De Novo Crohn’s Disease Diagnosed in the Setting of Acute SARSCov- 2 Infection Requiring Escalation of Infliximab Therapy Guided by Personalized Pharmacokinetics
Article Information
Martin Varea Romo1, Kishor Acharya2, Nathan R Shelman3, Phillip Minar4,5, Lee A Denson4,5, Samir Softic6,7*
1Kentucky Children’s Hospital, Lexington, KY, United States
2University of Kentucky College of Medicine, Lexington, KY, United States
3Department of Pathology and Laboratory Medicine, University of Kentucky, Lexington, KY, United States
4Division of Gastroenterology, Hepatology, and Nutrition, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA
5Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA
6Department of Pediatrics, Division of Pediatric Gastroenterology, University of Kentucky College of Medicine, Lexington, KY, United States
7Department of Pharmacology and Nutritional Sciences, University of Kentucky College of Medicine, Lexington, KY, United States
*Corresponding Author: Samir Softic, Assistant Professor of Pediatrics, University of Kentucky, Lexington, 40536, KY, United States.
Received: 21 December 2023; Accepted: 04 January 2024; Published: 12 January 2024
Citation:
Martin Varea Romo, Kishor Acharya, Nathan R Shelman, Phillip Minar, Lee A Denson, Samir Softic. De Novo Crohn’s Disease Diagnosed in the Setting of Acute SARS-Cov-2 Infection Requiring Escalation of Infliximab Therapy Guided by Personalized Pharmacokinetics. Journal of Pediatrics, Perinatology and Child Health. 8 (2024): 01-04.
View / Download Pdf Share at FacebookAbstract
Here, we describe a 7-year-old girl who was diagnosed with an earlyonset Crohn’s disease in the setting of COVID-19 illness. Her disease process responded poorly to standard infliximab dosing, necessitating repeat hospitalizations and red blood cell transfusions. Remission was subsequently induced using a personalized infliximab pharmacokinetic profile based on therapeutic drug monitoring. While the initial data does not support a link, several case reports suggest an association between COVID-19 illness and de-novo development of IBD, especially in young female patients. We report, to our knowledge, the youngest patient who developed early-onset Crohn’s disease in the setting of concomitant SARS-CoV-2 infection.
Keywords
COVID-19 illness; De Novo crohn’s disease; Inflammatory bowel disease
Article Details
1. Introduction
Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, some patients will inevitably present with a new onset of inflammatory bowel disease (IBD) in the setting of acute co-infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Whether COVID-19 illness can trigger de novo IBD and how the concurrent SARS-CoV-2 infection may influence the natural history of IBD are still unanswered questions. The initial evidence does not support a link between SARS-CoV-2 infection and an increased risk of developing de novo IBD [1]. Moreover, the patients with IBD who acquire SARS-CoV-2 infection are not at increased risk of adverse outcomes [2]. On the other hand, it is well described that SARS-CoV-2 can cause enteric infection via binding to angiotensin-converting enzyme receptor 2 (ACE2) abundantly expressed on the intestinal epithelial cells. Acute gastroenteritis is a well-recognized risk-factor for triggering de novo IBD, perhaps via inducing intestinal dysbiosis [3]. Furthermore, COVID-19 can cause immune dysregulation and trigger multisystem inflammatory syndrome, especially in children4. Lastly, several pathways associated with SARS-CoV2 infection overlap with IBD pathogenesis, advocating for shared molecular networks between the two conditions [5]. This interaction may be especially relevant in children with early onset IBD who have a higher prevalence of underlying immune dysregulation [6].
Unlike the ambiguity surrounding SARS-CoV-2 infection and IBD development, there is a clear relationship between IBD treatment and COVID-19 outcomes. For example, the use of corticosteroids or mesalamine is associated with significantly worse outcomes. In contrast, anti-tumor necrosis factor alpha (anti-TNFa) therapy is associated with more favorable outcomes [7]. Clearance of anti-TNFa therapy is an important predictor of IBD therapeutic response. Recently, individualized dosing based on pharmacokinetic models and therapeutic drug monitoring has emerged as a tool to improve outcomes in patients with IBD [8-10]. How concomitant SARS-CoV-2 infection affects the clearance of biologic therapy is not known, but some data suggest that anti-TNFa clearance could be modified by intestinal microbe [11].
