Copper Dressings to the Wound Rescue after Everything Else Failed: Case Report
Article Information
Cernica Chausha Weitman1*, Tohar Roth2, Gadi Borkow2
1Hadassah Medical Center, Jerusalem, Israel
2MedCu Technologies Ltd, Herzliya, Israel
*Corresponding Author: Cernica Chausha Weitman, Hadassah Medical Center, Jerusalem, Israel.
Received: 09 May 2022; Accepted: 25 May 2022; Published: 06 June 2022
Citation: Cernica Chausha Weitman, Tohar Roth, Gadi Borkow. Copper Dressings to the Wound Rescue after Everything Else Failed: Case Report. Archives of Clinical and Medical Case Reports 6 (2022): 466-473.
View / Download Pdf Share at FacebookAbstract
We report a dramatic case in which an initially minor superficial wound with an area of approximately 4 cm2 in diameter, increased in size to ~300 cm2 and more than 1cm deep, during 8 months of hospitalization. This deterioration occurred despite a wide range of standard of care procedures, such as OR debridement of necrotic tissue, systemic and local antibiotics administration, application of a variety of antimicrobial wound dressings, wound washes, Negative Pressure Wound Therapy, pressure chamber treatment, and two skin grafts after escharotomy. Resolution of the wound progression, subsequent granulation tissue formation, epithelialization, wound healing and almost complete wound closure- was achieved only after treatment with copper oxide impregnated wound dressings. This case strongly supports the potential role of copper in the healing process of hard-to-heal wounds.
Keywords
Wound dressings; Copper oxide; NPWT; Skin grafts; Pressure chamber; Lupus
Wound dressings articles; Copper oxide articles; NPWT articles; Skin grafts articles; Pressure chamber articles; Lupus articles
Wound dressings articles Wound dressings Research articles Wound dressings review articles Wound dressings PubMed articles Wound dressings PubMed Central articles Wound dressings 2023 articles Wound dressings 2024 articles Wound dressings Scopus articles Wound dressings impact factor journals Wound dressings Scopus journals Wound dressings PubMed journals Wound dressings medical journals Wound dressings free journals Wound dressings best journals Wound dressings top journals Wound dressings free medical journals Wound dressings famous journals Wound dressings Google Scholar indexed journals COVID-19 articles COVID-19 Research articles COVID-19 review articles COVID-19 PubMed articles COVID-19 PubMed Central articles COVID-19 2023 articles COVID-19 2024 articles COVID-19 Scopus articles COVID-19 impact factor journals COVID-19 Scopus journals COVID-19 PubMed journals COVID-19 medical journals COVID-19 free journals COVID-19 best journals COVID-19 top journals COVID-19 free medical journals COVID-19 famous journals COVID-19 Google Scholar indexed journals Cancer articles Cancer Research articles Cancer review articles Cancer PubMed articles Cancer PubMed Central articles Cancer 2023 articles Cancer 2024 articles Cancer Scopus articles Cancer impact factor journals Cancer Scopus journals Cancer PubMed journals Cancer medical journals Cancer free journals Cancer best journals Cancer top journals Cancer free medical journals Cancer famous journals Cancer Google Scholar indexed journals Copper oxide articles Copper oxide Research articles Copper oxide review articles Copper oxide PubMed articles Copper oxide PubMed Central articles Copper oxide 2023 articles Copper oxide 2024 articles Copper oxide Scopus articles Copper oxide impact factor journals Copper oxide Scopus journals Copper oxide PubMed journals Copper oxide medical journals Copper oxide free journals Copper oxide best journals Copper oxide top journals Copper oxide free medical journals Copper oxide famous journals Copper oxide Google Scholar indexed journals NPWT articles NPWT Research articles NPWT review articles NPWT PubMed articles NPWT PubMed Central articles NPWT 2023 articles NPWT 2024 articles NPWT Scopus articles NPWT impact factor journals NPWT Scopus journals NPWT PubMed journals NPWT medical journals NPWT free journals NPWT best journals NPWT top journals NPWT free medical journals NPWT famous journals