Cost-effectiveness of Colorectal Screening in a European Country. A Comparison of Five Alternative Screening Strategies
Article Information
Luís Lopes1,2,3*#, Manuela Certo4#, Paula Veiga5, Jorge Canena6,7,8
1Department of Gastroenterology, Hospital de Santa Luzia - Unidade Local de Saúde Alto Minho, Viana do Castelo, Portugal
2Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal
3ICVS/3B’s - PT Government Associate Laboratory, Braga/Guimarães, Portugal
4Department of Radiology, Hospital de Braga, Braga, Portugal
5School of Economics and Management, University of Minho, Braga, Portugal
6University Center of Gastroenterology, Hospital Cuf Tejo, Lisbon, Portugal
7Department of Gastroenterology, Professor Doutor Fernando Fonseca Hospital, Amadora, Portugal
8Department of Gastroenterology, Universidade Nova de Lisboa/Faculty of Medical Sciences, Lisbon, Portugal
#Luis Lopes and Manuela Certo contributed equally to this study and should be considered jointly first authors.
*Corresponding Author: Luís Lopes, Department of Gastroenterology, Hospital Santa Luzia, ULS Alto Minho, Estrada de Luzia, 504900 Viana do Castelo, Portugal
Received: 06 August 2022; Accepted: 08 August 2022; Published: 27 September 2022
Supplementary File
Citation: Luís Lopes, Manuela Certo, Paula Veiga, Jorge Canena. Cost-effectiveness of Colorectal Screening in a European Country. A Comparison of Five Alternative Screening Strategies. Journal of Surgery and Research 5 (2022): 529-540
View / Download Pdf Share at FacebookAbstract
Background: The implementation of an organized screening strategy should include a cost-effectiveness analysis for the governments to take decisions that promote health and better allocate resources which does not happen most of the times. This study aimed to evaluate the most costeffective strategy for CRC screening in a European Country.
Methods: A cost-effectiveness (CE) probabilistic Markov model was developed to compare the costs and the quality-adjusted life expectancy of 50-year-old average-risk individuals submitted to five alternative screening strategies based on colonoscopy, computed tomography (CT) and FIT, as well as no screening. We calculated the costs from the perspective of a third payer (Portuguese National Health Service) and populated the model with data from published literature. Probability of being cost-effective was estimated for different thresholds of willingness-to-pay.
Results: Colonoscopy 3/10 years is the most cost-effective strategy for colorectal screening in Portugal, with an estimated ICER of 802 €/ QALY when compared with colonoscopy every 10 years. The FIT and CT colonography based strategies are dominated by colonoscopy-based strategies. Biennial FIT, the strategy currently being used in Portugal, showed the smallest gains in life years gained (498.3 days) the smallest reduction in the incidence of CRC (-37%) and the smallest reduction in CRC mortality (-57%) between all the screening strategies. The findings were robust to probabilistic sensitivity analysis.
Conclusions: Colonoscopy based strategies offer the best value for the money in Portugal. Biennial FIT, the screening strategy in Portugal should be replaced by a colonoscopy-based strategy.
Keywords
Cost-effectiveness, Colorectal cancer, Screening, Cost-utility
Cost-effectiveness articles; Colorectal cancer articles; Screening articles; Cost-utility articles
Cost-effectiveness articles Cost-effectiveness Research articles Cost-effectiveness review articles Cost-effectiveness PubMed articles Cost-effectiveness PubMed Central articles Cost-effectiveness 2023 articles Cost-effectiveness 2024 articles Cost-effectiveness Scopus articles Cost-effectiveness impact factor journals Cost-effectiveness Scopus journals Cost-effectiveness PubMed journals Cost-effectiveness medical journals Cost-effectiveness free journals Cost-effectiveness best journals Cost-effectiveness top journals Cost-effectiveness free medical journals Cost-effectiveness famous journals Cost-effectiveness Google Scholar indexed journals Colorectal cancer articles Colorectal cancer Research articles Colorectal cancer review articles Colorectal cancer PubMed articles Colorectal cancer PubMed Central articles Colorectal cancer 2023 articles Colorectal cancer 2024 articles Colorectal cancer Scopus articles Colorectal cancer impact factor journals Colorectal cancer Scopus journals Colorectal cancer PubMed journals Colorectal cancer medical journals Colorectal cancer free journals Colorectal cancer best journals Colorectal cancer top journals Colorectal cancer free medical journals Colorectal cancer famous journals Colorectal cancer Google Scholar indexed journals mortality articles mortality Research articles mortality review articles mortality PubMed articles mortality PubMed Central articles mortality 2023 articles mortality 2024 articles mortality Scopus articles mortality impact factor journals mortality Scopus journals mortality PubMed journals mortality medical journals mortality free journals mortality best journals mortality top journals mortality free medical journals mortality famous journals mortality Google Scholar indexed journals health technologies articles health technologies Research articles health technologies review articles health technologies PubMed articles health technologies PubMed Central articles health technologies 2023 articles health technologies 2024 articles health technologies Scopus articles health technologies impact factor journals health technologies Scopus journals health technologies PubMed journals health technologies medical journals health technologies free journals health technologies best journals health technologies top journals health technologies free medical journals health technologies famous journals health technologies Google Scholar indexed journals National Institute for Health and Care Excellence articles National Institute for Health and Care Excellence Research articles National Institute for Health and Care Excellence review articles National Institute for Health and Care Excellence PubMed articles National Institute for Health and Care Excellence PubMed Central articles National Institute for Health and Care Excellence 2023 articles National Institute for Health and Care Excellence 2024 articles National Institute for Health and Care Excellence Scopus articles National Institute for Health and Care Excellence impact factor journals National Institute for Health and Care Excellence Scopus journals National Institute for Health and Care Excellence PubMed journals National Institute for Health and Care Excellence medical journals