Contribution of Vitamin C in the Treatment of Malignant Melanoma
Article Information
Hiba O. Osman1*, Neena E. Thomas2, Somtochi Udekwe3, Suzanne Habashy4, Aliya Jafri5, Stacey E. Heindl6
1California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
2Medical Graduate, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
3Endocrinology, Internal Medicine, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
4Family Medicine/Dermatology, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
5Biochemistry and Family Medicine, California Institute of Behavioral Neurosciences & Psychology, Fairfield, CA, USA
6California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
*Corresponding author: Hiba O. Osman, California Institute of Behavioral Neurosciences & Psychology, Fairfield, USA
Received: 27 June 2021; Accepted: 05 July 2021; Published: 20 July 2021
Citation: Hiba O. Osman, Neena E. Thomas, Somtochi Udekwe, Suzanne Habashy, Aliya Jafri, Stacey E. Heindl. Contribution of Vitamin C in the Treatment of Malignant Melanoma. Fortune Journal of Health Sciences 4 (2021): 383-393.
View / Download Pdf Share at FacebookAbstract
Malignant melanoma is a severe skin cancer with catastrophic complications due to its early distant metastasis and high mortality rate despite the development of different therapeutic approaches. Therefore, we need innovative ways to fight this cancer vigorously. It is time to collaborate with all the efforts to establish well-maintained guidelines and protocols that help eliminate the vicious cycle of melanoma. Vitamin C has demonstrated anti-tumor features that characterize it from other water and fat-soluble vitamins. Our principal objective was to assess how vitamin C supplementation could mutate the epigenetic base of melanoma cells and whether that could make them less aggressive. We also looked at how vitamin C could induce cell death within melanoma cell lines. Furthermore, we investigated the sensitizing sequel of vitamin C on melanoma and how they can improve other chemotherapy capacities. This review aims to contribute to vitamin C's launch as a potent anti-cancer that could limit melanoma morbidness and death rate.
Keywords
vitamin c, adjuvant treatment, malignant melanoma
vitamin c articles, adjuvant treatment articles, malignant melanoma articles, vitamin c articles vitamin c Research articles vitamin c review articles vitamin c PubMed articles vitamin c PubMed Central articles vitamin c 2023 articles vitamin c 2024 articles vitamin c Scopus articles vitamin c impact factor journals vitamin c Scopus journals vitamin c PubMed journals vitamin c medical journals vitamin c free journals vitamin c best journals vitamin c top journals vitamin c free medical journals vitamin c famous journals vitamin c Google Scholar indexed journals adjuvant treatment articles adjuvant treatment Research articles adjuvant treatment review articles adjuvant treatment PubMed articles adjuvant treatment PubMed Central articles adjuvant treatment 2023 articles adjuvant treatment 2024 articles adjuvant treatment Scopus articles adjuvant treatment impact factor journals adjuvant treatment Scopus journals adjuvant treatment PubMed journals adjuvant treatment medical journals adjuvant treatment free journals adjuvant treatment best journals adjuvant treatment top journals adjuvant treatment free medical journals adjuvant treatment famous journals adjuvant treatment Google Scholar indexed journals malignant melanoma articles malignant melanoma Research articles malignant melanoma review articles malignant melanoma PubMed articles malignant melanoma PubMed Central articles malignant melanoma 2023 articles malignant melanoma 2024 articles malignant melanoma Scopus articles malignant melanoma impact factor journals malignant melanoma Scopus journals malignant melanoma PubMed journals malignant melanoma medical journals malignant melanoma free journals malignant melanoma best journals malignant melanoma top journals malignant melanoma free medical journals malignant melanoma famous journals malignant melanoma Google Scholar indexed journals sentinel lymph node biopsy articles sentinel lymph node biopsy Research articles sentinel lymph node biopsy review articles sentinel lymph node biopsy PubMed articles sentinel lymph node biopsy PubMed Central