NPWT Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Lupus articles Lupus Research articles Lupus review articles Lupus PubMed articles Lupus PubMed Central articles Lupus 2023 articles Lupus 2024 articles Lupus Scopus articles Lupus impact factor journals Lupus Scopus journals Lupus PubMed journals Lupus medical journals Lupus free journals Lupus best journals Lupus top journals Lupus free medical journals Lupus famous journals Lupus Google Scholar indexed journals Skin grafts articles Skin grafts Research articles Skin grafts review articles Skin grafts PubMed articles Skin grafts PubMed Central articles Skin grafts 2023 articles Skin grafts 2024 articles Skin grafts Scopus articles Skin grafts impact factor journals Skin grafts Scopus journals Skin grafts PubMed journals Skin grafts medical journals Skin grafts free journals Skin grafts best journals Skin grafts top journals Skin grafts free medical journals Skin grafts famous journals Skin grafts Google Scholar indexed journals SARS-CoV2 articles SARS-CoV2 Research articles SARS-CoV2 review articles SARS-CoV2 PubMed articles SARS-CoV2 PubMed Central articles SARS-CoV2 2023 articles SARS-CoV2 2024 articles SARS-CoV2 Scopus articles SARS-CoV2 impact factor journals SARS-CoV2 Scopus journals SARS-CoV2 PubMed journals SARS-CoV2 medical journals SARS-CoV2 free journals SARS-CoV2 best journals SARS-CoV2 top journals SARS-CoV2 free medical journals SARS-CoV2 famous journals SARS-CoV2 Google Scholar indexed journals
Article Details
Abbreviations: COD: Copper oxide dressings; CRP: C-reactive protein; CT: Computed tomography; CTA: Computed tomography angiography; ER: Emergency room; NPWT: Negative pressure wound therapy; OR: Operation room; PAN: Polyarteritis nodosa; SLE: Systemic lupus erythematosus
1. Introduction
Copper, one of the eleven essential trace elements vital for the normal function of all human tissues [1], plays a key role in many of the wound healing-related processes [2, 3]. It stimulates angiogenesis [4, 5], secretion of fibrinogen, elastin and collagen by dermal fibroblasts [6, 7], upregulation of copper-dependent enzymes and polysaccharides important for matrix remodelling, cell proliferation and re-epithelization [8-11] and skin and stem cells migration [12, 13]. Copper also has a wide spectrum of potent biocidal properties [14, 15]. Antimicrobial wound dressings impregnated with Cuprous oxide microparticles (hereafter termed COD) have been produced [5, 16] and are clinically used in the treatment of a variety of acute and chronic wounds [17-19]. We report here a dramatic case of a minor wound on the distal shin of a patient, that despite 8 months of hospitalization and intensive standard of care modalities, deteriorated and increased in size and depth to such a degree that below-the-knee amputation was seriously considered. Strikingly, granulation tissue formation, epithelialization, wound healing and almost complete wound closure was achieved only after treatment with copper oxide impregnated wound dressings.
2. Patient Description
The female patient at the time of treatment was 58-year-old, with a past medical history of Systemic Lupus Erythematosus (SLE) since 2004, hypertension and hypothyroidism. She is a nonsmoker, nondrinker, does not take drugs and has no sleeping problems. The main SLE expressions from which she suffers are photosensitivity, minor aphthous ulcers, Raynaud phenomenon, arthralgia, and pancytopenia. Due to flares in her condition, she was hospitalized several times in the last few years, and treated with Iloprost and pulse-solumedrol. Since 2016 her disease was well-controlled under Imuran (Azathioprine) 50 mg twice a day. Additional regularly taken medications were as following: Atozet 10 mg/20 mg once a day, Ramipril 2.5 mg once a day, Caltrate 600 mg once a day, Euthyrox (Levothyroxine Sodium 2012) 100 mg once a day, Plaquenil 200 mg a day, Propranolol 80 mg once a day, Omepradex (Omeprazole) 20mg once a day, and Prednisone 5 mg once a day. Her serology over the years was ANA DSNDA RPN ANTI SMITH. C3 decreased sometimes. During the last few years her situation was stable and the dosage of Imuran was reduced. The patient is allergic to penicillin.