National Institute for Health and Care Excellence free journals National Institute for Health and Care Excellence best journals National Institute for Health and Care Excellence top journals National Institute for Health and Care Excellence free medical journals National Institute for Health and Care Excellence famous journals National Institute for Health and Care Excellence Google Scholar indexed journals low-risk adenomas articles low-risk adenomas Research articles low-risk adenomas review articles low-risk adenomas PubMed articles low-risk adenomas PubMed Central articles low-risk adenomas 2023 articles low-risk adenomas 2024 articles low-risk adenomas Scopus articles low-risk adenomas impact factor journals low-risk adenomas Scopus journals low-risk adenomas PubMed journals low-risk adenomas medical journals low-risk adenomas free journals low-risk adenomas best journals low-risk adenomas top journals low-risk adenomas free medical journals low-risk adenomas famous journals low-risk adenomas Google Scholar indexed journals preclinical stage CRC IV articles preclinical stage CRC IV Research articles preclinical stage CRC IV review articles preclinical stage CRC IV PubMed articles preclinical stage CRC IV PubMed Central articles preclinical stage CRC IV 2023 articles preclinical stage CRC IV 2024 articles preclinical stage CRC IV Scopus articles preclinical stage CRC IV impact factor journals preclinical stage CRC IV Scopus journals preclinical stage CRC IV PubMed journals preclinical stage CRC IV medical journals preclinical stage CRC IV free journals preclinical stage CRC IV best journals preclinical stage CRC IV top journals preclinical stage CRC IV free medical journals preclinical stage CRC IV famous journals preclinical stage CRC IV Google Scholar indexed journals adenoma polypectomy articles adenoma polypectomy Research articles adenoma polypectomy review articles adenoma polypectomy PubMed articles adenoma polypectomy PubMed Central articles adenoma polypectomy 2023 articles adenoma polypectomy 2024 articles adenoma polypectomy Scopus articles adenoma polypectomy impact factor journals adenoma polypectomy Scopus journals adenoma polypectomy PubMed journals adenoma polypectomy medical journals adenoma polypectomy free journals adenoma polypectomy best journals adenoma polypectomy top journals adenoma polypectomy free medical journals adenoma polypectomy famous journals adenoma polypectomy Google Scholar indexed journals
Article Details
Background
Colorectal cancer [CRC] is a major cause of cancer-associated morbidity and mortality worldwide and the 3rd leading cause of cancer-related death in Portugal [1]. Several screening methods are available: fecal occult-blood test (FOBT), fecal immunochemical test (FIT), flexible sigmoidoscopy, total colonoscopy, CT-colonography and stool DNA testing. CRC screening is endorsed by most health agencies, but recommendations are diverse [2]. Although the various screening methods have different levels of accuracy (colonoscopy is considered the gold standard by most), the consensus has long been that one test is better than no test at all [3]. Cost-effectiveness (CE) data for CRC screening in Europe are scarce, with diverging results, generating uncertainty about the best screening strategy and its own effectiveness. The decision to adopting a screening program in a country is complex, and must take into account several dimensions, such as, evidence of cost-effectiveness, feasibility, stakeholder support, and being part of a country’s public health priorities. An important step in the decision to start a screening program is to carry out a cost-effectiveness analysis (CEA) across different alternatives, using country-specific inputs. In several, the policies are implemented, without a clear strategical blueprint, as a reactive response to contingent political/public pressures, without a prior evaluation that appraised the impact of the policies under different scenarios. In Portugal (PT), an organized screening program, funded by the National Health Service (NHS) using FIT every 2-years was implemented in 2017 [4], without any CEA performed prior to its implementation. Give this lack of evidence sustaining the choice of screening strategy vis-à-vis other alternative strategies, we aim to estimate the most cost-effective strategy for CRC screening in Portugal.
Methods
Model structure overview
A Markov model was developed to simulate the natural history of colorectal cancer and to estimate the cost-effectiveness of five strategies for CRC screening in Portugal, as well as no screening, in 50-year-old individuals at average risk for colorectal cancer (Figure 1, S1). The CRC model includes a screening component and a natural history component. The screening component incorporates assumptions relevant to the screening programme, such as adherence, tests performance, screening intervals, type of tests used, while the natural history component incorporates assumptions about the progression from adenoma to CRC. The Markov model contained 23 mutually exclusive and exhaustive health states and the five screening strategies were superimposed on the natural history of the model. Screening began at age 50 years and stopped at age 75 years, and persons were followed until death or 100 years of age. If a screening test was positive, colonoscopy was offered, and biopsy or polypectomy were performed according to the findings. All colonoscopies were performed with anaesthesia assistance. The models run through a maximum of 50 cycles 1-year-cycles. The costs and outcomes are accrued within each cycle and accumulate throughout the life cycle of the model. At the end of each cycle, the members of the cohort return to the same health state and/or to other(s) health states, according with predetermined transition probabilities. Additional costs, outcomes and count progressions associated with relevant events occurring in the full set of pathways for each state during a cycle (‘transition subtrees’), are also accrued. The model uses a payer perspective (Portuguese NHS as the primary payer). All costs and outcomes were discounted at 3,0% to adjust for time preference. Half-cycle correction was used. Though there is no official maximum acceptable incremental cost-effectiveness ratio (ICER) in Portugal for health technologies evaluations, we assumed a threshold of 30 000€ per quality-adjusted life years (QALY), as recommended by The National Institute for Health and Care Excellence (NICE) [5]. The simulation was performed using TreeAge Pro HealthCare 2021, R2 (TreeAge Software Inc., Williamstown, MA, USA).
