food and Drug Administration approved the first COVID-19 vaccine, the Pfizer-BioNTech COVID-19 Vaccine [1]. As of 4 November 2021, a total of 7,027,377,238 vaccine doses have been administered worldwide [2]. The available vaccines are of four types, either inactivated viral vaccine, RNA and DNA vaccine, viral vector vaccine and protein based vaccine. There have been a number of reported side effects to these vaccines, and in particular relation to our article is the development of glomerulonephritis either de novo or relapse of an already existing glomerular disease. So far the reported cases were with the mRNA based vaccines. In our case series we report one case of de novo versus flare of IgA nephropathy which started after the first dose of mRNA vaccine the Pfizer-BioNTech and exacerbated after the second dose of the same vaccine, another case of de novo IgA vasculitis and nephropathy after the second dose of viral vector vaccine the Oxford-AstraZeneca, and a case of relapsing minimal change disease after a period of remission which occurred after the first dose of Pfizer-BioNTech vaccine.
2. Case 1
A 27-year-old male who had no past medical history presented with 2 weeks’ history of lower limb edema, periorbital edema and frothy urine. He had no history fever, GI or respiratory symptoms and no history of recent illness. He denied any history of drug, herbal or anabolic steroid intake. His symptoms started two weeks after the first dose of Pfizer-Biontech BNT162b2 vaccine and exacerbated 2 days after he took the second dose. His physical examination was unremarkable except for bilateral lower limbs edema. Laboratory investigations were unremarkable except for proteinuria 7.5 g/day, high cholesterol 377 mg/dL and low serum albumin 2.1 g/dL. His virology and serology screens were all negative. He was admitted with the impression of nephrotic syndrome for evaluation and renal biopsy was performed which was consistent with of IgA nephropathy with oxford classification M1 E0 S0 T0 (Figure 1). Lisinopril, atorvastatin and oral prednisolone 60 mg daily were prescribed for the patient.
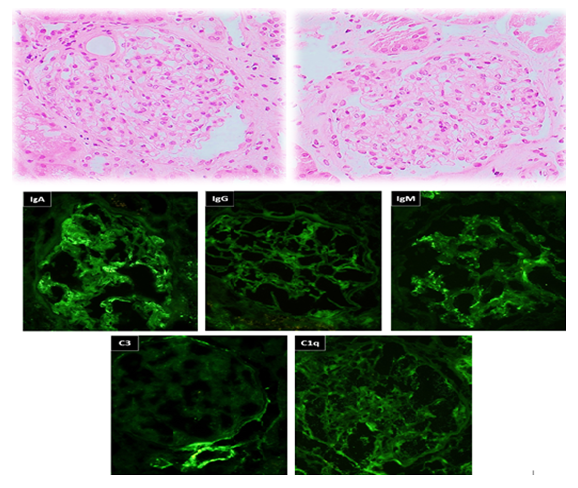
Figure 1: A & B (H&E-x400): Two glomeruli show mild mesangial matrix expansion and segmental mild mesangial hypercellularity “arrows”. No endocapillary hypercellularity or capillary wall changes. (Immunoflourescence-x400): The immunofluorescence stains show dominant IgA deposits in the mesangium.
3. Case 2
A 29-year-old male who had no past medical history, presented to ER complaining of 6 days’ history of abdominal pain not responding to analgesia which increased in severity, associated with nausea and 2 episodes of loose motion. He developed purpuric rash involving lower limbs, abdomen, chest, back and upper limbs, sparing the face. He also noted to have frothy urine for the same duration and lower limb edema. His symptoms started 2 days after he has received his second Oxford-AstraZeneca (AZD1222) vaccine. He sought a dermatologist advise and underwent a skin biopsy which was featuring leucocytoclasic vasculitis. On examination, he was afebrile with normal blood pressure. He had diffuse non-tender macular rash involving the lower limbs, abdomen, chest, back and upper limbs (Figure 2). Cardiac and chest examination were unremarkable. He had epigastric and right upper quadrant abdominal tenderness with no rigidity or rebound tenderness. Lab investigations showed normal CBC, negative virology and serology screens.