articles sentinel lymph node biopsy 2023 articles sentinel lymph node biopsy 2024 articles sentinel lymph node biopsy Scopus articles sentinel lymph node biopsy impact factor journals sentinel lymph node biopsy Scopus journals sentinel lymph node biopsy PubMed journals sentinel lymph node biopsy medical journals sentinel lymph node biopsy free journals sentinel lymph node biopsy best journals sentinel lymph node biopsy top journals sentinel lymph node biopsy free medical journals sentinel lymph node biopsy famous journals sentinel lymph node biopsy Google Scholar indexed journals BRAF articles BRAF Research articles BRAF review articles BRAF PubMed articles BRAF PubMed Central articles BRAF 2023 articles BRAF 2024 articles BRAF Scopus articles BRAF impact factor journals BRAF Scopus journals BRAF PubMed journals BRAF medical journals BRAF free journals BRAF best journals BRAF top journals BRAF free medical journals BRAF famous journals BRAF Google Scholar indexed journals HIF-1 alpha articles HIF-1 alpha Research articles HIF-1 alpha review articles HIF-1 alpha PubMed articles HIF-1 alpha PubMed Central articles HIF-1 alpha 2023 articles HIF-1 alpha 2024 articles HIF-1 alpha Scopus articles HIF-1 alpha impact factor journals HIF-1 alpha Scopus journals HIF-1 alpha PubMed journals HIF-1 alpha medical journals HIF-1 alpha free journals HIF-1 alpha best journals HIF-1 alpha top journals HIF-1 alpha free medical journals HIF-1 alpha famous journals HIF-1 alpha Google Scholar indexed journals qRT-PCR articles qRT-PCR Research articles qRT-PCR review articles qRT-PCR PubMed articles qRT-PCR PubMed Central articles qRT-PCR 2023 articles qRT-PCR 2024 articles qRT-PCR Scopus articles qRT-PCR impact factor journals qRT-PCR Scopus journals qRT-PCR PubMed journals qRT-PCR medical journals qRT-PCR free journals qRT-PCR best journals qRT-PCR top journals qRT-PCR free medical journals qRT-PCR famous journals qRT-PCR Google Scholar indexed journals melanoma articles melanoma Research articles melanoma review articles melanoma PubMed articles melanoma PubMed Central articles melanoma 2023 articles melanoma 2024 articles melanoma Scopus articles melanoma impact factor journals melanoma Scopus journals melanoma PubMed journals melanoma medical journals melanoma free journals melanoma best journals melanoma top journals melanoma free medical journals melanoma famous journals melanoma Google Scholar indexed journals
Article Details
Main Point of the Article
This review article studied the possibility of using vitamin C in the treatment of malignant melanoma by testing its ability to reprogram the epigenetic component of cancer cells.
1. Introduction
About 85,686 melanoma cases were discovered in the United States in 2017, out of which 8,056 lost their lives due to the disease. It is estimated that 23 in 100,000 individuals are diagnosed with melanoma [1]. Malignant melanoma is becoming more common and has an aggressive pattern because of its rapid spread and presentation. Hence, the focus of modern medicine has been evolving over the years to tackle this cancer. Despite this, there is no curative therapy that could prevent it from recurrence or metastases.
1.1 Surgery
As seen in figure 1, surgical removal of the initial lesion is the fundamental treatment for most melanoma stages. However, additional, more aggressive surgery may be needed based on the type and the clinical stage of the malignant melanoma [2]. For skin lesions larger than one millimeter, sentinel lymph node biopsy (SLNB) must be performed [2].
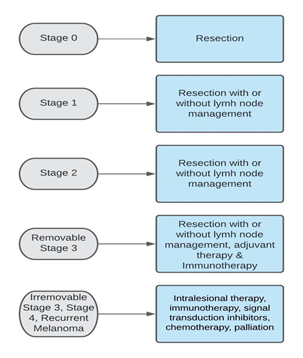
Figure 1: Treatment of cutaneous melanoma
1.2 Adjuvant Treatment
Adjuvant treatment with Interferon Alpha 2b has been considered for surgically excised melanoma, which is > 2mm in size, with or without local lymph node metastases [3,4]. The observed improvement in life expectancy and relapse-free survival, however, was minimal [3,5,6]. Nowadays, Nivolumab, Pembrolizumab, or Dabrafenib-Trametinib is advocated for surgically removed tumors with a high re-occurrence rate with BRAF V600E or V600K mutations [7]. For BRAF classified Melanoma, Nivolumab or Pembrolizumab is used [7].
1.3 Alternative Options
When a surgical option is not suitable, other treatment modes include radiotherapy, topical imiquimod and cryosurgery [2].