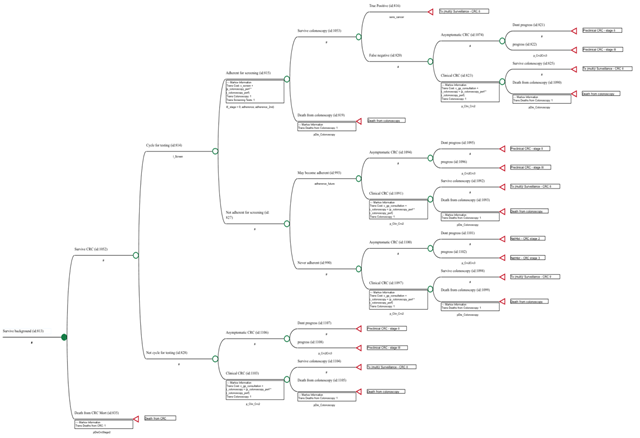
Figure 1: Pathway included in the colonoscopy 10/10y Markov node strategy
Screening strategies
We modelled five screening strategies: (1) FIT every 2 years (FIT 2/2y strategy), which is the strategy chosen in Portugal by the NHS [25]; 2) colonoscopy every 10 years (colonoscopy 10/10y strategy); 3) colonoscopy, with the second screening colonoscopy at a 3-year interval and thereafter every 10 years (colonoscopy 3/10y strategy); 4) CT-colonography every 5 years (CT 5/5y strategy); 5) FIT every year (FIT 1/1y strategy) (Table S1 - screening strategies). Under our model, all colonoscopies are performed with anaesthesia assisted sedation administered by an anaesthesiologist, as it is common practice in Portugal since 2014.
Model population
An arbitrary cohort of average-risk persons for CRC at the age of 50 entered the model and was distributed at 7 health states at model initiation. The initiation states were defined according to the prevalence of adenomas and CRC in the population: (1) normal mucosa, (2) low-risk adenomas, (3) advanced adenomas (defined as either >= 1cm, villous histology or high-grade dysplasia), (3) preclinical CRC stage I, (5) preclinical CRC stage II, (6) preclinical CRC stage III and (7) preclinical stage CRC IV [6]. The cohort population transitioned between 23 health states, in annual cycles, based on transition probabilities estimated from the literature. The model run until death or by 50 cycles (Table S2 - distribution of patients across health states).
Health states
The model aims to replicate the natural history of CRC and CRC screening using the following health states: (1) Normal mucosa; (2) Low-risk adenomas; (3) Advanced adenomas (defined as either >= 1cms, villous histology or high-grade dysplasia); (4) Preclinical CRC stage I; (5) Preclinical CRC stage II; (6) Preclinical CRC stage III; (7) Preclinical stage CRC IV; (8) Treatment/Surveillance CRC stage I; (9) Treatment/Surveillance CRC stage II; (10) Treatment/Surveillance CRC stage III; (11) Treatment/Surveillance CRC stage IV; (12) Natural history no polyp; (13) Natural history low-risk adenomas; (14) Natural history advanced adenoma; (15) Natural history preclinical CRC stage I; (16) Natural history CRC stage II; (17) Natural history CRC stage III; (18) Natural history CRC stage IV; (19) Surveillance after low-risk adenoma polypectomy; (20) Surveillance after advanced adenoma polypectomy; (21) Death from background mortality; (22) Death from CRC and (23) Death from colonoscopy.
Transition probabilities
The probabilities of transition between health states, the compliance rate, the age- and stage specific incidence and mortality rates of CRC, and other input parameters were based on a systematic literature review (Table S3- annual transition probabilities). The probability of background death was obtained from Portuguese official life tables [7]. The probabilities of CRC-related death were stage specific, and it was assumed that after six years of survival the probability of death was fixed (Table S4 - probability of death).
Test performance and compliance
The performance and the compliance rate with the screening tests were based on a systematic literature review (Table 1). In the base case, the adherence for initial screening varied according to the test (Table 2). For the colonoscopy assisted with anaesthesia, since there is no available data with which to estimate the initial adherence in an organized screening program, we assumed an initial adherence of 60%. At each screening interval (after the initial) we assumed that a random 73% underwent screening, independent of whether they were compliant with past tests. We assumed that 20% of the persons were never adherent to screening (drop out). This sub- cohort of patients transited to the natural history health states of the model immediately after the first screening round. For the follow-up colonoscopy after a positive screening test, we assumed a compliance rate of 80%. The same compliance rate was assumed for the surveillance colonoscopy after polyp removal or CRC diagnosis. Among patients who had symptomatic presentation of CRC, it was assumed that all patients undergo colonoscopy with biopsy.
