Cutaneous Hemangioendothelioma with Intramammary Node Metastasis: Case Report and Review of Literature
Article Information
Melisa Camaño1*, Carlos Palacio2, Marta Gonda3, Clara Delbene4, Florencia Rébora5, Daniela De Boni6
1Medical Oncology Department, National Cancer Institute, 11600 Montevideo, Uruguay
2Plastic Surgery Department, National Cancer Institute, 11600Montevideo, Uruguay
3Surgery Department, National Cancer Institute, 11600 Montevideo, Uruguay
4Pathology Department, National Cancer Institute, 11600 Montevideo, Uruguay
5Diagnostic Imaging Department, National Cancer Institute, 11600 Montevideo, Uruguay
6Dermathology Department, National Cancer Institute, 11600 Montevideo, Uruguay
*Corresponding Author: Ivanna Melisa Camaño, Medical Oncology Department, National Cancer Institute, 11600 Montevideo, Uruguay.
Received: 24 November 2023; Accepted: 04 December 2023; Published: 14 December 2023
Citation: Melisa Camaño, Carlos Palacio, Marta Gonda, Clara Delbene, Florencia Rébora, Daniela De Boni. Cutaneous Hemangioendothelioma with Intramammary Node Metastasis: Case Report and Review of Literature. Journal of Surgery and Research. 6 (2023): 432-436.
View / Download Pdf Share at FacebookAbstract
Epithelioid hemangioendothelioma is a rare malignant vascular neoplasm. The skin presentations often have a delayed diagnosis because of their nonspecific presentation and have a more indolent evolution.
We present the case of a 50-years-old woman with an unplanned skin EHE resection. She presented a relapse on the previous scar and a mammary gland metastasis. Both lesions were successfully removed and follow-up was decided. To our knowledge this is the first report of a breast metastasis.
There is no solid prospective evidence for the selection of the systemic treatment in case of unresectability and the recommendation based on retrospective studies is antiangiogenic therapy like pazopanib.
Keywords
Hemangioendothelioma, Skin, Breast metastasis, Surgery, Antiangiogenic therapy
Hemangioendothelioma articles; Skin articles; Breast metastasis articles; Surgery articles; Antiangiogenic therapy articles
Hemangioendothelioma articles Hemangioendothelioma Research articles Hemangioendothelioma review articles Hemangioendothelioma PubMed articles Hemangioendothelioma PubMed Central articles Hemangioendothelioma 2023 articles Hemangioendothelioma 2024 articles Hemangioendothelioma Scopus articles Hemangioendothelioma impact factor journals Hemangioendothelioma Scopus journals Hemangioendothelioma PubMed journals Hemangioendothelioma medical journals Hemangioendothelioma free journals Hemangioendothelioma best journals Hemangioendothelioma top journals Hemangioendothelioma free medical journals Hemangioendothelioma famous journals Hemangioendothelioma Google Scholar indexed journals Skin articles Skin Research articles Skin review articles Skin PubMed articles Skin PubMed Central articles Skin 2023 articles Skin 2024 articles Skin Scopus articles Skin impact factor journals Skin Scopus journals Skin PubMed journals Skin medical journals Skin free journals Skin best journals Skin top journals Skin free medical journals Skin famous journals Skin Google Scholar indexed journals Breast metastasis articles Breast metastasis Research articles Breast metastasis review articles Breast metastasis PubMed articles Breast metastasis PubMed Central articles Breast metastasis 2023 articles Breast metastasis 2024 articles Breast metastasis Scopus articles Breast metastasis impact factor journals Breast metastasis Scopus journals Breast metastasis PubMed journals Breast metastasis medical journals Breast metastasis free journals Breast metastasis best journals Breast metastasis top journals Breast metastasis free medical journals Breast metastasis famous journals Breast metastasis Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals Antiangiogenic therapy articles Antiangiogenic therapy Research articles Antiangiogenic therapy review articles Antiangiogenic therapy PubMed articles Antiangiogenic therapy PubMed Central articles Antiangiogenic therapy 2023 articles Antiangiogenic therapy 2024 articles Antiangiogenic therapy Scopus articles Antiangiogenic therapy impact factor journals Antiangiogenic therapy Scopus journals Antiangiogenic therapy PubMed journals Antiangiogenic therapy medical journals Antiangiogenic therapy free journals Antiangiogenic therapy best journals Antiangiogenic therapy top journals Antiangiogenic therapy free medical journals Antiangiogenic therapy famous journals Antiangiogenic therapy Google Scholar indexed journals antiangiogenic therapy articles antiangiogenic therapy Research