2. Case Presentation
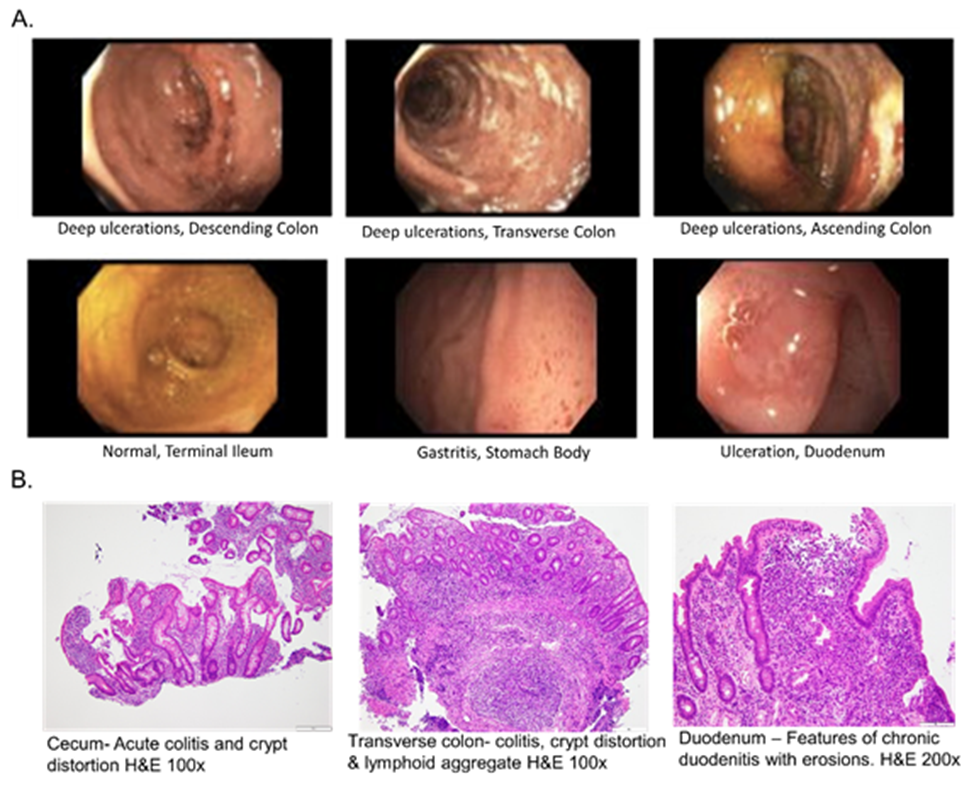
A previously healthy 7-year-old female presented with diarrhea, hematochezia and a seven-pound weight loss over the preceding two months. She was found to have hemoglobin of 6.6 mg/dL with a 1.6 mg/dL hemoglobin drop in a two-day interval. She was sent to the Emergency Department, where she was diagnosed with SARS-CoV-2 infection by PCR. One week before the presentation, her family members had symptomatic COVID-19 illness. The patient was admitted to the hospital and received a packed red blood cell (PRBC) transfusion. A workup identified an increased erythrocyte sedimentation rate (ESR) of 31 mm/hr, high c-reactive protein (CRP) of 8.5 mg/L, low serum albumin of 3.5 g/dL, and elevated stool calprotectin of 1860 ug/g. An esophagogastroduodenoscopy identified mild gastritis and ulcerations in the duodenum. A colonoscopy revealed severe pan-colitis and normal terminal ileum (Figure 1A).
Histological assessment was consistent with Crohn’s disease (Figure 1B), and the patient was started on infliximab 5 mg/kg induction therapy. Two days later, her hematochezia subsided. Infliximab concentration before the second infusion was 16.3 μg/ml. Twenty-one days after receiving the second infliximab infusion, hematochezia recurred. She was readmitted for repeat PRBC transfusion, and due to the recurrence of symptoms, the patient received an early and higher dose of infliximab, 7.5 mg/kg. Subsequently, the infliximab level was found to be 4.3 μg/ml, confirming rapid drug clearance. She was SARS-CoV-2 negative at that time, although she was found to have Clostridium difficile enteric infection and completed a 21-day antibiotic therapy.
