Conservation of the Functional Properties of Okra Powder by Local Storage Techniques
Article Information
Elizabeth Ufuoma Etaware1, Peter Mudiaga Etaware2*
1Department of Food Technology, School of Technology, Yaba College of Technology, Lagos, Nigeria
2Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Nigeria
*Corresponding author: Peter Mudiaga Etaware, Department of Botany, Faculty of Science, University of Ibadan, Ibadan, Nigeria
Received: 07 December 2019; Accepted: 18 December 2019; Published: 21 December 2019
Citation:
Elizabeth Ufuoma Etaware, Peter Mudiaga Etaware. Conservation of the Functional Properties of Okra Powder by Local Storage Techniques. Journal of Nanotechnology Research 1 (2019): 136-143
View / Download Pdf Share at FacebookAbstract
Okra is an essential vegetable plagued by postharvest losses with a total of 25-30% loss accrued between handling and consumption. Drying is one process that can reduce postharvest losses. Sadly, several chemicals and structural changes occur during drying, which are not accounted for, and some moisture mediated biological reactions are halted. The current research was poised to determine the functionality of the functional properties of okra powder under diverse processing techniques/local storage conditions. Standard food assessment techniques were conducted using the principles of AOAC. There was no significant difference (P<0.05) in the water absorption capacity [WAC] and water solubility index [WSI] of the freshly prepared okra powder and those stored in cupboards and on the shelf for one month. The bulk density of Kubewa okra powder [stored on the Shelf (0.76cm3) and in the Cupboard (0.76cm3), respectively] showed no significant difference with that of the freshly prepared okra powder [sun-dried (0.79cm3) and oven-dried (0.78cm3), respectively] regardless of the drying technique used in the removal of moisture from the raw okra samples. The current research was able to determine a suitable storage method for processing okra powder in places where there are power insurgences, poor urban amenities or rural areas with poor market facilities.
Keywords
Postharvest, Monosaccharide, Sundried, Pulverized Okra, Okra Powder
Postharvest articles Postharvest Research articles Postharvest review articles Postharvest PubMed articles Postharvest PubMed Central articles Postharvest 2023 articles Postharvest 2024 articles Postharvest Scopus articles Postharvest impact factor journals Postharvest Scopus journals Postharvest PubMed journals Postharvest medical journals Postharvest free journals Postharvest best journals Postharvest top journals Postharvest free medical journals Postharvest famous journals Postharvest Google Scholar indexed journals Monosaccharide articles Monosaccharide Research articles Monosaccharide review articles Monosaccharide PubMed articles Monosaccharide PubMed Central articles Monosaccharide 2023 articles Monosaccharide 2024 articles Monosaccharide Scopus articles Monosaccharide impact factor journals Monosaccharide Scopus journals Monosaccharide PubMed journals Monosaccharide medical journals Monosaccharide free journals Monosaccharide best journals Monosaccharide top journals Monosaccharide free medical journals Monosaccharide famous journals Monosaccharide Google Scholar indexed journals Sundried articles Sundried Research articles Sundried review articles Sundried PubMed articles Sundried PubMed Central articles Sundried 2023 articles Sundried 2024 articles Sundried Scopus articles Sundried impact factor journals Sundried Scopus journals Sundried PubMed journals Sundried medical journals Sundried free journals Sundried best journals Sundried top journals Sundried free medical journals Sundried famous journals Sundried Google Scholar indexed journals Pulverized Okra articles Pulverized Okra Research articles Pulverized Okra review articles Pulverized Okra PubMed articles Pulverized Okra PubMed Central articles Pulverized Okra 2023 articles Pulverized Okra 2024 articles Pulverized Okra Scopus articles Pulverized Okra impact factor journals Pulverized Okra Scopus journals Pulverized Okra PubMed journals Pulverized Okra medical journals Pulverized Okra free journals Pulverized Okra best journals Pulverized Okra top journals Pulverized Okra free medical journals Pulverized Okra famous journals Pulverized Okra Google Scholar indexed journals Okra Powder articles Okra Powder Research articles Okra Powder review articles Okra Powder PubMed articles Okra Powder PubMed Central articles Okra Powder 2023 articles Okra Powder 2024 articles Okra Powder Scopus articles Okra Powder impact factor journals Okra Powder Scopus journals Okra Powder PubMed journals Okra Powder medical journals Okra Powder free journals Okra Powder best journals Okra Powder top journals Okra Powder free medical journals Okra Powder famous journals Okra Powder Google Scholar indexed journals physiological articles physiological Research articles physiological review articles physiological PubMed articles physiological