2.1. Patient Condition during Hospitalization
During the hospitalization, the patient did not suffer from an active disease, as she was receiving high doses of steroids. Her blood pressure on hospitalization was 137/81 sitting down and she had 98% blood saturation. Heart and lung activities were normal. ANA, ENA SMITH were positive; C3 and C4 were low. Blood counts were fine, besides suffering from chronic non- hemolytic anemia (low folic acid levels). Due to her anemia, the patient received 5 times blood transfusions and constant folic acid administration during her hospitalization. When her condition seemed to deteriorate due to systemic infections, urine samples were taken for bacterial swabs, and antibiotic treatment was administered accordingly. She was treated with Cephazolin i.v. and then per os for two weeks. Due to suspected thrombosis, eco heart was performed; APLA determination was negative. Treatment with preventive Clexane (40 mg/day) was initiated. Due to the immune-suppressive steroid treatment in high doses, the patient received one tablet of Co Trimoxazole forte (800/160) three times a week, and osteoporosis treatment (continuous 1000 units of vitamin D3 and 600 mg of calcium carbonate administration per day, Teriparatide [Forteo] 20 mg/day sub-cutaneous for one month followed by once-a- month Rizedronet administration). Since the patient suffered from substantial pain, she was treated with Targin (40 mg x 2/day), Oxicodone Hel (20 mg x 2/day), Dipyrone, i.v. Paracetamol, and Fentanyl patches.
2.3. Wound Condition and Treatment
The patient suffered from a painful ~ 1 cm2 ulcer above the malleolus medially right leg, apparently from an insect bite, without inflammation or odor and normal CRP when she first arrived to the emergency room (ER) of the hospital (6.9.2019). She was given antibiotics (Naproxen); the wound was dressed with hydrocolloid dressings and the patient was released home. She returned to the hospital two weeks later with no improvement in the wound condition. She was then given Clindamycin and the wound was dressed with Hydroclean® Advance dressing. Four weeks later, the patient arrived again to the ER with the wound that increased in size to ~ 4 cm2, with 1 cm diameter of edema and light cellulitis (5 mm), with no fever and normal C-reactive protein (CRP) (Figure 1, D0). The patient was hospitalized and 4 skin biopsies were taken from the wound under sedation. No specific pathology was identified. In common to all biopsies was the lack of the epidermal layer, wide necrosis of the fat layer, metaplastic ossification, without calciphylaxis, increase in blood vessels with fibrinoid thrombi. The findings from micro analysis of single blood vessels indicated potential Polyarteritis nodosa (PAN), but the macroanalysis did not. The findings could not be attributed to SLE due to lack of hyaline changes in the fat tissue and lack of lymphocytes localization. The alterations could also not be attributed to vasculitis or Georgian ulcer. The following bacteria colonized the wound: Escherichia coli, Stenotrophomonas maltophilia and Aeromonas punctate. However no deep infection was noted, including of the bones. Computed tomography angiography (CTA) and two computed tomography (CT) scanning did not reveal any vascular problem. It was suspected that the patient suffers from Pyoderma gangrenosum and it was decided to treat her with systemic prednisone steroids (Figure 2). Initially with a high dose of 80 mg/day and gradually the dose was decreased. However, due to lack of improvement and continued increase in the size of the wound, the administration of Azathioprine for her lupus condition was stopped and treatment with Cyclosporin was initiated. Initially with a dose of 3 mg/kg and gradually the dose was increased to 5 mg/kg. Nevertheless, the wound continued to grow to approximately 45 cm2 within a month without clearing the infection and with increase in the necrotic tissue (Figure 1., D39). At Day 60, the necrotic fat was removed by operation room (OR) debridement all the way to the necrotic wound edge and the wound was covered with saline dressings and Sulfamylon® cream. Again, no significant improvement in the wound was noted (Figure 1, D62). The possibility that the patient suffers