articles antiangiogenic therapy review articles antiangiogenic therapy PubMed articles antiangiogenic therapy PubMed Central articles antiangiogenic therapy 2023 articles antiangiogenic therapy 2024 articles antiangiogenic therapy Scopus articles antiangiogenic therapy impact factor journals antiangiogenic therapy Scopus journals antiangiogenic therapy PubMed journals antiangiogenic therapy medical journals antiangiogenic therapy free journals antiangiogenic therapy best journals antiangiogenic therapy top journals antiangiogenic therapy free medical journals antiangiogenic therapy famous journals antiangiogenic therapy Google Scholar indexed journals systemic treatment articles systemic treatment Research articles systemic treatment review articles systemic treatment PubMed articles systemic treatment PubMed Central articles systemic treatment 2023 articles systemic treatment 2024 articles systemic treatment Scopus articles systemic treatment impact factor journals systemic treatment Scopus journals systemic treatment PubMed journals systemic treatment medical journals systemic treatment free journals systemic treatment best journals systemic treatment top journals systemic treatment free medical journals systemic treatment famous journals systemic treatment Google Scholar indexed journals histopathologic diagnosis articles histopathologic diagnosis Research articles histopathologic diagnosis review articles histopathologic diagnosis PubMed articles histopathologic diagnosis PubMed Central articles histopathologic diagnosis 2023 articles histopathologic diagnosis 2024 articles histopathologic diagnosis Scopus articles histopathologic diagnosis impact factor journals histopathologic diagnosis Scopus journals histopathologic diagnosis PubMed journals histopathologic diagnosis medical journals histopathologic diagnosis free journals histopathologic diagnosis best journals histopathologic diagnosis top journals histopathologic diagnosis free medical journals histopathologic diagnosis famous journals histopathologic diagnosis Google Scholar indexed journals mammography articles mammography Research articles mammography review articles mammography PubMed articles mammography PubMed Central articles mammography 2023 articles mammography 2024 articles mammography Scopus articles mammography impact factor journals mammography Scopus journals mammography PubMed journals mammography medical journals mammography free journals mammography best journals mammography top journals mammography free medical journals mammography famous journals mammography Google Scholar indexed journals breast cancer articles breast cancer Research articles breast cancer review articles breast cancer PubMed articles breast cancer PubMed Central articles breast cancer 2023 articles breast cancer 2024 articles breast cancer Scopus articles breast cancer impact factor journals breast cancer Scopus journals breast cancer PubMed journals breast cancer medical journals breast cancer free journals breast cancer best journals breast cancer top journals breast cancer free medical journals breast cancer famous journals breast cancer Google Scholar indexed journals
Article Details
Introduction
Epithelioid hemangioendothelioma (EHE) is a malignant vascular neoplasm composed of epithelioid endothelial cells within a myxohyaline stroma; in most cases the WWTR1-CAMTA1 gene fusion is present and in a small portion of cases YAP1-TFE3 \fusion is present [1].
Most frequently affects somatic soft tissue, lung, and liver, but any site or organ can be affected. Skin and soft tissue presentations are usually solitary, whereas visceral and bone are can be multifocal or even metastatic at presentation. Skins EHE series reported between 33-58% of the primary tumors arising in the extremities, whereas 17-12% were found on the head and neck. However, lesions of the palm, trunk, anogenital region, and foot have also been reported [2]. Diagnosis is often delayed because of its rarity, its nonspecific presentation, and the difficulty of histopathologic diagnosis.
We describe a case with cutaneous presentation.
Case Report
Our patient is a 50-years-old woman with a 6 months history of a gradually enlarging skin node of the left breast, she was firstly assisted in another center. The mammography showed an isodense, ovoid focal asymmetry in the cutaneous projection of the internal sector of the left breast. BI-RADS 2 (Figure 1). The ultrasound showed a collected, hypoechoic 16 mm lesion in the skin.
The lesion had an infectious complication and an excision biopsy was performed. The pathology diagnosis was EHE and the patient was referred to our center.