PubMed Central articles physiological 2023 articles physiological 2024 articles physiological Scopus articles physiological impact factor journals physiological Scopus journals physiological PubMed journals physiological medical journals physiological free journals physiological best journals physiological top journals physiological free medical journals physiological famous journals physiological Google Scholar indexed journals Pulverization articles Pulverization Research articles Pulverization review articles Pulverization PubMed articles Pulverization PubMed Central articles Pulverization 2023 articles Pulverization 2024 articles Pulverization Scopus articles Pulverization impact factor journals Pulverization Scopus journals Pulverization PubMed journals Pulverization medical journals Pulverization free journals Pulverization best journals Pulverization top journals Pulverization free medical journals Pulverization famous journals Pulverization Google Scholar indexed journals absorption capacity articles absorption capacity Research articles absorption capacity review articles absorption capacity PubMed articles absorption capacity PubMed Central articles absorption capacity 2023 articles absorption capacity 2024 articles absorption capacity Scopus articles absorption capacity impact factor journals absorption capacity Scopus journals absorption capacity PubMed journals absorption capacity medical journals absorption capacity free journals absorption capacity best journals absorption capacity top journals absorption capacity free medical journals absorption capacity famous journals absorption capacity Google Scholar indexed journals physicochemical articles physicochemical Research articles physicochemical review articles physicochemical PubMed articles physicochemical PubMed Central articles physicochemical 2023 articles physicochemical 2024 articles physicochemical Scopus articles physicochemical impact factor journals physicochemical Scopus journals physicochemical PubMed journals physicochemical medical journals physicochemical free journals physicochemical best journals physicochemical top journals physicochemical free medical journals physicochemical famous journals physicochemical Google Scholar indexed journals molecules articles molecules Research articles molecules review articles molecules PubMed articles molecules PubMed Central articles molecules 2023 articles molecules 2024 articles molecules Scopus articles molecules impact factor journals molecules Scopus journals molecules PubMed journals molecules medical journals molecules free journals molecules best journals molecules top journals molecules free medical journals molecules famous journals molecules Google Scholar indexed journals
Article Details
1. Introduction
Vegetables and fruits are essential components of human diet required in large proportions to generate biofuel to power most of the complex physiological and metabolic reactions in the body [1]. Okra [Abelmoschus esculentus L. (Moench)] is an essential vegetable [2] plagued by postharvest losses with a total of 25-30% loss accrued between handling and consumption [1]. In order to prevent postharvest loss, okra pods are usually dried to remove excess moisture; which will prevent growth and proliferation of organisms that can cause decay, and also halt most of the moisture-mediated deteriorative reactions during preservation [1]. Drying technique is probably the oldest and the most important method of food preservation practiced by humans from time immemorial, with substantial decrease in weight/volume, reduced packaging slots, storage and transportation costs, and enhanced shelf life under optimum storage conditions [3]. Drying (systematic dehydration by a coordinated water removal process) can alter the structural (morphological), biochemical, proximate, vitamins, minerals and physicochemical components of the preserved food substance(s), with a complete modification of the product’s quality and composite component’s quantity compared to the freshly harvested crops [1]. Mucilage (a plant hydrocolloid) is a polymer of distinct/different monosaccharide units [4] or a polysaccharide with high molecular weight and numerous hydrophilic components capable of sublimation in water due to its ability to form effective bonds with water molecules. Thus, mucilage can be used as food additives to improve food quality, optimize food stability, increase food texture and enhance food appearance [1]. The chemical composition, molecular structure, monosaccharide sequence, and glycoside linkages in the backbone structure and the side chains of natural plant mucilage such as okra are some of the factors that can affect its functional properties [5]. Therefore, this research seeks to carefully analyse the functional properties of dried, pulverized okra pods preserved under diverse storage conditions with a view to identify the best storage form for optimization of the colloidal, emulsifying and functional properties of okra mucilage.