|
Variable |
Base case (range) |
SD |
Source |
|
Fecal immunochemical test: |
|||
|
Sensitivity to detect CRC |
0.73 (0.603-0.839) |
0.035 |
[23] |
|
Sensitivity to detect advanced carcinoma |
0.238 (0.208-0.270) |
0.02 |
[23] |
|
Sensitivity to detect low risk adenoma |
0.076 (0.0607-0.086) |
0.01 |
[23] |
|
Specificity |
0.964 (0.958-0.969) |
0.01 |
[23] |
|
Colonoscopy assisted with anaesthesia: |
|||
|
Sensitivity to detect CRC |
0.95 (0,900-0,970) |
0.02 |
[17] |
|
Sensitivity to detect advanced adenoma |
0.90 (0.900-0.970) |
0.015 |
[17] |
|
Sensitivity to detect low risk adenoma |
0.85 (0.850-0.950) |
0.015 |
[17] |
|
Specificity |
1 |
[17] |
|
|
CT-Colonography: |
|||
|
Sensitivity to detect CRC |
0.84 (0.756-0.924) |
0.03 |
[24] |
|
Sensitivity to detect |
0.84 (0.756-0.924) |
0.03 |
[24] |
|
Sensitivity to detect low-risk adenoma |
0.57 (0.489-0.716) |
0.03 |
[24] |
|
Specificity |
0.88 (0.777-0.883) |
0.02 |
[25] |
Table 1: Screening test performance
|
Variable |
Base case (range) |
SD |
Source |
|
Adherence with initial screening: |
|||
|
Fecal immunochemical test |
0.42 (0.34-0.51) |
0.08 |
[13] |
|
Colonoscopy |
0.60 (0.3-0.7) |
0.1 |
[13,26] |
|
CT-Colonography |
0.22 (0.15-0.32) |
0.22 |
|
|
Probability of undergoing each screening round after initial screening |
0.73 (0.5-0.9) |
0.03 |
[13] |
|
Follow-up colonoscopy for a positive screening test |
0.80 (0.50-0.90) |
0.02 |
[26,27] |
|
Never adherent to a screening test |
0.20 (0.10-0.50) |
0.2 |
Assumption |
|
Follow-up colonoscopy after polyp removal |
0.80 (0.60-0.90) |
0.2 |
[26,27] |
Table 2: Adherence to screening (Real-World Scenario)
Costs inputs
The costs were calculated after measuring the quantities of resources used, using the appropriate physical units (Table S5- Medical Costs). Only direct medical costs are included. Costs came derived from two national reference costs datasets and the contractual framework for hospital care produced by the Portuguese Ministry of Health [8-10]. Costs for the initial two years of CRC treatment (stages II to stage IV) use the amount that hospitals receive under the prospective payment pilot program for CRC treatment. For stage I CRC, we assumed a different reimbursement, as no treatment, other than surgery is performed (e.g., chemotherapy or radiotherapy). In stage 1, hospitals were reimbursed in the first year, for the surgery performed, according to the cost of the appropriate Portuguese classification for Diagnosis-Related Group (DRG) (code 221 named in portuguese, “procedimentos major do intestino grosso”, severity 2) plus the price of the consultations and tests required for surveillance. The cost of a a death related with CRC was assumed to be reimbursed under DRG code 240, severity 4. We assumed that there is no co-payments supported by patients, since in Portugal, all consultations and tests requested by primary care physicians are free of charge for patients in point-of-care.
Utility inputs
We assigned utilities to the health states used in the model, to calculate quality adjusted life-years (QALYs). We assigned a utility of 1 for patients without a diagnosis of cancer [11]. When CRC patient became symptomatic, he would be promptly diagnosed, and a different utility would be assigned according to the CRC stage. (Table S6 - Utilities).
Modelled scenarios
Two scenarios were considered to evaluate the impact of adherence on screening: (i) Scenario 1- Real World Scenario: where real-world adherence rates are assumed for all screening tests (initial and uptake) and follow-up colonoscopies; (ii) Scenario 2- Perfect Adherence Scenario: where 100% adherence rates are assumed for all screening tests (initial and uptake) and follow-up colonoscopies (Table S7 – Scenarios).
Outcomes
The following clinical outcomes were estimated: QALY gained, life years gain (LYG), number of CRC cases, number of CRC deaths, CRC incidence reduction, CRC mortality reduction, number of screening tests, number of colonoscopies for follow-up/ surveillance and costs of screening strategy. The outcomes will be presented for the two modelled scenarios. Costs and health benefits are combined to estimate the ICER between different screening strategies. The numerators were the differences in costs for each strategy in relation to the previous strategy (ranked in order of costs), and the denominators were the differences effectiveness. CE ranking tables will be presented, as well as CE planes. In this diagrams differences in effect between alternative screening strategies are plotted along the horizontal axis, and the difference in cost on the vertical axis. In addition, clinical outcomes were presented as totals over 1000 patients over the model lifetime.
Sensitivity analysis
Uncertainty was evaluated using deterministic and probabilistic sensitivity analysis (PSA) to assess the robustness of the conclusions to changes in critical input parameters in the base case scenario (scenario 1). Results for the deterministic sensitivity analysis are presented in a Tornado chart displaying the results of those analysis (using the intervals defined in the input tables) with largest impact in the model output (results presented as incremental net monetary benefits) between the pair of strategies: Colo 3/10y vs FIT 1/1y. The individual parameters were varied across a plausible range and threshold values (at which two strategies have equal expected values) were identified if occurred. For the PSA analysis, we performed a probabilistic Monte Carlo simulation recalculating the model with 10,000 trials in which multiple parameters (probabilities and utilities) sampled from beta distributions (representing uncertainty around the base case estimate; using the base case as the mean and standard deviations (SD) described in tables) are varied simultaneously, with the resulting cost and effect pairs being recorded. Results were presented using acceptability curves, acceptability at willingness-to-pay threshold (WTP). An incremental cost-effectiveness scatterplot between the most CE strategy and FIT2/2y will be presented. The perfect adherence scenario, compared to the real-world scenario (base case), will give information to the decision makers, about the impact of adherence on the outcomes of the model. In case the best strategy in this scenario of perfect adherence differs from the baseline scenario, it may be a strong argument for the decision-makers to support policies to increase patient adherence to this strategy.