Examination revealed a purple, slightly elevated, 5 mm lesion arising in the scar. It was painless and without infection signs. On the upper outer quadrant of the same breast a 2 cm firm, mobile, painless node was found. The axillary examination was normal.
Computer Tomography informed the presence of a solid 18 x16 mm node in the left breast. No other alterations were found. There was no evidence of distant metastasis (Figure 2).
The mammography revealed a dense 16x 24mm nodule with irregular morphology and poorly defined margins, in the left breast´s axillary extension. With the ultrasound it was a vascularized solid nodule. BI-RADS 4c (Figure 1).
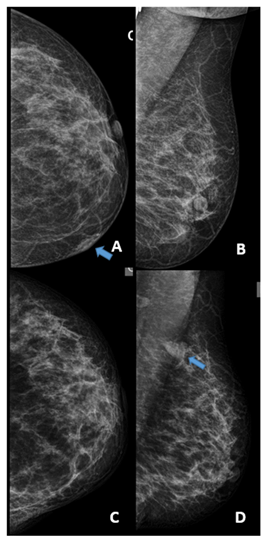
Figure 1: A) Mammography at diagnosis, the craniocaudal view shows a focal asymmetry in the cutaneous projection of the internal sector of the left breast. B) The mediolateral oblique view of the same study. BI-RADS 2 C) Mammography at relapse, the carniocaudal view does show the cutaneous alteration. D) The mediolateral oblique view of the same study shows an intramammary nodule with poorly defined margins not present in the previous study. BI-RADS 4c
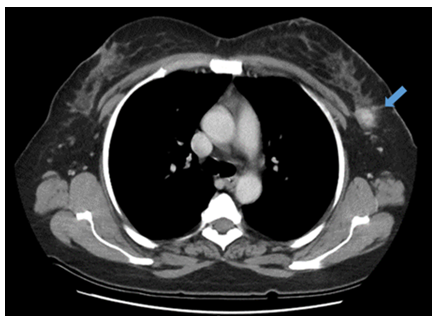
Figure 2: Computer Tomography of the relapse. It showed a solid node in the left breast.
Both lesions were biopsied and the pathology confirmed the skin relapse and the mammary gland metastasis of the hemangioendothelioma.
Simple mastectomy was performed in the macroscopic evaluation a 23x17x12cm intramammary lesion was found. The histopathology found an infiltrating cell proliferation consisting of medium-sized epithelioid-looking cells arranged in solid nests and rudimentary trabecular structures where red blood cells are recognized in their lumen. Cells with little cytoplasm and pleomorphic nuclei with evident nucleoli. The immunohistochemistry found CD 31 membranes positive, FLI 1 Nuclear positive, CD34 and GATTA 3 were both negative. The hemangiodeothelioma was confirmed. (Figure 3).
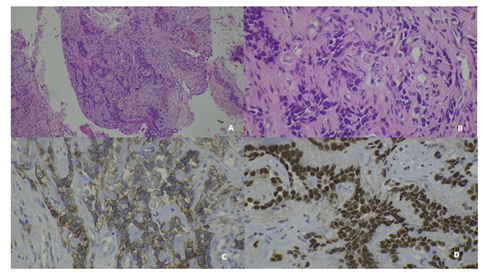
Figure 3: A) Hematoxylin and Eosin 10x shows a cell proliferation consisting of medium-sized epithelioid-looking cells arranged in nests with trabecular structures and red blood cell in their lumen. B) Hematoxylin and Eosin 40 x shows cells with little cytoplasm and pleomorphic nuclei with evident nucleoli C) CD 31 membranes positive D) FLI 1 Nuclear positive
No adjuvant treatment was needed and follow-up was decided. The patient persists without imaging or clinical evidence of relapse after 2 years.
Discussion
EHE arises from both vascular endothelial and pre-endothelial cells; therefore, the clinical behavior of EHE tumors can be quite diverse. This neoplasm is often misdiagnosed, as it accounts for less than 1% of all vascular tumors [2]. Skin metastasis has been reported, but primary cutaneous involvement is rare. In our case the mammary gland was previously evaluated and no lesions were found, the breast compromise in the relapse confirmed that the skin was the primary lesion and the mammary gland was the metastasis.