1.4 Vitamin C
Vitamin C is a water-soluble vitamin that has antioxidant, immune enhancement, wound healing and anti-inflammatory effects. It was speculated that a large amount of intravenous vitamin C could cure complicated cancers by Ewan Cameron and Linus Pauling back in 1970. However, its use for this purpose remains debatable [8, 9].
A high level of hypoxia-inducible factor-1 alpha (HIF-1 alpha) is being revealed in melanoma cases under normal oxygen conditions as opposed to other cancers [10]. The suppression of 5-hydroxymethycystosine (5-hmc) is the epigenetic characteristic of melanoma, which can help diagnose and predict the outcome of the disease [11]. It is noted that vitamin C (ascorbic acid) diminishes the role of HIF-1 alpha in malignant melanoma [12]. Moreover, it augments the 5hmc levels in melanoma cells and reduces melanoma's virulent shape [13]. Furthermore, sodium ascorbate stimulates apoptosis in A375.S3 melanoma cells through catalyzation of caspase 3, raising p53, p21, and cellular Ca and weakening the mitochondrial membrane potential [14].
1.5 Clinical Application of Vitamin C in Melanoma Treatment
The fatality rate related to malignant melanoma has been a considerable burden even with today's medical development and technology. Crucial steps should be taken to combat this cancer and improve the overall survival rate. Vitamin C is anticipated to play a vital role in cancer treatment in general and malignant melanoma in particular due to the above observed molecular effects. Therefore, further studies need to be conducted to correctly evaluate vitamin C's role in transforming the genetic nature of melanoma. This review article considers different types of research that investigate vitamin C's role as adjuvant therapy in the treatment of melanoma.
2. Review
2.1 Discussion
The mortality rate from melanoma is worsening more than ever because of the lack of curable and efficient treatment options. This review focuses on vitamin C’s role, that has been tested in several studies, in the treatment of cutaneous melanoma. Our databases included PubMed, which yielded 136 studies related to vitamin C using the keywords "vitamin C" and "treatment of malignant melanoma" alone or in combination. We included studies between 1990 to 2020, abstract and free full-text papers. Our search also included human and animal studies. After manually reviewing each paper for relevance to our topic, we were left with 11 papers. All the obtained articles were in English, and none of them is duplicated.
2.2 Epigenetic Programming of Melanoma by Vitamin C
Melanoma and other neoplasms have been marked by deprivation of 5hmc [13, 15-18]. The reduced level of Ten-eleven Translocation (TET) family dioxygenase contributed enormously to the suppression of 5hmc as TET enzymes stimulate its production [13]. This review article investigated whether vitamin C can re-program the epigenetic content of melanoma cells by reversing its pathophysiology. A study in 2015 by Gustafson et al. was conducted using in vitro testing of basically cultivated normal human cells and multiple melanoma cells utilizing qRT-PCR (Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction) [13]. It concluded that a small amount of vitamin C at bodily need could substitute TETs in repairing 5hmc levels in melanoma cells (Table 1) [13]. It also proved that vitamin C at this small dose could modify A2058 melanoma cells' aggressive pattern by hampering their relocation and impeding anchorage-independent proliferation [13]. This research shows us the effect of a water-soluble vitamin on cancer cells. However, the limitation of this study is that it was conveyed in a stimulating environment rather than in real patients.
Another in vitro study conducted by Venturelli et al. in 2014 revealed that ascorbate's blood level is usually lesser in stage 4 melanoma patients than in the average population, and melanoma treatments make it even worse [19,20]. Their study also clarified that modifying the epigenetic component of melanoma cells can be attained through injecting a large amount of ascorbate intravenously [19]. It showed that BLM melanoma cells entered into two apoptosis levels within 12 hours apart following 8mM ascorbate dose [19]. Moreover, this dose obstructs DNA methyl transferase (DNMT) action in melanoma cells (Table 1) [19]. We can see the tremendous prospect that vitamin C could play in modifying how we treat cancer. Again this study was conducted in vitro, which is a limitation, as we require evidence-based trials to demonstrate vitamin C efficacy.
An exploratory study by Cavicchio et al. in 2017 showed that potassium ascorbate with ribose (PAR) could improve the expression of Connexin 43 on A375 melanoma cells in a 2D sample, which consequently led to blocking cell growth (Table 1) [21]. This result was also verified in 3D sample testing [21]. This kind of research spots light on how PAR might impact the changing genetic behavior of melanoma. Nonetheless, experimental studies have different conditions from that of real patients, which could be considered a weak area of this study.