Results
Scenario 1- Real world scenario
In the real-world scenario, the most expensive strategy was no screening costing 6,683€ per person. The cheapest strategy was colonoscopy 10/10y which costs 974.25€ per person. The most effective strategy was colonoscopy 3/10y providing 20.55 QALYs and the least effective strategy, was no screening providing 20.22 QALYs. Colonoscopy 3/10y is the most cost-effective strategy with an ICER of 802.07€ per QALY (clearly below the WTP of 30 000€/ QALY), when compared with colonoscopy 10/10y. All the other strategies (all stool-based and CT screening strategies) are absolutely dominated by the colonoscopy screening strategies. FIT 1/1y, the strategy immediately above colonoscopy 3/10y in the CE ranking table, is absolutely dominated by colonoscopy 3/10y, as it is more costly (incremental cost of € 1,395.28€) and less effective (incremental effectiveness of -0.003 QALYs) (Table 3). In the cost-effectiveness plane we observe the cost-effectiveness frontier displayed as a line that connects colonoscopy 10/10y and colonoscopy 3/10y (Figure 2). FIT 2/2 y, the CRC screening strategy used in Portugal, is the second with highest cost (3,533.33€), and the second least effective with 20.46 QALYs only surpassed by no screening. The total number of LYG gained per 1000 persons was highest for FIT 1/1y (661.3 days) (Table 4). Colonoscopy 3/10y resulted in the highest CRC incidence reduction (-64%). As expected, the total number of colonoscopies (screening and surveillance) were higher for colonoscopy 3/10 y strategy (n=2,503). FIT 2/2 y showed the smallest gains in LYG (498.3 days) of all strategies, as well as the smallest reduction in the incidence of CRC (-37%) and the smallest reduction in mortality (-57%).
|
Dominance |
Strategy |
Cost(€) |
Incr Cost |
Eff(QALYs) |
Incr Eff |
ICER |
NMB |
|
undominated |
Colo 10/10y |
974.257 |
20.484 |
613,555.843 |
|||
|
undominated |
Colo 3/10y |
1,031.18 |
56.917 |
20.555 |
0.071 |
802.07 |
615,627.823 |
|
abs. dominated |
FIT 1/1y |
2,426.46 |
1395.282 |
20.552 |
-0.003 |
dominated |
614,138.406 |
|
abs. dominated |
CT 5/5y |
3,450.67 |
2,419.49 |
20.496 |
-0.059 |
dominated |
611,431.756 |
|
abs. dominated |
FIT 2/2y |
3,533.34 |
2,502.16 |
20.459 |
-0.096 |
dominated |
610,242.881 |
|
abs. dominated |
No screening |
6,683.81 |
5,652.63 |
20.218 |
-0.338 |
dominated |
599,849.913 |
Abreviations: Incr, incremental; Eff, effectiveness; NMB, net monetary benefit
Table 3: Cost-effectiveness Report - Real-World Scenario
Table 4: Clinical outcomes per 1000 patients - Real-World Scenario
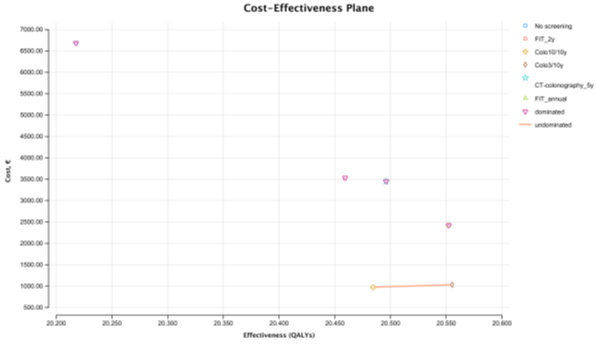
Figure 2: Cost-effectiveness plane - Real World Scenario
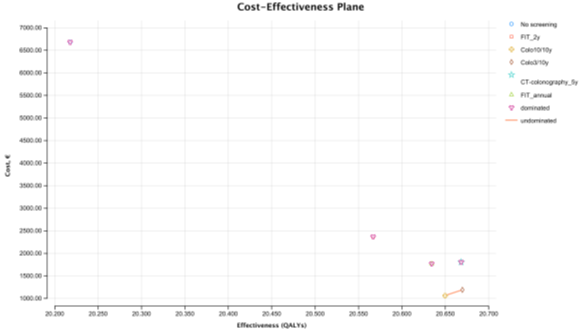
Figure 3: Cost-effectiveness plane - Perfect Adherence Scenario
Scenario 2- Perfect adherence scenario
In the perfect adherence scenario, assuming 100% adherence for screening and follow-up colonoscopies (when indicated), colonoscopy 3/10y is the most cost-effective strategy with an ICER of 6,361.08€ per QALY (at WTP of 30 000€/ QALY), compared with colonoscopy 10/10y (Table S8- Cost-effectiveness Report-Perfect Adherence Scenario). All other strategies are absolutely dominated by the colonoscopy screening strategies (Figure 3). FIT 1/1y, the strategy immediately below colonoscopy 3/10y in the CE ranking table, is absolutely dominated by colonoscopy 3/10y. FIT 2/2 y, the strategy used in Portugal, is the second with highest cost (2,369.17€), and the second least effective with 20.567 QALYs only surpassed by no screening. The most expensive strategy was no screening costing 6,683.8€ per person. The cheapest strategy was colonoscopy 10/10y which costs € 1063 per person. The most effective strategy was colonoscopy providing 20.67 QALYs and the least effective strategy, was no screening providing 20.22 QALYs. The total number of LYG gained per 1000 persons was highest for colonoscopy 3/10y (812.2 days) (Table S9- Clinical outcomes per 1000 patients – Perfect Adherence Scenario]. Colonoscopy 3/10y resulted in the highest CRC incidence reduction (-80%) and greatest CRC mortality reduction (-91%). The total number of colonoscopies (screening and surveillance) were higher for colonoscopy 3/10 y strategy (n= 3,566). FIT 2/2 y showed the smallest gains in LYG (687.5 days) of all strategies, as well as the smallest reduction in the incidence of CRC (-55%) and the smallest reduction in mortality (-76%). The no screening strategy had the highest cost (6,683,805.71€), with colonoscopy 10/10y being the least expensive (1,063,366.85€), followed by colonoscopy 3/10y (1,190,483.53€).