EHE is characterized by a t(1;3) (p36;q23-q25) resulting in a WWTR1-CAMTA1 fusion in > 90% of cases. The WWTR1 (TAZ) protein is an effector of the tumor-suppressive Hippo pathway. The Hippo pathway phosphorylates TAZ and YAP1 (encoded by YAP1), resulting in cytoplasmic sequestration and degradation. TAZ and YAP1 are transcription factors that promote a pro-oncogenic transcriptional program. The WWTR1-CAMTA1 fusion results in dysregulation of the Hippo pathway with the consequent oncogenic transformation. Another translocation, YAP1-TFE3, has been identified in a subset of EHE with distinct morphological features. YAP1, is also a downstream transcriptional regulator of the Hippo pathway. TFE3, a well-known oncogenic transcription factor, is upregulated as a result of this fusion [1-3].
EHE are angiocentric tumors that expand the vessel wall, obliterate the lumen, and spread centrifugally into surrounding tissue, where they induce a sclerotic response. EHE is characterized by cords and nests of epithelioid cells in a myxohyaline stroma. Tumor cells have moderate to small amounts of eosinophilic cytoplasm and round nuclei with inconspicuous nucleoli. Intracytoplasmic vacuoles are sometimes present and may contain erythrocytes. In most cases there is only minimal atypia and the mitotic count is very low. All these characteristics were found in our patient. The stroma is variably myxoid and hyaline; in some cases, hyaline stroma may obscure the tumor cells. Cystic degeneration, hemorrhage, sclerosis, and metaplastic ossification may be present. In the EHE with YAP1-TFE3 the tumor cells usually have brightly eosinophilic cytoplasm and are more likely to show solid growth and often form vascular spaces [1,2,4].
In less than 10% of the cases there are atypical histological features, including nuclear pleomorphism like in our patient, increased mitotic activity, solid sheet-like growth, and necrosis that may resemble epithelioid angiosarcoma. In such cases, CAMTA1 expression supports the diagnosis. This latter group of lesions is often associated with more aggressive behavior [1].
EHE express CD34, CD31, podoplanin (D2-40), FLI1, and ERG. Epithelial antigens are expressed in as many as 40% of cases (CK7, CK8, CK18, pan-keratin), but EMA is rare. SMA expression is present in approximately 50% of cases. When WWTFI1-CAMTA1 is present it typically shows diffuse strong nuclear expression of CAMTA1, which is a surrogate for the WWTR1-CAMTA1 fusion protein. Tumors with YAP1-TFE3 fusion show diffuse nuclear TFE3 expression [1,3]. Our patient was CD34 and FLI 1 positive as expected. GATTA 3 was performed because the lesion was in the mammary gland to despite the possibility of a breast cancer.
When complete resection is possible the surgery is the indicated treatment [5,6]. Wide margins are recommended. The use of Mohs surgery has been reported in head and neck lesions [4,7]. In our case the wide margins were not difficult to obtain because of the location of the relapse and the metastasis. The simple mastectomy allowed to remove both lesions with adequate margins.
Although the primary lesion was resected our patient had mainly two risk factors that can explain the relapse. The most important one is that the first surgery was an unplanned resection tumor and therefore there was no emphasis on the margins. We must also take into consideration the presence of nuclear pleomorphism which as we already discussed is related to higher aggressiveness.
The prognosis is linked to the presentation form. When arises in soft tissues can be indolent; however, approximately 21% will metastasize and pursue an aggressive clinical course, with death up to 17% of patients [1,2,8]. Dissemination can be hematogenous or via lymphatics, and the most frequent sites of metastases include the liver and bones [7,9], this is to our knowledge the first report of a breast metastasis.
Risk stratification into high-risk and low-risk groups based on a combination of mitotic activity (> 3 mitoses per 10 mm2; > 3 mitoses per 50 high-power fields, assuming a field diameter of 0.5 mm and an area of 0.2 mm2) and tumor size (> 3 cm) has shown a worse prognosis for patients with both features (5-year disease-specific survival rate 59%) compared with patients with neither feature (5-year disease-specific survival rate: 100%) [1,10]. This stratification is based on the primary tumor´s characteristics so this does not apply to our case not only because we treated the relapse but also because we had a distant metastasis.
However, EHE arising in the lung or bone has a worse prognosis than soft tissue tumors, and patients often present with metastatic disease. By contrast, cutaneous presentation has an excellent prognosis [11-13].
When wide margins cannot be achieved or the patient is in the high-risk group adjuvant radiotherapy can be considered but there is not enough evidence for systematic indication [2,4,10].