Table 1: Description of selected studies on epigenetic programming
5hmc: 5-hydroxymethycystosine, DNMT: DNA methytransferase, PAR: potassium ascorbate with ribose
|
Study |
Year |
Conclusion |
|
Gustafson et al. [13] |
2015 |
Vitamin C rebuilds 5hmc in melanoma cells and changes the physical composition of melanoma |
|
Venturelli et al. [19] |
2014 |
There is a surge of BLM melanoma cells in apoptosis, which impedes DNMT in melanoma cells |
|
Cavicchio et al. [21] |
2017 |
PAR raises Connex in 43 levels leading to hindering of cell growth |
2.3 Vitamin C Causes Melanoma Cell Apoptosis
Studies done by Mark Levine's group at the National Cancer Institute proved that vitamin C could destroy malignant cells while being less harmful to normal body cells [22-25]. Some surveys tried to inspect how vitamin C can induce melanoma cells' death, making it less disruptive. As we can see in table 2, an in vitro study by Chen et al. in 2019 found that the A375 melanoma cell line of humans was destroyed, and their proliferation was hindered when treated with vitamin C [22]. The cell death in the melanoma cell line was attributed to diminishing the mitochondrial membrane potential, stimulation of caspase-9 and caspase-3 and amplifying the proportion of Bax/Bcl-2 [22]. This paper reflects the high likelihood that vitamin C may be considered an anti-cancer treatment one day, provided that its apoptotic effect is being demonstrated in a clinical setting. This brings us to the point that although this study seems promising for the future of cancer therapy, more studies need to investigate vitamin C efficacy further.
Consistent with Chen et al. result, another study in an artificial environment setting by Mustafi et al. in 2017 also concluded that a physiological dose of ascorbate promoted Bax and caspases stimulation within the membrane of the mitochondria with Bcl-xl detachment from the membrane [26]. This effect is associated with cell death in A2058 melanoma cells due to the downgrading effect of ascorbate on Clusterin (CLU) gene expression (Table 2) [26]. The similarity of these two studies in some aspects asserts that vitamin C could be a potential apoptotic agent that can reverse various pathological conditions via catalyzing the Bax and caspases processes. However, the limitation of every in vitro study is that it still lacks the in vivo factors that display different biological interactions with various environmental and genetic elements.
Table 2: Selected studies on melanoma cell apoptosis
|
Study |
Year |
Conclusion |
|
Chen et al. [22] |
2019 |
Vitamin C results in apoptosis of A375 melanoma cell line |
|
Mustafi et al. [26] |
2017 |
Ascorbate produces cell death in A2058 melanoma cells |
2.4 Sensitizing of Melanoma cells by Vitamin C
The anti-tumor features of bromodomain and extra terminal inhibitors (BETi) have created motivation and demand for researchers to assess its strength and effectiveness in treating cancers [27,28]. Mustafi et al. in 2018 tested a group of cultivated melanoma cells where some of the cells were exposed to ascorbate while others were not [27]. Their study demonstrated that ascorbate improved BETi effectiveness by minimizing histone H4 acetylation, and thus bromodomain (BRD) proteins will be left with fewer binding spots (table 3)[27]. Ascorbate could facilitate the way that other chemotherapy and immunotherapy work making their job easier.
The worst scenarios of cancer attacking various structures are adherence to other cells, downregulation of extracellular matrix (ECM) and moving freely between broken matrix [29,30]. Ascorbic acid synthesized in many animals is essential for collagen manufacturing and ECM integrity [29,31]. Cha et al. conducted an animal study in 2013 where they compared the impact of ascorbate supplementary on female gluconolactone oxidase (Gulo) knockout mice versus a control group of mice with no ascorbate in their diet [29]. They were further subdivided into two more groups; BT6FO melanoma was administered to one group while 4T breast cancer cells were given to the second group [29]. The study indicated that the melanoma group showed an enormous drop in cancer metastases, interleukin (IL)-6 and vascular endothelial growth factor (VEGF) compared to the ascorbate deficient mice (Table 3) [29]. The breast cancer group revealed a fall in IL-6 amount and cancer mass and proliferation [29]. Although this study explored multiple areas in the vitamin C mechanism of action in tumors, a comprehensive case-control study in humans would give a thorough understanding of how ascorbate could fight these tumor cells.