Sensitivity analysis
Univariate analysis
The impact of adherence and cost parameters on the cost-effectiveness of colonoscopy 3/10 strategy versus FIT 1/1y strategy (Figure 4). We evaluated adherence to first screening test, uptake tests, dropout from screening and adherence to follow-up colonoscopy when FIT was positive. Cost of the CRC treatment and cost of the screening colonoscopy were also evaluated. Alteration of adherence to baseline colonoscopy had the greatest impact on the base-case ICER, being the only input that changes the most cost-effective strategy, but only when the rate of adherence is below 39,8%. In that case FIT 1/1y becomes the most cost-effective strategy. The results were sensitive to variation of the other parameters (over the defined parameter’s uncertainty range) supporting the evidence that colonoscopy 3/10y is the most cost-effective strategy. Interestingly, even with colonoscopy costing 2,000€ (the upper limit of the range used in this sensitivity analysis), colonoscopy 3/10y is still the most cost-effective strategy.

Figure 4: Tornado diagram: colonoscopy 3/10y versus FIT 1/1y
Probabilistic sensitivity analysis
In the probabilistic sensitivity analysis, colonoscopy 3/10 screening strategy was cost-effective over all other strategies in 84.77% of the simulations at a WTP of €30,000/QALY (Figure 5). FIT 1/1y screening strategy was only cost-effective in 15.23% of the simulations. To evaluate the percentage of iterations WTP that favour each screening strategy over a range WTP, an acceptability curve was produced (Figure 6). Again, in an WTP range (€0 – 60,000€), colonoscopy 3/10y is the more cost-effective strategy. FIT 2/2 y, was never a cost-effective strategy, in the 10,000 iterations (Figure 7).
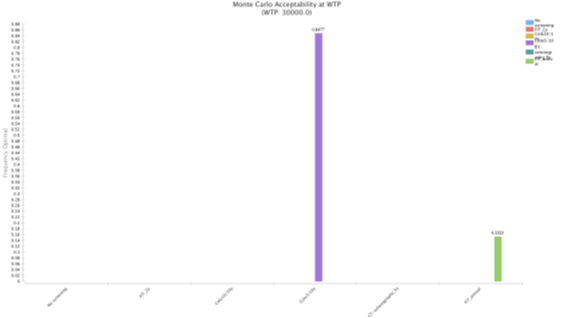
Figure 5: Monte Carlo acceptability at WTP
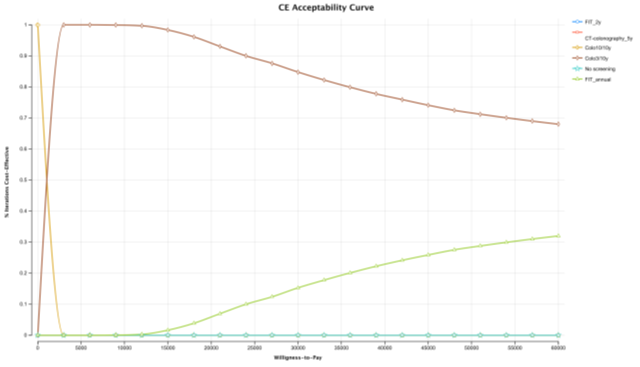
Figure 6: CE acceptability curve
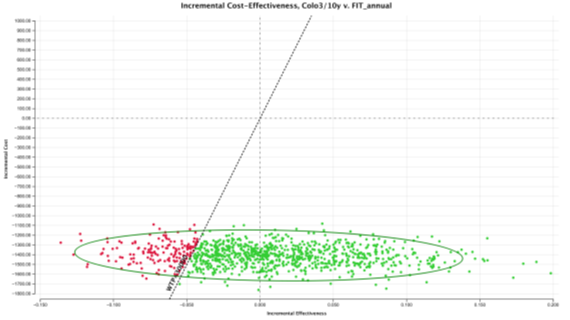
Figure 7: ICE scatterplot: Colonoscopy3/10y versus FIT1/1y
Discussion
The results of our model, indicate that the Portuguese context, the most cost-effective strategy for CRC screening is colonoscopy every 10 years, with the first follow-up colonoscopy performed 3 years after completion of the baseline colonoscopy. The colonoscopy based-strategies (colonoscopy 3/10y and colonoscopy 10/10y) absolutely dominate all stool-based strategies as well as CT-colonography, with ICERs clearly below the WTP threshold of 30,000€ per QALY and the largest net monetary benefits (NMB). Choosing a different strategy resulted in lower effectiveness and higher costs, making such an option not rational and undesirable. The biennial FIT strategy, although positive in reducing CRC incidence and mortality compared with no screening, is the least cost-effective choice among the 5 strategies modelled. Our findings are consistent with previous findings in the literature, although there are studies with different results [12]. Teldford et al, used a Markov probabilistic model to estimate the most cost-effective strategy for CRC screening in Canada; colonoscopy every 10 years was the most cost-effective strategy with an ICER of 6,133€ per QALY when compared to FIT performed annually [13]. In a study by Barzi et al evaluating 13 screening strategies, including FIT, colonoscopy was the most effective strategy with the highest effectiveness, but also the lowest total cost per patient ($2861) [14]. They showed that annual FIT was dominated by colonoscopy every 10 years. In our study, we found similar results, as annual FIT is absolutely dominated by colonoscopy, with an ICER of – 44,461.97€ per QALY (FIT 1/1y versus colonoscopy 3/10y). The absolute dominance of colonoscopy over all other screening strategies in Portugal, observed in our model, is probably explained by two main factors acting simultaneously in the Portuguese context: (1) relatively low cost of anaesthetic-assisted colonoscopy (compared to other developed countries), and (2) the high reimbursement received by NHS hospitals during the first two years of CRC treatment. In our model colonoscopy 3/10y is more cost-effective than colonoscopy 10/10y, with an ICER of 802.7€ per QALY, in the base case scenario. Colonoscopy every 10 years is the strategy generally evaluated in CRC models. As far as we know, this is the first time, a colonoscopy 3/10 y strategy has been included in a model, aiming to decrease the number of missing lesions in the baseline colonoscopy [2]. Our results are reinforced by the perfect adherence scenario. Colonoscopy-based strategies continue to dominate all other strategies and the cost-effectiveness ranking remains unchanged. These results are further supported by PSA, in which colonoscopy 3/10y is the most cost-effective strategy in more than 80% of the iterations. The only situation in which FIT is more cost-effective than colonoscopy is for initial adherence for colonoscopy below 39.8% ceteris paribus, reinforcing the importance of health policies to increase adherence. The colonoscopy adherence rate used in the cost-effectiveness models for base case estimates is variable, from 22% to 60% [12,15,16]. In our study, we assumed the upper limit of this range, as colonoscopy was modelled under anaesthesia