Coupling Between Atrial Function and Exercise Tolerance in a Patient with Heart Failure and Atrial Fibrillation Treated with Sacubitril-Valsartan and Combined Exercise
Article Information
Caminiti G1, Iellamo F1,2, D’Antoni V1, Catena M1, Morsella V1, Volterrani M1*
1Cardiology Rehabilitation Unit, S. Raffaele IRCCS, Rome, Italy
2Dipartimento di Scienze Cliniche e Medicina Traslazionale, Università Tor Vergata, Roma, Italy
*Corresponding Author: Ferdinando Iellamo, Dipartimento di Scienze Cliniche e Medicina Traslazionale, Università Tor Vergata, Via Montpellier 1, 00173 Roma, Italy
Received: 11 September 2021; Accepted: 22 September 2021; Published: 28 September 2021
Citation: Caminiti G, Iellamo F, D’Antoni V, Catena M, Morsella V, Volterrani M. Coupling Between Atrial Function and Exercise Tolerance in a Patient with Heart Failure and Atrial Fibrillation Treated with Sacubitril- Valsartan and Combined Exercise. Cardiology and Cardiovascular Medicine 4 (2021): 530-534.
View / Download Pdf Share at FacebookAbstract
A 50 years old male patient with heart failure with reduced ejection fraction and permanent atrial fibrillation, referred to our centre for a cardiac rehabilitation program. He had a very limited exercise tolerance and presented a very compromised atrial function at 2D spleckle tracking echocardiography. Both exercise tolerance and left atrial function improved greatly after a comprehensive intervention including sacubitrilvalsartan administration and exercise training. These benefits, partially, persisted 8 weeks after training cessation. This is the first demonstration of an improvement in left atrial function in a patient, with heart failure and atrial fibrillation, produced by a therapeutic intervention.
Keywords
Atrial function; Cardiac Rehabilitation Program; Echocardiography
Article Details
1. Introduction
The assessment of left atrial (LA) function, through two dimension speckle tracking echocardiography (2D STE), has received a growing interest as a prognostic index in patients with cardiovascular disease [1]. In particular, in patients with heart failure (HF), a reduced peak atrial longitudinal strain (PALS) is considered a predictor of poor exercise tolerance and of increased rate of adverse clinical events [2,3]. In addition, in patients with atrial fibrillation (AF) LA strain and strain rate are lower compared to those of subjects in sinus rhythm, and have been related with the extent of myocardial remodelling and atrium fibrosis [4]. Finally, PALS has also been associated with AF burden, sinus rhythm maintenance, response rate after catheter ablation and risk of ischemic stroke [5,6]. In light of these findings, reversing LA dysfunction, in particular increasing PALS, appears to be a potential therapeutic target in both HF and AF. However, until now there are no prospective studies investigating the impact of a therapeutic intervention on PALS in subjects with concomitant HF and AF. Here, we present the case of a patient with both HF and permanent AF in whom a comprehensive pharmacological and non-pharmacological treatment determined changes in exercise tolerance and clinical conditions that were associated with LA function as assessed by 2D STE.
2. Case Report
We report the case of a 50 years old male patient who was screened on September 2020, for participating in a cardiac rehabilitation program. His medical record was as follows: heart failure with reduced ejection fraction (HFrEF) secondary to non-ischemic cardiomyopathy; hypertension, permanent AF, type II diabetes and obesity. At the first visit, therapy included: bisoprolol 5 mg/day, ramipril 5 mg/day, furosemide 50 mg/day, rosuvastatin 10 mg/day, ezetimibe 10 mg/day, metformin 1 gr/day, and apixaban 10 mg/day. Heart rate (HR) was 81 bpm, blood pressure 145/90 mmHg, body mass index (BMI) 46.8 kg/m2. He presented severe exercise intolerance due mainly to dyspnea (NYHA class III). A blood sample showed high NT-proBNP (2089 pg/ml); normal renal function (eGFR= 78 ml/min) and emoglobin levels (14.1 g/dl). Echocardiographic examination showed: left ventricular (LV) and LA enlargement, low ejection fraction (41%), and low LV global longitudinal strain (GLS) -11%; impaired LA function (PALS 7.2%); mild mitral and tricuspid regurgitations; preserved right ventricular function (TAPSE= 21 mm). An ergometric test was prematurely interrupted for exhaustion at low exercise level (METs=2.9). We decided to stop ramipril and to start sacubitril/valsartan 100 mg twice/day two days later. Two weeks later the dose of sacubitril/valsartan was up-tritated to 200 mg twice/day. One week after the first visit the patient started an exercise-based cardiac rehabilitation program that consisted in three sessions/week for 12 weeks of within-session combined aerobic plus resistance exercises. At each exercise session, the patient walked for 25 minutes on a treadmill at 55–70% of peak HR, then he performed resistance exercises with arms and legs, consisting in 2 sets of 10 repetitions at 60% of 1 repetition maximum (1 RM), with 2 min rest between sets. Resistance training consisted in the following exercises: leg press and extension, shoulder press, chest press, low row and vertical traction (Technogym Wellness System, Technogym, Cesena, Italy).