Impressively, an in vitro study by Tremante et al. in 2015 stated that negligible apoptosis causing dose of 1.5 µM Alpha-tocopheryl succinate (Alpha-TOS), 0.2 µM vitamin K3 (VK3) and 20 µM ascorbic acid (AA) admixture could stimulate natural killer (NK) cell ligands [32]. It also demonstrated that it could induce apoptosis in melanoma cells [32]. The idea of having naturally inter-mixture supplements that can combat cancer by positively motivating our immune system to fight it sounds encouraging; however, this study's limitation is the down-regulation of NK ligands in a few melanoma line samples instead of up-regulating them makes it a feeble proposal.
Several research pieces linked cancer AA amount, low HIF-1 alpha and improving relapse-free survival [33,34]. Slackened AA level within a cancer cell is postulated to take part in the lack of HIF-1 alpha balancing in melanoma [33]. A study by Miles et al. in 2015 investigated cultured melanoma cell lines, and they advocated the effect of AA and ascorbate 2-phosphate (A2P) on shrinking HIF-1 alpha protein and transcription in widely spread melanoma cells [33]. Despite the encouraging results of AA and A2P in organizing HIF-1 alpha in tumor cells, this experiment's testing environment makes the suggested hypothesis weak unless empowered by evidence-based studies.
A review article by Wang et al. in 2018 was compatible with what we had obtained from other studies for this paper. They highlighted vitamin C's role in attenuating B16FO melanoma metastasis and halting 4T1 breast cancer proliferation in mice [29,35]. The article also mentioned how vitamin C could minimize cancer size and aggressiveness in mice via halting HIF-1 alpha transcriptional activity [33,35,36]. The study further emphasized that vitamin C boosts 5hmc in melanoma cells, consistent with our previous findings [13,35]. Contrary to the common notion, their study stated that caucasian females who consume a large amount of vitamin C from food have a high chance of having melanoma and that vitamin C level can solely be raised via intravenous administration (Table 3) [35,37,38]. Although this article has some identical findings with ours, the last part disregards the importance of vitamin C, which we are trying to endorse as conventional medicine in treating melanoma. Additionally, we question their conclusions, as vitamin C has already demonstrated multiple anticancer features in several research papers.
Table 3: Description of selected studies on melanoma cell sensitization
BETi: Bromodomain and extraterminal inhibitors, glu KO: gulonolactone oxidase knockout, Alpha-TOS: Alpha-tocopheryl succinate, VK3: Vitamin K3, AA: Ascorbic Acid, NK: natural killer, A2P: ascorbate 2-phosphate, mets: metastases, HIF-1alpha : Hypoxia-Inducible Factor-1alpha
|
Study |
Year |
Conclusion |
|
Mustafi et al. [27] |
2018 |
Ascorbate makes melanoma cells more sensitive to BETi treatment |
|
Cha et al. [29] |
2013 |
Supplementary ascorbate impeded metastasis, cancer proliferation and cytokine production in gluo KO mice |
|
Tremante et al. [32] |
2015 |
Alpha-TOS, VK3 and AA admixture can stimulate NK |
|
Miles et al. [33] |
2015 |
AA and A2P diminishes HIF-1 alpha role in melanoma |
|
Wang et al. [35] |
2018 |
Vitamin C reduces melanoma mets and reduces breast cancer in mice by decreasing HIF-1 alpha and improving 5hmc. Contradicting view: higher risk of melanoma in white women taking large amounts of vitamin C |
2.5 Vitamin C as Adjuvant Therapy to IL-2
Interleukin-2 (IL-2) is one of the most archaic tumor immunotherapies that showed efficacy in improving high-stage melanoma and renal cell carcinoma outcomes. However, its adverse side effects make it less desirable as a cancer therapy [39]. A review article by Wagner et al. in 2014 suggested the utilization of intravenous AA as adjuvant therapy to IL-2 [39]. It supported its view that IL-2 results in a critical plunge of blood AA content, and giving AA injection will spike it and improve cytotoxicity associated with IL-2 as well (Table 4) [39]. Also, vitamin C has a frank anticancer property, making it more favorable instead of other agents introduced to control IL-2 toxicity and might inhibit its treatment mechanism [39]. With its optimistic view about vitamin C as an adjuvant to IL-2, this article requires supporting clinical and practical investigating studies that reinforce its speculation.