assistance (in Portugal, almost all colonoscopies requested by NHS primary care physicians are performed under anaesthesia in an open access basis, free of charge at the point of care), which will very plausibly increase the adherence rate. In 2015, a US survey found that 45.3% of adults aged 50 to 54 years reported screening with either colonoscopy or sigmoidoscopy [2]. In our model, the assumption of adherence for initial screening with colonoscopy-based strategies was 60%. Individual adherence can be influenced by many factors, such as, education about screening, test characteristics and physician recommendation. Regarding colonoscopy adherence, two major factor influencing patient compliance is ensure that they will not have of pain and safety. Our results are in line with previous studies on the clinical impact of different screening strategies on the natural history of CRC. Screening colonoscopy 3/10y, prevented 64% of CRCs (129.7 CRC cases in the unscreened cohort) and gained 337.5 QALYs (per 1000 screened patients); improving screening for a perfect adherence scenario would prevent 80% of the CRCs cases and gain 451.9 QALYs. In a study by Ladabaum et al, the number of cases of CRC per 1000 persons was 105 in the unscreened cohort; colonoscopy every 10 years, averted 60 cases of CRC (57.2% reduction) [17]. Again as expected, the least effective strategy, biennial FIT avoided only 55% of CRCs, and gained only 77.2% of the QALYs obtained by the colonoscopy 3/10y strategy, being more than six times more expensive compared to colonoscopy 3/10y. As far as we know, our study is the first to estimated cost-effectiveness analysis for the Portuguese context, using the NHS perspective, using a Markov model replicating the natural history of colon cancer, as well as its current treatment according to CRC staging, where the five strategies were superimposed. Areia et al compared only 2 strategies in the Portuguese context: biennial FIT and colonoscopy every 10 years (using no screening as baseline) [18]. They concluded that biennial FIT was the most cost-effective strategy, as colonoscopy presented an ICER (103,633€ per QALY) above the WTP threshold. The study used a societal perspective, which despite strong economic theoretical arguments for its use, is not the perspective used and recommended by most government agencies, such as NICE or the Infarmed-National Portuguese Authority of Medicines and Health Products, due to its lack of consistency and objectivity, as it introduces a large number of discretionary costs [19-21]. Their analysis is limited to 2 strategies, as they argue that including more strategies in the model would compromise the evaluation of the 2 strategies. This argument has no practical or theoretical support, as the quality of the model analysis is not dependent on the number of strategies included, as each strategy is an independent node in a Markov model. In our opinion, FIT 1/1 y should have been included, not least because FIT 2/2y is not endorsed by most medical societies [22]. Moreover in their model they only considered a very low compliance rate for colonoscopy, which seems not realistic given that in Portugal, colonoscopy is performed under anaesthesia, free of charge. The 38% adhesion rate used in the base case estimate was also used as the upper limit in the model's sensitivity analysis; it does not seem plausible that the baseline value is, at the same time, the upper limit in a sensitivity analysis. Furthermore, using the initial adherence rate value of 38%, as the adherence rate for subsequent colonoscopies is surprising. In most studies, the rate of adhesion used for subsequent colonoscopies is higher between 60%-80% [12]. The cost per patient of the 2 modelled strategies is very low: 11.9€ in the FIT strategy, 199.4€ in the colonoscopy strategy and 7.9€ in unscreened patient. This is difficult to understand as the cost of the cancer treatment, is the main driver of the total costs in any CRC screening program, give the price of new drugs and treatments. They should have used, as we did in our model, for objectivity and simplicity, the fixed reimbursement for the first 2 years of CRC treatment that it is being progressively implemented in Portugal. In their work, the cost of CRC per stage is not explicitly reported, as only cost data is showed in a supplementary table, with unit cost for several tests and procedures. We should note that this study is a a modelling exercise, using multiple health states, patients’ pathways, inputs and assumptions, and using published data. The costs are derived from the Portuguese NHS, so care must be exercised when applying these results to other countries. Despite the limitations, we believe that the study can contribute to the design of better and more efficient screening policies. Our results, strong suggest, that biennial FIT, the screening strategy used in Portugal, should be abandoned as it is the most expensive and less effective strategy for the NHS, and replaced by one of the two colonoscopy strategies. Finally, the implementation of an organized screening strategy should include a cost-effectiveness analysis for the governments to take more rational decisions and better allocate resources in healthcare.