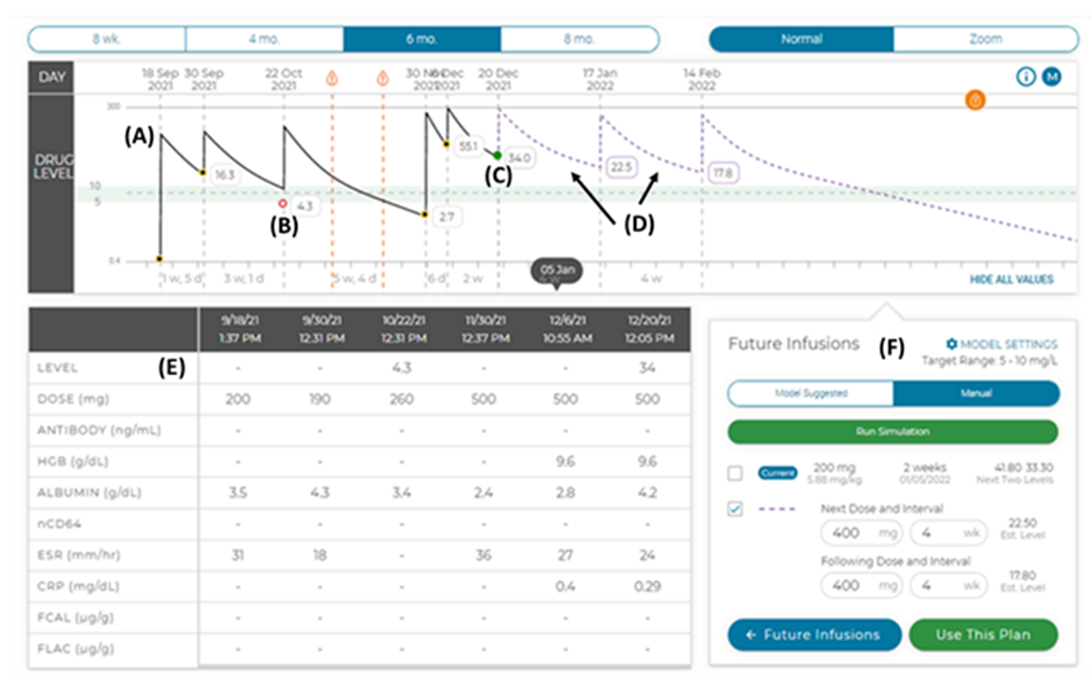
Four weeks later, rectal bleeding recurred, and the patient was readmitted for the third time. Infliximab level was low at 2.7 μg/ml despite previously increased dosing. The infliximab dose was increased to 15 mg/kg, and the administration interval decreased to every four weeks. This regimen was based on poor clinical response to standard dosing and the patient’s pharmacokinetic (PK) profile (Figure 2) based on the therapeutic drug monitoring (TDM) protocol developed at Cincinnati Children’s Hospital Medical Center [12].
Figure 2: The patient’s pharmacokinetic (PK) profile. (A) The patient’s previous infusions (solid line) are shown on the concentration-time curve with the (B & C) measured (actual) trough concentrations shown, The PK platform then uses the patient's infusion history, past biomarkers of drug clearance and prior drug concentrations to predict the concentration-time curve for (D) two future infusions (dashed line). (E) The table displays past doses and key biomarkers. (F) Future infusions and predicted trough concentrations are also shown and can be personalized by the user in manual mode.
Six months after initiation of the dosing regimen based on PK, the patient was asymptomatic. Her hemoglobin was 12.5 g/dL, ESR was 9 mm/h, CRP was <3 mg/L and albumin was 4.3 mg/dL. Infliximab was stepwise decreased to 10 mg/kg, given every six weeks. She had a new exacerbation of GI symptoms after a second infection with SARS-CoV-2. The infliximab level was again low and she was treated with systemic steroids. Infliximab infusion frequency was decreased back to four weeks. After this interval adjustment, her symptoms improved, and her infliximab level was in the desired range. The patient is currently ten-years-old, she is off steroids, receiving 10/mg/kg infliximab infusions every four weeks and is taking sulfasalazine.
3. Discussion
Here, we describe a case of a seven-year-old girl newly diagnosed with Crohn’s disease in the setting of concomitant SARS-CoV-2 infection. She had poor response to standard infliximab therapy. Our team successfully guided her infliximab treatment with a personalized pharmacokinetic profile based on therapeutic drug monitoring and a clinical decision support tool. We are unsure how much the concomitant SARS-CoV-2 infections contributed to her low infliximab level and poor clinical response. Increased clearance of biologic medication is driven primarily by patient-related factors, such as a degree of systemic inflammation, serum albumin concentration, body weight, and the presence of anti-drug antibodies. More recent evidence suggests intestinal microbiome factors may contribute to the clearance of biologic medications [8]. SARS-CoV-2 is well-known to infect enterocytes, and any condition that results in significant intestinal inflammation, leading to poor barrier function, could theoretically contribute to increased drug clearance. Whatever the underlying cause, personalized pharmacokinetics based on therapeutic drug monitoring can potentially improve disease-specific outcomes by ensuring therapeutic drug levels.