from PAN and Georgian ulcers was raised again, as her parents were immediate relatives. However, the patient was negative to Adenosine Deaminase 2 (ADA2). It was decided to stop the Cyclosporin treatment, increase the dose of Prednisone to 1 mg/kg, and to administer Ilomedin i.v. to improve the blood circulation (gradually increasing the dose from 10 mg to 50 mg by slow dripping for one week). The wound was then treated by Hyperbaric Oxygen Therapy (pressure chamber) for 5 days. By then, the wound reached an approximate size of 220 cm2 and ~2 cm depth. As the wound continued to be contaminated without showing any improvement, it was decided to perform another OR debridement of the necrotic and contaminated fat up to the facia (Figure 1, D92). This was followed by 3 days of Negative Pressure Wound Therapy (NPWT) wash procedure (Figure 1, D93 D96). Wound swabs taken then revealed that the wound was contaminated with Pseudomonas aeruginosa and Proteus mirabilis. The patient was administered with one dose of Vancomycin, one dose of Amikacin and 2 grams Meropenem treatment. After two weeks of antibiotic treatment and escharotomy, a skin graft was performed and the wound was covered with saline dressings. NPWT was continued on top of the grafted skin (Figure 1, D110). However, the grafted skin cells were rejected and the treatment failed (Figure 1, D116). The wound size by then was approximately 300 cm2. Two weeks later the wound continued to be treated by Hyperbaric Oxygen Therapy 5 days a week for 3 weeks (Figure 1, D162). Another escharotomy was performed, followed by Neomycin washes, Amikacin and Meropenem administration, Iodoform dressings and multiple layer bandage treatment into the upper cavity of the wound. This was followed by an additional debridement of necrotic fat in the edges of the wound, washing with a water jet and NPWT for 3 days, and a second skin graft (Figure 1, D184). The patient was then administered with Amikacin and Chlorhexidine. The wound was covered with Vaseline gauze. Yet, the skin cells once again were rejected and no significant improvement in the wound condition was noted (Figure 1, D220). Due to suspected pseudomonas infection, another OR debridement was performed. Amikacin and i.v. injections of immunoglobulins were administered. During the patient hospitalization until the 29.5.20 (Figure 1, D223), the wound itself was managed by several different dressings: HydroClean®, PolyMem®, Medihoney™, AQUACEL®Extra™, Hydrocolloid and Iodoform dressings; and by the following irrigation solutions/creams/gels: Sulfamylon®, Octanelin®, Prontosan®, Flaminal®, and Askina® Gel. By this time the wound size of ~300 cm2 surrounded almost the whole lower extremity on the front and side of the leg below the knee. Basically, the subcutaneous and dermal layers were gone. At this stage, just before the Copper Oxide Dressings (COD) started to be applied on the wound (Figure 1, D229 (COD-0)), amputation below the knee was being considered. The COD were changed with fresh COD every 2 to 3 days. Prontozan® gel was applied at every dressing change for 15 minutes. While initially there were high amounts of wound exudate secreted from the wound, the amount of secretions gradually decreased. In parallel, some granulation tissue started to appear in the wound edges together with new epithelia crawling from the wound edges, covering the muscle (Figure 1, COD-24-42). The size of the wound continued to gradually decrease (Figure 1, COD-78-119), and the patient experienced significant reduction in pain. Thirty-four days after the commencement of the COD treatment (D263) the patient was finally released to home after being hospitalized for almost 9 months. The wound continued to be treated in the Chronic and Complicated Wound Clinic with the COD, which was replaced with a fresh COD every 3-4 days. After 3 months of treatment most of the wound was covered with epithelia (Figure 1; COD-119). While the wound was still not completely closed after 7 months of COD treatment, strikingly, gradually new skin tissues were formed beneath the covering epidermal layer reducing significantly the wound depression (Figure 1, COD-224). No antibiotics were administered during the period when the wound was managed with the COD.