In case our patient had an unresectable disease relapse the slow growth rate of these tumors justifies observation, with initiation of systemic therapy at the onset of new lesions, accelerated growth of existing lesions, or symptomatic disease. Limited prospective data exist to clarify this rare tumor´s approach.
Vascular endothelial growth factor (VEGF) and the VEGF receptor were found on EHE tumor cells, which is the basis for the recommendation of antiangiogenic therapy using VEGFR Tyrosine kinase inhibitors (TKI) as the first line of treatment. In a retrospective study with Pazopanib a response rate of 20 % was described, a clinical benefit rate of 60% and a median progression-free survival of 26.3 months. Pazopanib was active both in the first and in second line [14,15]. Sorafenib was also tested in a phase II study. The median treatment duration was 124 days, the 6-months-progression-free rate was 46.6% [16]. A phase II study of Bevacizumab, a recombinant humanized antibody against VEGF not a TKI but still an antiangiogenic drug found a partial response rate of 17% with stable disease rate of 50%. The mean progression-free survival was 26 weeks [17]. According to these studies the recommended first line of treatment is Pazopanib.
There are reports of efficacy of cytotoxic chemotherapy in patients with advanced or metastatic EHE Gemcitabine [18], Carboplatin plus Etoposide [19], Pemetrexed [20], other observational studies have demonstrated limited response rates for doxorubicin-based chemotherapy and metronomic oral cyclophosphamide [6,21,22]. When patients are not candidates for Pazopanib Gemcitabine is a good option with complete responses reported.
Sirolimus, an inhibitor of the mammalian target of rapamycin (mTOR) serine/threonine kinase, was evaluated in a retrospective study. It had a partial response rate of 6%, a stable disease rate of 75% and a median progression-free survival of 12 months. There was no benefit among the patients that presented serosal effusions [23,24].
Interferon alfa has also been reported to achieve clinical response and stable disease [6,22].
Lenalidomide, an antineoplastic drug with antiproliferative and cytotoxic effects, immunomodulatory properties, and anti-angiogenic activity, demonstrated long-term disease stability up to three years in some case reports [25-27].
Conclusions
Skin EHE is an extremely rare sarcoma. To our knowledge this is the first report of the presentation with a breast metastasis. When possible, surgery is the best treatment, but it has to be performed in the context of a pathology diagnosis with the correct planning, and with full stratification.
In case of unresectable disease there is little to no prospective evidence so participation in clinical trials should be encouraged. With the current data available antiangiogenic therapy like Pazopanib is the first option, other options include cytotoxic therapy like Gemcitabine. Sirolimus, Interferon alfa and Lenalidomide have little evidence.
Author contributions
Conceptualization, M.C and DDB..; methodology, All the authors; formal analysis, M.C., DDB ; investigation, all the authors.; resources, all the authors; data curation, all the authors.; writing-original draft preparation, M.C.; writing-review and editing, all the authors; supervision, DDB.
Funding
Not applicable
Institutional review board statement
The study was conducted according to the guidelines of the Declaration of Helsinki.
Informed consent statement
Informed consent was obtained from the patient reported here.
Conflicts of interest
No conflict of interest is reported.
References
- Rubin B, Deyrup A, Doyle L. Soft Tissue and Bone Tumours WHO Classification of Tumours (5th ed). Tumours WCo, editor: World Health Organization (2020).
- Snyder M, Lyle S, Dinh T, et al. Primary cutaneous epithelioid hemangioendothelioma with lymph node metastasis. JAAD Case Reports 6 (2020): 1125-1128.
- Costigan D, Doyle L. Advances in the clinicopathological and molecular classification of cutaneous mesenchymal neoplasms. Histopathology 68 (2016): 776-795.
- Sung-Min P, Hoon-Soo K, Hyun-Chang K, et al. Cutaneous epithelioid hemangioendothelioma treated with Mohs micrographic surgery. International Journal of Dermatology 56 (2017): 97-99.
- García-Arpaa M, Rodríguez-Vázqueza M, Cortinaa P, et al. Hemangioendotelioma epitelioide cutáneo. Actas Dermosifiliogr 96 (2005): 386-391.
- Yousaf N, Maruzzo M, Judson I, et al. Systemic Treatment Options for Epithelioid Haemangioendothelioma: The Royal Marsden Hospital Experience. Anticancer Research 35 (2015): 473-480.