2. Methodology
2.1 Sample collection
Two (2) local varieties of okra were used for this experiment. A total of 5kg of fresh, healthy (Disease free) and matured green okra pods from each variety was randomly collected from three (3) major distributors in mile 12 market, Lagos State, Nigeria. The collected samples were physically screened for disease infection, decay symptoms, insects and pests/rodents’ encroachment, discolouration and mouldiness, aseptically packaged in sterile collecting vessels, labelled accordingly and transported to the Food Technology laboratory of the Department of Food Technology, School of Technology, Yaba College of Technology, Yaba, Lagos State, Nigeria for further diagnostic analysis and other prognostic measures.
2.2 Sorting
Okra pods showing signs of disease infection, insects and pests/rodents’ encroachment, mouldiness, discolouration and other morphological symptoms/signs were isolated and discarded. Fresh, healthy and viable green pods were thoroughly washed with warm sterile distilled water and Teepol (A mild but highly effective liquid detergent recommended for food industries) to remove germs and contaminants from the okra pod samples picked up within the market environment, rinsed in several changes of sterile distilled water to remove excess detergent, plotted using absorbent papers and air-dried in a laminar air flow chamber for 1hr.
2.3 Sample preparation
The method adapted for sample preparation was as described by Etaware and Etaware [6].
2.3.1 Blanching: The air-dried samples were blanched using hot water at 100oC for 15mins so as to deactivate enzyme producing cells and destroy chlorophyll pigments present in the green pods in order to avoid self-induced senescence, which might result in nutrient depletion, loss of vitamins, demineralization, deactivation of important physicochemical components and the denaturing of other medically important constituents.
2.3.2 Shredding: The blanched okra pods were shredded to a thickness of about 2mm radial diameter using a manual shredding machine.
2.3.3 Preservation technique: The method of Etaware and Etaware [6] was used. The shredded samples were weighed and 5kg from each sample was sundried between 11am and 4pm in a control environment (Microcosm) for one week. Also, 5kg of the remaining samples from each variety was oven dried at 50oC for 48hrs.
2.3.4 Dry weight determination: Random samples from each drying techniques were collected intermittently, physically assessed and weighed (i.e. daily measurements for the sun-dried samples, while the oven dried samples were measured every 4hrs). At constant weight measurement after three (3) successive trials, the drying of okra pods was terminated. The dried okra pods were packed, labelled according to their varieties and stored in a controlled environment to avoid contamination.
2.3.5 Pulverization: After drying using the various preservation techniques, the samples were pulverized using the Laboratory Disk Mill.
2.4 Determination of the functional properties of okra
2.4.1 Reconstitution index (RI): The reconstitution Index (RI) was determined by the method of Kulkarni et al. [7]. 100ml of sterile distilled water was measured into a clean sterilized measuring cylinder and 10g of dried pulverized okra was aseptically added to the liquid. The mixture was stirred vigorously using a sterile glass rod and allowed to stand in a water bath at 40oC for the 90s. The mixture was removed and aseptically transferred to a laminar air flow chamber where it was allowed to stand for 10mins. When a clear distinction between the supernatant and residue was observed, the volume of the residue (okra powder beneath the clear supernatant) was recorded and noted as the reconstitution index. The experiment was carried out in replicates.