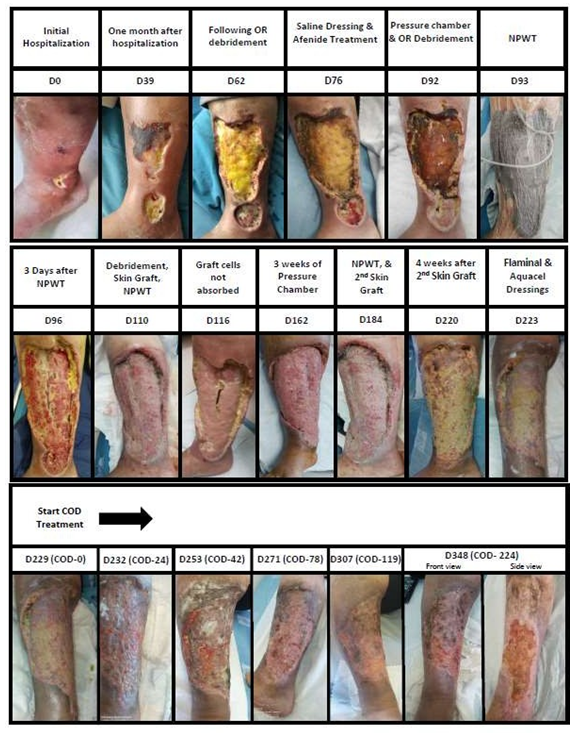
Figure 1: Sequential pictures of the wound are shown, together with a brief description of the treatments conducted, days of treatment since the hospitalization of the patient and the days of treatment since the beginning of the use of the COD.
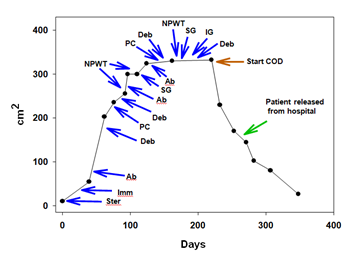
Figure 2: Time plot showing the wound area. The various treatments conducted are depicted. The COD treatment continued also after the patient was released home in the wound care clinic. Ster: Steroid treatment; Imm: Immunodepression treatment; Ab: Antibiotic treatment; Deb: Debridement; PC: Pressure chamber treatment; NPWT: Negative pressure wound therapy; SG: Skin grafting; IG: Immunoglobulin treatment; COD: Copper oxide dressing.
4. Discussion
We describe here a dramatic case of wound healing that occurred in a very large lower extremity wound only when copper oxide impregnated dressings were used in a patient suffering from Lupus, hypertension and hypothyroidism. This occurred after 9 months of hospitalization during which a small wound increased from a few cm2 to almost 300 cm2, basically eliminating all the skin layers, all the way to the muscle, below the knee in the front and side sides of the right leg extremity, despite a wide range of different wound healing treatment modalities applied. The treatments used included debridement of necrotic tissue, systemic and local antibiotics administration, wound washes, Negative Pressure Wound Therapy, pressure chamber treatment, skin grafting and use of silver and honey antimicrobial wound dressings. After almost 9 months of hospitalization, it was decided to use the COD, as a last resort before amputating the limb below the knee. The COD were just then incorporated into the series of antimicrobial wound dressings used in the hospital. Why the wound then started to heal? Already in 2008 a hypothesis was made that chronic wounds may not heal because to too amounts of systemic copper reach the wound [2]. This hypothesis was based mainly in the physiological role that copper plays in angiogenesis, dermal fibroblasts proliferation and migration, secretion of collagen and elastin fibers by dermal fibroblasts, and its role as a cofactor of Lysyl oxidase needed for efficient dermal extracellular matrix protein cross-linking [2;5;17]. The essential role of copper in wound healing has since then gotten significant recognition by the scientific community [3]. In the current case, we hypothesize that the COD had a dual activity. First, the COD conferred protection to the wound from microorganisms and maintained the wound clear from infection. Secondly, and even more importantly, the copper ions eluting from the COD induced to some degree formation of granulation tissue despite the lack of all the skin layers including the subcutaneous layer. And more notorious was the induction of the gradual and continuous migration of the epidermis from the skin surrounding the wound. This led to the eventual closure of almost all the wound within three months of treatment with the COD. The patient had a significant reduction in pain, was able to walk and was dismissed from the hospital to continue the treatment in the clinic. During this period of COD treatment, no other wound treatment modalities were used other than applying Protozan gel to assure removal of biofilm if existent. Taken together, this case strongly supports the potential role of copper in the healing process of hard-to-heal wounds.