Table 4: Depiction on the selected study on adjuvant therapy
IL-2: Interleukin-2
|
Study |
Year |
Conclusion |
|
Wagner et al. [39] |
2014 |
Supporting the use of vitamin C as adjuvant treatment to IL-2 to lower the noxious side effects of IL-2 |
3. Limitations
Our data source comprises in vitro studies, one experimental animal study and two review articles. This entails the difficulty we have faced in making a factual and concise evidence-based outcome regarding vitamin C in its use in cancer therapy. Nevertheless, to support our hypothetical theory about vitamin C in treating melanoma as monotherapy or adjuvant therapy, we still need a considerable amount of retrospective and prospective researches in humans.
4. Conclusion
This review article intended to study the potential use of vitamin C as an adjuvant treatment for malignant melanoma. It was shown that vitamin C could rearrange the epigenetic pattern of melanoma cells by re-establishing its 5hmc content, modifying the harshness and severity of the disease, blocking its DNMT activity and enhancing connexin 43 expression. Vitamin C also causes apoptosis of A375 and A2058 melanoma cell lines via activation of the caspase pathway and rising bax/bcl-2 ratio. It sensitizes melanoma by slumping HIF-1 alpha protein and transcriptional factor, decreasing inflammatory cytokines and reducing cancer metastasis. Moreover, it ameliorates the anti-tumor BETi effect and lowers IL-2 toxicity. Our future recommendations involve more longitudinal experimental research with clinical trials that test the efficacy, safety, and mechanism of vitamin C in real melanoma patients. Additionally, providing more accurate findings and eliminating bias.
Currently, vitamin C is suggested to be given as a supplement in any melanoma patients as part of their treatment regime and perhaps consider intravenous vitamin C in the near future when clinical evidence from various studies supports its use. We think this review article can benefit the studies already devoted to vitamin C use as anticancer therapy. It highlights the role that vitamin C could play in transforming the way we treat malignant melanoma, especially since there is no definite cure, and the number of patients affected by melanoma is increasing steeply.
References
- (Centers for Disease Control and Prevention: 2020, Accessed). Accessed: December 12, 2020.
- Bichakjian CK, Halpern AC, Johnson TM et al. M., … American Academy of Dermatology. Guidelines of care for the management of primary cutaneous melanoma. American Academy of Dermatology. Journal. of the American Academy of Dermatology 65 (2011):1032-1047.
- Garbe C, Eigentler TK, Keilholz U et al. Systematic review of medical treatment in melanoma: current status and future prospects. The Oncologist 16:5-24.
- Molife R, Hancock BW. Adjuvant therapy of malignant melanoma. Crit Rev Oncol Hematol 44 (2002):81-102.
- Wheatley K, Ives N, Hancock B et al. Does adjuvant interferon-alpha for high-risk melanoma provide a worthwhile benefit? A meta-analysis of the randomised trials. Cancer Treat Rev 29 (2003):241-252.
- Mocellin S, Pasquali S, Rossi CR, et al. Interferon alpha adjuvant therapy in patients with high-risk melanoma: a systematic review and meta-analysis. J Natl Cancer Inst. 102 (2010):493-501.
- Petrella TM, Fletcher GG, Knight G et al. Systemic adjuvant therapy for adult patients at high risk for recurrent cutaneous or mucosal melanoma: an Ontario Health (Cancer Care Ontario) clinical practice guideline. Current oncology (Toronto, Ont.) 27:43-52.
- Wilson MK, Baguley BC, Wall C et al. Review of high-dose intravenous vitamin C as an anticancer agent: High-dose IV vitamin C as anticancer agent. Asia-Pacific Journal of Clinical Oncology 10:22-37.
- Cameron E, Pauling L. Supplemental ascorbate in the supportive treatment of cancer: Prolongation of survival times in terminal human cancer. Proceedings of the National Academy of Sciences. of the United States of America 73:3685-3689.
- Kuphal S, Winklmeier A, Warnecke C et al. Constitutive HIF-1 activity in malignant melanoma. European Journal of Cancer (Oxford. England 46 (1990):1159-1169.
- Lian CG, Xu Y, Ceol C et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. G Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM (2012):5-150.
- Fischer AP, Miles SL. Ascorbic acid, but not dehydroascorbic acid increases intracellular vitamin C content to decrease Hypoxia Inducible Factor -1 alpha activity and reduce malignant potential in human melanoma. Biomed Pharmacother. 86 (2017):502-513.