2.4.2 Determination of bulk density (BD): The bulk density of equal volume of each okra powder was determined using the methods of AOAC [8]. The weight of the unloaded centrifuge tube was measured using a milligram capacity laboratory measuring scale and subsequently filled with the pulverized okra sample. The tube was tapped gently and loaded continuously until its compartment was filled to the brim. The tube together with the content was then weighed and the volume of the sample measured using a graduated measuring cylinder. The experiment was conducted in replicates and the bulk density was determined by the formula below:
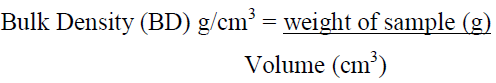
2.4.3 Determination of water absorption capacity (WAC): The Water Absorption Capacity (WAC) was determined using the modified method of Kohn et al. [9]. Dried pulverized okra sample weighing 1g was loaded into a centrifuge tube of known weight (Mo) and 10ml of sterile distilled water was added while the mixture was stirred intermittently to aid homogenization. The mixture was allowed to stand for 30mins and the weight of the final mixture was recorded (M1). The loaded tube was placed in the centrifuge and spin at 2,500rpm for 15mins. The supernatant was decanted into a sterile test tube and the weight of tube containing the sediment was again weighed (M2). WAC was estimated by the formula below:

Where
Mo = Weight of empty tube
M1 = Weight of loaded tube (Sample + 10ml DH20)
M2 = Weight of loaded tube (Sediments only)
2.4.4 Determination of water solubility index (WSI): The Water Solubility Index (WSI) was determined using a modification of the method described by Yousf et al. [10]. The amount of polysaccharide dissolved in water was determined by weighing the supernatant decanted from the WAC experiment and subtracting the weight of equal Volume of distilled water from the value obtained. WSI was determined as the percentage of solid okra powder dissolved in the supernatant. The formula given below was used to estimate %WSI.
WSI (%) = 
Where
Wo = Weight of Supernatant
W1 = Weight of equal Volume of distilled water
2.4.5 Data analysis: The data obtained from the experiment were in replicates and was represented as means and standard deviation in different tables. Quantitative data were analysed using COSTAT 6.451 analytical software and the homogeneity of statistical significant means was determined using Duncan Multiple Range Test (DMRT). Graphs and figures were extrapolated from Microsoft Excel workbook 2010 service pack.
3. Results
There was no significant difference (P<0.05) in the reconstitution index [RI] of the oven dried okra powder stored on the shelf for one month [Kubewa (93.50cm3), Ila-Iwo (92.50cm3), respectively] and the freshly prepared oven dried okra powder [Kubewa (93.00cm3), Ila-Iwo (95.00cm3), respectively] for both varieties as shown in Table 1. The sun dried Kubewa okra samples stored on the shelf for one month had significant decrease in its reconstitution index [92.00cm3] compared to the freshly prepared sundried Kubewa okra samples [96.00cm3]. All the samples stored in the cupboard showed significant decrease in their reconstitution index as shown in Table 1. There was no significant difference (P<0.05) in the water absorption capacity [WAC] and water solubility index [WSI] of the freshly prepared okra powder and those stored in the cupboard and on the shelf for one month as shown in Table 2 and 3. The bulk densities of all the okra samples were reduced during storage with the exception of Kubewa okra samples stored on the shelf and in the cupboard. The Kubewa okra powder [stored on Shelf (0.76cm3) and in the Cupboard (0.76cm3), respectively] showed no significant difference with that of the freshly prepared okra powder [sundried (0.79cm3) and oven-dried (0.78cm3), respectively] regardless of the drying technique used in the removal of moisture from the raw okra samples (Table4).
|
Storage |
Okra Variety |
Drying |
Reconstitution Index |
||
|
Type |
Duration |
Method |
Method |
(cm3) |
|
|
None [Control] |
0 Month |
None [Fresh Samples] |
Kubewa |
Sun |
96.00 ± 2.83a |
|
Ila-Iwo |
93.50 ± 0.71a-c |
||||
|
Kubewa |
Oven |
93.00 ± 1.41a-c |
|||
|
Ila-Iwo |
95.00 ± 1.41ab |
||||
|
Closed Storage |
1 Month |
Cupboard |
Kubewa |
Sun |
84.50 ± 0.71e |
|
Ila-Iwo |
85.50 ± 0.71e |
||||
|
Kubewa |
Oven |
88.50 ± 0.71d |
|||
|
Ila-Iwo |
89.00 ± 1.41d |
||||
|
Open Storage |
1 Month |
Shelf |
Kubewa |
Sun |
92.00 ± 1.41bc |
|
Ila-Iwo |
90.50 ± 0.71cd |
||||
|
Kubewa |
Oven |
93.50 ± 0.71a-c |
|||
|
Ila-Iwo |
92.50 ± 0.71bc |
||||
Means with the same alphabets down the COLUMN are not significantly different at P<0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only
Table 1: The reconstitution index of each variety of pulverized okra.