Conflict of Interests
G.B. and T.R. are employees of MedCu Technologies, the company that developed the COD. C.C.W. has no conflict of interests.
References
- Uauy R, Olivares M, Gonzalez M. Essentiality of copper in humans. Am J Clin Nutr 67 (1998): 952S-959S.
- Borkow G, Gabbay J, Zatcoff RC. Could chronic wounds not heal due to too low local copper levels? Med Hypotheses 70 (2008): 610-613.
- Kornblatt AP, Nicoletti VG, Travaglia A. The neglected role of copper ions in wound healing. J Inorg Biochem 161 (2016): 1-8.
- Parke A, Bhattacherjee P, Palmer RM, et al. Characterization and quantification of copper sulfate-induced vascularization of the rabbit cornea. Am J Pathol 130 (1988): 173-178.
- Borkow G, Gabbay J, Dardik R, et al. Molecular mechanisms of enhanced wound healing by copper oxide-impregnated dressings. Wound Repair Regen 18 (2010): 266-275.
- Harris ED, Rayton JK, Balthrop JE, et al. Copper and the synthesis of elastin and collagen. Ciba Found Symp 79 (1980): 163-182.
- Philips N, Samuel P, Parakandi H, et al. Beneficial regulation of fibrillar collagens, heat shock protein-47, elastin fiber components, transforming growth factor-beta1, vascular endothelial growth factor and oxidative stress effects by copper in dermal fibroblasts. Connect Tissue Res 53 (2012): 373-378.
- Lansdown AB. Metallothioneins: potential therapeutic aids for wound healing in the skin. Wound Repair Regen 10 (2002): 130-132.
- Rucker RB, Kosonen T, Clegg MS, et al. Copper, lysyl oxidase, and extracellular matrix protein cross-linking. Am J Clin Nutr 67 (1998): 996S-1002S.
- Simeon A, Wegrowski Y, Bontemps Y, et al. Expression of glycosaminoglycans and small proteoglycans in wounds: modulation by the tripeptide-copper complex glycyl-L- histidyl-L-lysine-Cu (2+). J Invest Dermatol 115 (2000): 962-968.
- Simeon A, Emonard H, Hornebeck W, et al. The tripeptide-copper complex glycyl- L-histidyl-L-lysine-Cu2+ stimulates matrix metalloproteinase-2 expression by fibroblast cultures. Life Sci 67 (2000): 2257-2265.
- Chen M, Li R, Yin W, et al. Copper promotes migration of adipose-derived stem cells by enhancing vimentin-Ser39 phosphorylation. Exp Cell Res 388 (2020): 111859.
- Alizadeh S, Seyedalipour B, Shafieyan S, et al. Copper nanoparticles promote rapid wound healing in acute full thickness defect via acceleration of skin cell migration, proliferation, and neovascularization. Biochem Biophys Res Commun 517 (2019): 684-690.
- Borkow G, Gabbay J. Copper as a biocidal tool. Curr Med Chem 12 (2005): 2163-2175.
- Borkow G. Using copper to fight microorganisms. Curr Chem Biol 6 (2012): 93-103.
- Borkow G, Okon-Levy N, Gabbay J. Copper oxide impregnated wound dressings: biocidal and safety studies. Wounds 22 (2010): 310-316.
- Borkow G, Melamed E. Copper, an abandoned player returning to the wound healing battle. In: Shahin A, editor. Scars and Kelloids. 1st ed. London: IntechOpen 2021.
- Melamed E, Kiambi P, Okoth D, et al. Healing of Chronic Wounds by Copper Oxide- Impregnated Wound Dressings-Case Series. Medicina 57 (2021): 296-310.
- Melamed E, Rovitsky A, Roth T, et al. Stimulation of Healing of Non-Infected Stagnated Diabetic Wounds by Copper Oxide-Impregnated Wound Dressings. Medicina 57 (2021): 1129-1138.