Methods and Declarations
Ethics approval and consent to participate
Study performed in accordance with relevant guidelines and regulations and approved by Hospital de Santa Luzia, ULS Alto Minho, Ethics committee.
Consent for publication
Not applicable
Availability of data and materials:
The datasets generated during/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
No competing interests to declare
Funding
No funding
Authors’ contribution
LL was responsible for the conception, design, acquisition, analysis, interpretation of the data and draft of the work. MC contributed to the design and interpretation of the data. PV contributed to the analysis and interpretation of the data. JC contributed to the interpretation of the data. All authors read and approved the final manuscript.
Acknowledgements
We would like to acknowledge Prof. Ricardo Gonçalves, from Porto Católica Business School, for his support and Andrew Munzer from TreeAge Software, LLC, for helping to build the model.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians 68 (2018): 394-424.
- Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians 68 (2018): 250-281.
- Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. American Journal of Gastroenterology 112 (2017): 1016-1030.
- Ministério da Saúde. Rastreios Oncológicos (2017).
- Guide to the methods of technology appraisal (2013).
- Sharma T. Analysis of the effectiveness of two noninvasive fecal tests used to screen for colorectal cancer in average-risk adults. Public Health 182 (2020): 70-76.
- Instituto Nacional de Estatística. Tábuas de Mortalidade (2021).
- ACSS. Regulamentos e as Tabelas de Preços das Instituições e Serviços Integrados no Serviço Nacional de Saúde (2021).
- ACSS. Tabela MCDT convencionados (2021).
- ACSS. Termos de Referência para contratualização de cuidados de saúde no SNS para 2021 (2021).
- Ness RM, Holmes AM, Klein R, Dittus R. Utility valuations for outcome states of colorectal cancer. The American Journal of Gastroenterology 94 (1999): 1650-1657.
- Zhong GC, Sun WP, Wan L, et al. Efficacy and cost-effectiveness of fecal immunochemical test versus colonoscopy in colorectal cancer screening: a systematic review and meta-analysis. Gastrointestinal Endoscopy 91 (2020): 684-697.
- Telford JJ, Levy AR, Sambrook JC, et al. The cost-effectiveness of screening for colorectal cancer. Cmaj 182 (2010): 1307-1313.
- Barzi A, Lenz HJ, Quinn DI, et al. Comparative Effectiveness of Screening Strategies for Colorectal Cancer. Cancer 123 (2017): 1516-1543.
- Greuter MJE, Berkhof J, Fijneman RJA, et al. The potential of imaging techniques as a screening tool for colorectal cancer: A cost-effectiveness analysis. British Journal of Radiology 12 (2016): 89-82.
- Sekiguchi M, Igarashi A, Matsuda T, et al. Optimal use of colonoscopy and fecal immunochemical test for population-based colorectal cancer screening: A cost-effectiveness analysis using Japanese data. Japanese Journal of Clinical Oncology 46 (2016): 116-125.
- Ladabaum U, Mannalithara A, Meester RGS et al. Cost-Effectiveness and national effects of initiating colorectal cancer screening for average-risk persons at age 45 years instead of 50 Years. Gastroenterology 157 (2019): 137-148.
- Areia M, Fuccio L, Hassan C, et al. Cost-utility analysis of colonoscopy or faecal immunochemical test for population-based organised colorectal cancer screening. United European Gastroenterology Journal 7 (2019): 105-113.
- NICE. Principles for the development of NICE guidance (2008).
- Gray AM, Clarke PM, Wolstenholme JL, et al. Applied methods of cost-effectiveness analysis in healthcare. Oxford University Press (2011).
- Briggs A, Sculpher M, Claxton K. Decision modelling for health economic evaluation. Oup Oxford (2006).
- Bibbins DK, Grossman DC, Curry SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. JAMA 315 (2016): 2564-2575.
- Imperiale TF, Ransohoff DF, Itzkowitz SH, et al. Multitarget Stool DNA Testing for Colorectal-Cancer Screening. New England Journal of Medicine 370 (2014): 1287-1297.
- Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of benefits, burden, and harms of colorectal cancer screening strategies: Modeling study for the US preventive services Task Force. Journal of the American Medical Association 315 (2016): 2595-609.
- Martín-López JEE, Beltrán-Calvo C, Rodríguez-López R, et al. Comparison of the accuracy of CT colonography and colonoscopy in the diagnosis of colorectal cancer. Colorectal Disease 16 (2014): 82-89.
- Frazier ALL, Colditz GA, Fuchs CSCS, et al. Cost-effectiveness of screening for colorectal cancer in the general population. Journal of the American Medical Association 284 (2000): 1954-1961.
- Wong CKH, Lam CLK, Wan YF, et al. Cost-effectiveness simulation and analysis of colorectal cancer screening in Hong Kong Chinese population: Comparison amongst colonoscopy, guaiac and immunologic fecal occult blood testing. BMC Cancer 15 (2015).
- Krilaviciute A, Stock C, Brenner H. International variation in the prevalence of preclinical colorectal cancer: Implications for predictive values of noninvasive screening tests and potential target populations for screening. The Authors International Journal of Cancer 141 (2017): 1566-1575.
- Lee D, Muston D, Sweet A, et al. Cost effectiveness of CT colonography for UK NHS colorectal cancer screening of asymptomatic adults aged 6069 years. Applied Health Economics and Health Policy 8 (2010): 141-154.
- Leshno M, Halpern ZZ, Arber N. Cost-effectiveness of colorectal cancer screening in the average risk population. Health Care Management Science 6 (2003): 165-174.
- NCRAS - National Cancer Registration and Analyses Service. Colorectal Cancer Survival by Stage (2018).