This case brings into question a possible link between early-onset IBD and COVID-19. While the initial evidence does not support a correlation, much remains to be learned about the long-term effects of SARS-CoV-2 infection. The development of autoimmune diseases and inflammatory conditions after recovery from COVID-19 has been described [13]. A dysregulated immune response has been proposed as the underlying pathogenesis. SARS-CoV-2 infection leads to immune cell activation and redistribution of immune cells to the lungs and intestines, leading to relative peripheral lymphopenia and loss of tolerance to self. Indeed, several case reports describe an association between SARS-CoV-2 infection and de novo development of IBD, especially in younger female patients [14]. As we continue to learn more about the long-term effects of SARS-CoV-2 infection, the relationship between early-onset IBD and COVID-19 needs to be further investigated in longitudinal studies.
Acknowledgement:
This work was supported in part by the COCVD Pilot and Feasibility Grant (GM127211) awarded to SS for his basic science studies [15]. Informed consent was obtained from the family for this report.
References
- Ungaro RC, Kappelman MD, Rubin DT, et al. COVID-19 and Inflammatory Bowel Disease: Lessons Learned, Practical Recommendations, and Unanswered Questions. Gastroenterology 160 (2021): 1447-1451.
- Brenner EJ, Pigneur B, Focht G, et al. Benign Evolution of SARS-Cov2 Infections in Children With Inflammatory Bowel Disease: Results From Two International Databases. Clin Gastroenterol Hepatol 19 (2021): 394-396 e5.
- Garcia Rodriguez LA, Ruigomez A, Panes J. Acute gastroenteritis is followed by an increased risk of inflammatory bowel disease. Gastroenterology 130 (2006): 1588-1594.
- Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol 16 (2020): 413-414.
- Suarez-Farinas M, Tokuyama M, Wei G, et al. Intestinal Inflammation Modulates the Expression of ACE2 and TMPRSS2 and Potentially Overlaps With the Pathogenesis of SARS-CoV-2-related Disease. Gastroenterology 160 (2021): 287-301 e20.
- Lekbua A, Ouahed J, O'Connell AE, et al. Risk-factors Associated With Poor Outcomes in VEO-IBD Secondary to XIAP Deficiency: A Case Report and Literature Review. J Pediatr Gastroenterol Nutr 69 (2019): e13-e18.
- Tripathi K, Godoy Brewer G, Thu Nguyen M, et al. COVID-19 and Outcomes in Patients With Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm Bowel Dis 28 (2022): 1265-1279.
- Dotan I, Ron Y, Yanai H, et al. Patient factors that increase infliximab clearance and shorten half-life in inflammatory bowel disease: a population pharmacokinetic study. Inflamm Bowel Dis 20 (2014): 2247-2259.
- Xiong Y, Mizuno T, Colman R, et al. Real-World Infliximab Pharmacokinetic Study Informs an Electronic Health Record-Embedded Dashboard to Guide Precision Dosing in Children with Crohn's Disease. Clin Pharmacol Ther 109 (2021): 1639-1647.
- Colman RJ, Tsai YT, Jackson K, et al. Achieving Target Infliximab Drug Concentrations Improves Blood and Fecal Neutrophil Biomarkers in Crohn's Disease. Inflamm Bowel Dis 27 (2021): 1045-1051.
- Colman RJ, Mizuno T, Fukushima K, et al. Real world population pharmacokinetic study in children and young adults with inflammatory bowel disease discovers novel blood and stool microbial predictors of vedolizumab clearance. Aliment Pharmacol Ther 57 (2023): 524-539.
- Colman RJ, Samuels A, Mizuno T, et al. Model-informed Precision Dosing for Biologics Is Now Available at the Bedside for Patients With Inflammatory Bowel Disease. Inflamm Bowel Dis (2022).
- Liu Y, Sawalha AH, Lu Q. COVID-19 and autoimmune diseases. Curr Opin Rheumatol 33 (2021): 155-162.
- Tursi A, Lopetuso LR, Vetrone LM, et al. SARS-CoV-2 infection as a potential trigger factor for de novo occurrence of inflammatory bowel disease. Eur J Gastroenterol Hepatol 34 (2022): 883-884.
- Helsley RN, Park SH, Vekaria HJ, et al. Ketohexokinase-C regulates global protein acetylation to decrease carnitine palmitoyltransferase 1a-mediated fatty acid oxidation. J Hepatol 79 (2023): 25-42.