Urinalysis: Protein 3+, Blood 2+, WBC 0-2, RBC 0-2. 24-hour urine protein: 9168 mg/day.
CT abdomen was done which was showing segmental bowel wall thickening and hyper enhancement involving the terminal ileum, cecum and ascending colon with minimal peripheral fat stranding and multiple enlarged regional lymph nodes, the findings are highly suggestive of inflammatory/ infectious process. He was admitted with the impression of IgA vasculitis (Henoch-Schonlein Purpura) and IgA nephropathy. Renal biopsy was performed which was consistent with IgA nephropathy with Oxford classification M0 E1 S0 T0 C0 (Figure 3). Intravenous Methylprednisolone 500mg daily was prescribed for 3 days followed by oral prednisolone 60mg daily. His symptoms improved dramatically after initiation of steroids and the rash started to subside (Figure 4).
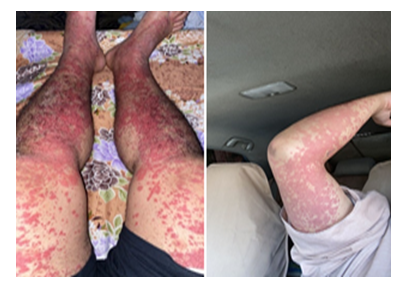
Figure 2: Maculopapular rash involving upper and lower limbs.
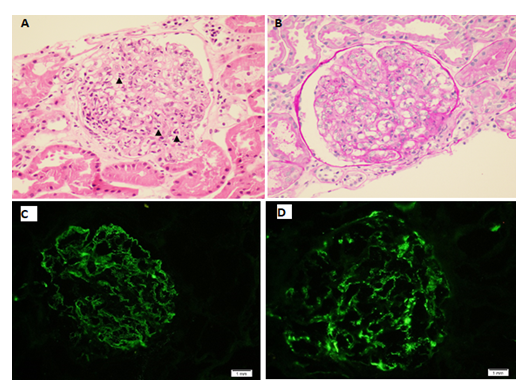
Figure 3A: A glomerulus depicting prominent endocapillary hypercellularity with predominant neutrophils (arrowheads) (Hematoxylin and eosin, original magnification x 400). B: Endocapillary hypercellularity with neutrophils and occasional mononuclear cells (PAS, original magnification x 400). C: IgA immunofluorescence staining showing granular mesangial and capillary loops staining (original magnification x 400). D: C3 immunofluorescence staining showing granular mesangial staining (original magnification x 400).
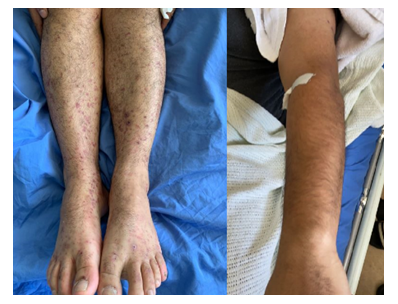
Figure 4: Resolving rash after corticosteroid initiation.
4. Case 3
A 32-year-old female diagnosed with biopsy proven minimal change disease in June 2019 who responded to steroids and was in remission since November 2019, presented to ER complaining of fatigue associated with mild periorbital and lower limb edema which started 3 weeks after she has received the first dose of Pfizer-biontech BNT162b2 vaccine. Her symptoms exacerbated 2 days after receiving her second vaccine dose which was one after her first dose. Her physical examination was unremarkable except for bilateral lower limb edema and lab investigations showed a 24-hour urine protein of 1698 mg/day. Oral prednisolone 20 mg daily was prescribed after which her proteinuria subsided, and her latest 24-hour urine is negative for protein.