- Gustafson CB, Yang C, Dickson KM et al. Epigenetic reprogramming of melanoma cells by vitamin C treatment. Clinical epigenetics 7:51-10.
- Lin SY, Lai WW, Chou CC et al. Sodium ascorbate inhibits growth via the induction of cell cycle arrest and apoptosis in human malignant melanoma A375.S2 cells. Melanoma Research 16:509-519.
- Ko M, Huang Y, Jankowska AM et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 468:839-843.
- Xu W, Yang H, Liu Y et al. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell 19:17-30.
- Lian CG, Xu Y, Ceol C, et al. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell 150:1135-1146.
- Gambichler T, Sand M, Skrygan M, et al.: Loss of 5-hydroxymethylcytosine and ten-eleven translocation 2 protein expression in malignant melanoma. Melanoma Research 23:218-220.
- Venturelli S, Sinnberg TW, Berger A et al. Epigenetic impacts of ascorbate on human metastatic melanoma cells. Frontiers in oncology 4:227.
- Schleich T, Rodemeister S, Venturelli S et al. Decreased plasma ascorbate levels in stage IV melanoma patients. Metabolism and Nutrition in Oncology. 1:2-6.
- Cavicchio C, Benedusi M, Pambianchi E, et al.: Potassium Ascorbate with Ribose: Promising Therapeutic Approach for Melanoma Treatment. Oxidative medicine and cellular longevity 2017:4256519.
- Chen XY, Chen Y, Qu CJ et al. Vitamin C induces human melanoma A375 cell apoptosis via Bax- and Bcl-2-mediated mitochondrial pathways. Oncology letters 18:3880-3886.
- Chen Q, Espey MG, Krishna MC et al. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proceedings of the National Academy of Sciences. of the United States of America 102:13604-13609.
- Chen Q, Espey MG, Sun AY et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proceedings of the National Academy of Sciences. of the United States of America 104:8749-8754.
- Chen Q, Espey MG, Sun AY, et al. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proceedings of the National Academy of Sciences. of the United States of America 105:11105-11109.
- Mustafi S, Sant DW, Liu ZJ, et al.: Ascorbate induces apoptosis in melanoma cells by suppressing Clusterin expression. Scientific reports 7 1038:41598-017.
- Mustafi S, Camarena V, Volmar CH et al. Vitamin C Sensitizes Melanoma to BET Inhibitors. Cancer research 78:572-583.
- Jain AK, Barton MC: Bromodomain histone readers and cancer. Journal of Molecular Biology 429:2003-2010.
- Cha J, Roomi MW, Ivanov V et al. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. International journal of oncology 42:55-64.
- Fidler IJ. Molecular biology of cancer: invasion and metastasis. In: De Vita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. 5th edition. Lippincott-Raven; Philadelphia PA (1997):135-152.
- Rath M, Pauling L. Plasmin-induced proteolysis and the role of apoprotein(a), lysine and synthetic analogs. Orthomol Med. 1992;7:17-23.
- Tremante E, Santarelli L, Lo Monaco E et al. Sub-apoptotic dosages of pro-oxidant vitamin cocktails sensitize human melanoma cells to NK cell lysis. Oncotarget 6:31039-31049.
- Miles SL, Fischer AP, Joshi SJ et al. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC cancer 15 1186:12885-015.
- Kuiper C, Dachs GU, Munn D et al. Increased tumor ascorbate is associated with extended disease-free survival and decreased hypoxia-inducible factor-1 activation in human colorectal cancer. Frontiers in Oncology 4:10.
- Wang K, Jiang H, Li W et al. Role of Vitamin C in Skin Diseases. Frontiers. in physiology 9:819.
- Cha J, Roomi MW, Ivanov V et al. Ascorbate depletion increases growth and metastasis of melanoma cells in vitamin C deficient mice. Exp Oncol 33:226-230.
- Feskanich D, Willett WC, Hunter DJ et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer 88 1381:1387.
- Ohno S, Ohno Y, Suzuki N et al. High-dose vitamin C (Ascorbic Acid) therapy in the treatment of patients with advanced cancer. Anticancer Res 29:809-816.
- Wagner SC, Markosian B, Ajili N et al. Intravenous ascorbic acid as an adjuvant to interleukin-2 immunotherapy. Journal of translational medicine 12:127.