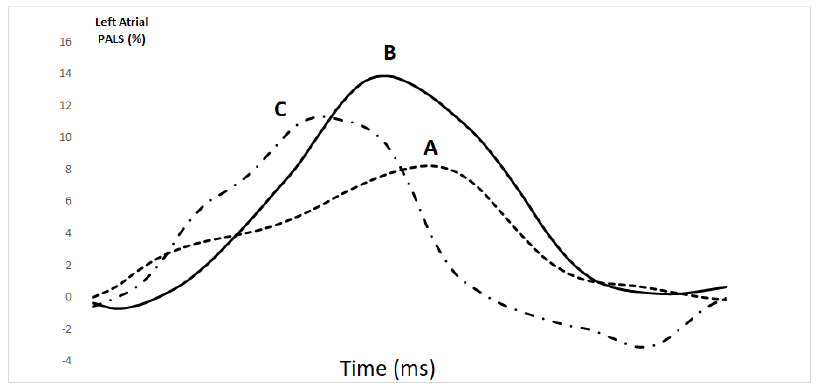
At the end of 12th week, the patient underwent a second evaluation. He was still in NYHA class II, HR was 78 bpm, BP 130/80 mmHg and BMI was 46.1 kg/m2. Compared to the first visit, LVEF and LV GLS were mildly changed to 47% and -14%, respectively. Diastolic LV volume was decreased (from144.4 to 128.9 ml) while LA volume remained unchanged (from 121.2 to 121.5 ml). PALS increased to 13.8% (Figure 1). Exercise tolerance at the ergometric test increased to 6.0 METs.
Eight weeks after the end of exercise training, the patient underwent a third visit. During the period following exercise training, the patient was not engaged in any structured physical activity program, while continued his pharmacological therapy. He remained in NYHA class II. HR was 74 bpm, BP 120/70 mmHg. At echocardiography evaluation, LA and LV volumes were 123.6 and 135.8 ml, respectively. PALS was 11.6%, GLS -14.1% and LVEF 47%, respectively. Exercise tolerance decreased to 4.8 METs.
3. Discussion
In this case report we observed that our comprehensive intervention, that included the administration of sacubitril-valsartan combined with a 12-weeks exercise training program, led to a 75% increase of LA PALS in a patients with HF and permanent AF. To our knowledge, this is the first prospective demonstration of an improvement in LA PALS in a patients with both HF and AF produced by a therapeutic intervention. Recent retrospective data suggests that the administration of sacubitril/valsartan may improve LA PALS in HF subjects in sinus rhythm compared to ACE inhibitors or ARBS therapy [7]. Conversely, no data are available about changes in LA PALS in patients with HF and concomitant AF. Interestingly, the increase of LA PALS that we observed in this patient at the end of our pharmacological and exercise intervention, was paralleled by a substantial improvement of symptoms, exercise tolerance and NT-proBNP levels. In contrast, changes in the other echocardiography parameters were only slightly improved or unchanged. The assessment in this patient of LA function through 2D STE prompted us to speculate on the potential mechanism(s) brought about by our intervention: we could reasonably hypothesize that the increase in LA PALS, by reflecting an increased atrium distensibility during the atrial reservoir phase, determined an increase in LV filling and, in turn, promoted an improvement of LV systolic performance. This central mechanism could concur to the improvement in exercise tolerance observed in such a complex patient. The improvement in exercise tolerance cannot be attributed to changes of HR because HR values were only slightly reduced during the follow-up period and they were not coupled with changes in exercise tolerance at the third visit, when both HR and METs decreased. Given the concomitant beginning of sacubitril-valsartan and of exercise training it is difficult to establish the relative contribution of each intervention to the improvement of PALS in our patient. It is worthy of note however, that on third visit, performed 8 weeks after the end of the exercise training program, while the patient was continuing to take sacubitril-valsartan, PALS was still decreased compared to that observed at the second visit, nevertheless it remained 30% higher in comparison to the first visit. According to these findings, we can hypothesize that it was the administration of sacubitril-valsartan that determined the PALS increase in our patient and that its effects were amplified by the exercise training; this hypothesis should be verified in properly designed studies. Our result encourage further prospective studies aimed to investigate changes in atrial function. Moreover further studies are needed in order to verify the usefulness of PALS for monitoring the effects of pharmacological and non-pharmacological interventions in patients with HF and permanent AF.
In conclusion we observed that in a patient with HFrEF and permanent AF, exercise tolerance and NT-proBNP levels improved after a combined pharmacological (specifically, sacubitril-valsartan) and non-pharmacological (exercise) intervention, with the improvement being associated with an increase in LA reservoir phase assessed by PALS.
Conflict of Interest
Authors have no conflict of interest to notify.
References
- Bolog MI, Dumitrescu M, Pacuraru E, et al. Prognostic utility of routine assessment of left ventricular global longitudinal strain and peak atrial reservoir strain in stable heart disease patients. European Heart Journal 41 (2020): 7-11.
- Hasselberg NE, Haugaa KH, Sarvari SI, et al. Left ventricular global longitudinal strain is associated with exercise capacity in failing hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging 16 (2015): 217-224.
- Santos AB, Roca GQ, Claggett B, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail 21 (2016): 9-15.
- Kuppahally SS, Akoum N, Burgon NS, et al. Left atrial strain and strain rate in patients with paroxysmal and persistent atrial fibrillation: relationship to left atrial structural remodeling detected by delayed-enhancement MRI. Circ Cardiovasc Imaging 3 (2010): 231-239.
- Yoon YE, Oh IY, Kim SA, et al. Echocardiographic predictors of progression to persistent or permanent atrial fibrillation in patients with paroxysmal atrial fibrillation (E6P Study). J Am Soc Echocardiogr 28 (2015): 709-717.
- Dell'Era G, Rondano E, Franchi E, et al. Novara Atrial Fibrillation (NAIF) Study group. Atrial asynchrony and function before and after electrical cardioversion for persistent atrial fibrillation. Eur J Echocardiogr 11 (2010): 577-583.
- De Vecchis R, Paccone A, Di Maio M. Favorable effects of Sacubitril/Valsartan on the peak atrial longitudinal strain in patients with chronic heart failure and a history of one or more episodes of Atrial Fibrillation: A retrospective cohort study. J Clin Med Res 12 (2020): 100-107.