5. Discussion
The pathophysiological mechanism of COVID-19 vaccine induced glomerular disease is unknown, however its temporal association with the vaccine suggest a vaccine related immune mediated glomerular injury. To date there are reported cases of minimal change disease [3,4], IgA nephropathy [5], ANCA vasculitis [6] and lupus nephritis flare [7] in association with COVID-19 vaccination, specifically with mRNA vaccines (Pfizer-BioNTech and Moderna vaccines). To our knowledge, case number 2 is the first case of IgA vasculitis and nephropathy associated with adeno viral vector vaccine Oxford-AstraZeneca vaccine. The association between some vaccinations and the development of glomerular disease has been described in the past such as a reported case of minimal change disease (MCD) after administration of influenza vaccine [8]. The underlying mechanism of MCD in general is unclear. It is suggested that it is due to T-cell dysfunction resulting in production of permeability factors that in turn will lead to foot process fusion. However, with secondary cause of MCD, immunization is one of the documented causes of MCD, possibly due to hypersensitivity syndrome. While with IgA nephropathy, the data indicate that patients have a higher than normal immune response to influenza vaccine, limited to the IgA1 isotype and this hyper responsiveness does not appear to be limited to influenza, but applies to various viral antigens [9].
6. Conclusion
In view of the limited reported COVID-19 vaccine associated glomerular disease (CVAGD) we are reporting these three cases of either a de novo glomerular disease plus flare of an already existing glomerular disease following COVID-19 vaccines, with both the mRNA and adeno viral vector vaccine. This is to highlight an issue that require further investigation in order to better understand the mechanism of glomerular injury associated with these vaccines, and to provide the proper management in a timely manner. The mechanism of glomerular injury remains unknown. However, this should not discourage our patients from receiving the COVID-19 vaccine, as the risk of the COVID-19 disease itself and its complications far more exceeds the risk of the vaccine complications. Furthermore, given the limited number of the reported cases so far in comparison to the massive amount of administered vaccine doses worldwide, the incidence of CVAGD although serious remains very low. Nevertheless, an insight about the occurrence of such complications must be brought to the physicians’ attention to observe any symptoms that manifest after the vaccine. In addition, patients should be aware to report any symptoms that indicate a renal involvement in order to obtain prompt and proper management.
Declaration of Conflicting Interest
The authors declare no potential conflict of interest in relation to the research, authorship or publication of this article.
Consent for Publication
The manuscript contains pictures of a patient who gave the consent for publication.
Funding
The authors received no financial support for the research, authorship or for publication of this article.
References
- FDA: FDA Approves First COVID-19 Vaccine.
- World Health Organization: WHO Coronavirus (COVID-19) Dashboard | WHO Coronavirus (COVID-19) Dashboard With Vaccination Data.
- Lebedev L, Sapojnikov M, Wechsler A, et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am J Kidney Dis 78 (2021): 142-145.
- D'Agati VD, Kudose S, Bomback AS. Minimal change disease and acute kidney injury following the Pfizer-BioNTech COVID-19 vaccine. Kidney Int 100 (2021): 461-463.
- Negrea L, Rovin BH. Gross hematuria following vaccination for severe acute respiratory syndrome coronavirus 2 in 2 patients with IgA nephropathy. Kidney Int 99 (2021): 1487.
- Sekar A, Campbell R, Jad Tabbara and Prerna Rastogi: ANCA glomerulonephritis after the Moderna COVID-19 vaccination. Kidney International 100 (2021) : 473-474.
- Tuschen K, Bräsen JH, Schmitz J, et al. Alexander Weidemann: Relapse of class V lupus nephritis after vaccination with COVID-19 mRNA vaccine: Kidney International 100 (2021): 941-944.
- Gutiérrez S, Dotto B, Petiti JP, et al. Minimal change disease following influenza vaccination and acute renal failure: just a coincidence?. Nefrologica 32 (2012): 414-415.
- Bake AW, Masurel N, L A van Es. Humoral immune response to influenza vaccination in patients with primary immunoglobulin A nephropathy. An analysis of isotype distribution and size of the influenza-specific antibodies. J Clin Invest 84 (1989): 1070-1075.
- Tan HZ, Tan RY, Choo JCJ, et al. Is COVID-19 vaccination unmasking glomerulonephritis? Kidney Int 100 (2021): 469-471.