- Kumar V, Kachhawa D, Rekha S, et al. Cutaneous epithelioid hemangioendothelioma: A rare presentation. Indian Journal of Dermatology. Venereology and Leprology 84 (2018): 739-742.
- Kanik A, Hwan C, J Bhawan. Disseminated cutaneous epithelioid hemangioma. Journalof the American Academyof Dermatology 35 (1996): 851-853.
- Requena L, D?´az JL, Manzarbeitia F, et al. Cutaneous composite hemangioendothelioma with satellitosis and lymph node metastases. Journal of Cutaneous Pathology 35 (2008): 225-230.
- Senaratne R, Casey M, Kelly J. Malignant epithelioid hemangioendothelioma of the ear. Journal of Surgical Case Reports 3 (2022): 1-3.
- Fenniche S, Benmously R, Marrak H, et al. Hémangioendothéliome épithélioïde cutané. Ann Dermatol Venereol 131 (2004): 818-821.
- Polk P, Webb M. Isolated cutaneous epithelioid hemangioendothelioma. Journal of the American Academy of Dermatology 36 (1997): 1026-1028.
- Clarke L, Lee R, Militello G, et al. Cutaneous epithelioid hemangioendothelioma. Journal of Cutaneous Pathology 35 (2008): 236-240.
- Kollár A, Jones R, Stacchiotti S, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncologica 56 (2017): 88-92.
- Semenisty V, Naroditsky I, Keidar Z, et al. Pazopanib for metastatic pulmonary epithelioid hemangioendothelioma- A suitable treatment option: case report and review of anti-angiogenic treatment options. BMC Cancer 15 (2015): 402-407.
- Chevreau C, Cesne AL, Ray-Coquard I, et al. Sorafenib in Patients With Progressive Epithelioid Hemangioendothelioma A Phase 2 Study by the French Sarcoma Group (GSF/GETO). Cancer 5 (2013): 2639-2645.
- Agulnik M, Yarber JL, Okuno SH, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Annals of Oncology (2013): 257-263.
- Pranteda G, Magri F, Muscianese M, et al. The management of pseudomyogenic hemangioendothelioma of the foot: A case report and review of the literature. Dermatologic Therapy 11 (2018): e12725.
- Pinet C, Magnan A, Garbe L, et al. Aggressive form of pleural epithelioid haemangioendothelioma: complete response after chemotherapy. European Respiratory Journal 14 (1999): 237-238.
- Kanemura S, Kuribayashi K, Moriya Y, et al. Pemetrexed for epithelioid haemangioendothelioma of the pleura. Respirology Case Reports 4 (2016): e00191.
- Lakkis Z, Kim S, Delabrousse E, et al. Metronomic cyclophosphamide: An alternative treatment for hepatic epithelioid hemangioendothelioma. Journal of Hepatology 58 (2013): 1254-1257.
- Frezza A, Ravi V, Vullo SL, et al. Systemic therapies in advanced epithelioid haemangioendothelioma: A retrospective international case series from the World Sarcoma Network and a review of literature. Cancer Medicine 10 (2021): 2645-2659.
- Stacchiotti S, Provenzano S, Dagrada G, et al. Sirolimus in Advanced Epithelioid Hemangioendothelioma: A retrospective case-series analysis from the italian rare cancer network database. Annals Surgical Oncology 23 (2016): 2735-2744.
- Stacchiotti S, Simeone N, Vullo SL, et al. Activity of Sirolimus in Patients With Progressive Epithelioid Hemangioendothelioma: A Case-Series Analysis Within theItalian Rare Cancer Network. Cancer 19 (2021): 569-576.
- Pallotti M, Nannini M, Agostinelli C, et al. Long-term durable response to lenalidomide in a patient with hepatic epithelioid hemangioendothelioma. World Journal Gastroenterol 20 (2014): 7049-7054.
- Sumrall A, Fredericks R, Berthold A, et al. Lenalidomide stops progression of multifocal epithelioid hemangioendothelioma including intracranial disease. Journal of Neurooncology 97 (2010): 277-285.
- Hettmer S, Andrieux G, Hochrein J, et al. Epithelioid hemangioendotheliomas of the liver and lung in children and adolescents. Pediatr Blood Cancer 12 (2017): e26675.