|
Storage |
Okra Variety |
Drying |
Water Absorption |
||
|
Type |
Duration |
Method |
Method |
Capacity (g) |
|
|
None [Control] |
0 Month |
None [Fresh Samples] |
Kubewa |
Sun |
9.77 ± 1.20ab |
|
Ila-Iwo |
10.57 ± 0.06a |
||||
|
Kubewa |
Oven |
10.07 ± 0.31ab |
|||
|
Ila-Iwo |
10.60 ± 0.03a |
||||
|
Closed Storage |
1 Month |
Cupboard |
Kubewa |
Sun |
9.71 ± 0.72ab |
|
Ila-Iwo |
10.57 ± 0.16a |
||||
|
Kubewa |
Oven |
10.17 ± 0.08ab |
|||
|
Ila-Iwo |
10.03 ± 0.03ab |
||||
|
Open Storage |
1 Month |
Shelf |
Kubewa |
Sun |
10.65 ± 0.04a |
|
Ila-Iwo |
9.82 ± 0.14ab |
||||
|
Kubewa |
Oven |
9.39 ± 0.02b |
|||
|
Ila-Iwo |
9.81 ± 0.13ab |
||||
Means with the same alphabets down the COLUMN are not significantly different at P<0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only
Table 2: The water holding/absorption capacities of the analysed okra samples.
|
Storage |
Okra Variety |
Drying |
Water Solubility |
||
|
Type |
Duration |
Method |
Method |
Index (%) |
|
|
None [Control] |
0 Month |
None [Fresh Samples] |
Kubewa |
Sun |
12.35 ± 11.95ab |
|
Ila-Iwo |
4.35 ± 0.64b |
||||
|
Kubewa |
Oven |
9.30 ± 3.11ab |
|||
|
Ila-Iwo |
4.00 ± 0.28b |
||||
|
Closed Storage |
1 Month |
Cupboard |
Kubewa |
Sun |
12.90 ± 7.21ab |
|
Ila-Iwo |
4.30 ± 1.56b |
||||
|
Kubewa |
Oven |
8.35 ± 0.78ab |
|||
|
Ila-Iwo |
9.70 ± 0.28ab |
||||
|
Open Storage |
1 Month |
Shelf |
Kubewa |
Sun |
3.55 ± 0.35b |
|
Ila-Iwo |
11.80 ± 1.41ab |
||||
|
Kubewa |
Oven |
16.15 ± 0.21a |
|||
|
Ila-Iwo |
11.70 ± 1.56ab |
||||
Means with the same alphabets down the COLUMN are not significantly different at P<0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only
Table 3: The water solubility index of the stored okra powder.
|
Storage |
Okra Variety |
Drying |
Bulk Density |
||
|
Type |
Duration |
Method |
Method |
(g/cm3) |
|
|
None [Control] |
0 Month |
None [Fresh Samples] |
Kubewa |
Sun |
0.79 ± 0.01a |
|
Ila-Iwo |
0.75 ± 0.01c-e |
||||
|
Kubewa |
Oven |
0.78 ± 0.01ab |
|||
|
Ila-Iwo |
0.74 ± 0.02c-f |
||||
|
Closed Storage |
1 Month |
Cupboard |
Kubewa |
Sun |
0.69 ± 0.01i |
|
Ila-Iwo |
0.70 ± 0.01hi |
||||
|
Kubewa |
Oven |
0.76 ± 0.01a-c |
|||
|
Ila-Iwo |
0.71 ± 0.01g-i |
||||
|
Open Storage |
1 Month |
Shelf |
Kubewa |
Sun |
0.76 ± 0.01a-c |
|
Ila-Iwo |
0.72 ± 0.01f-h |
||||
|
Kubewa |
Oven |
0.73 ± 0.02d-g |
|||
|
Ila-Iwo |
0.73 ± 0.01d-g |
||||
Means with the same alphabets down the COLUMN are not significantly different at P<0.05 using Duncan Multiple Range Test (DMRT) for separation of statistically significant means. Data collected were represented as “Means ± SD” only
Table 4: The bulk density values of the stored okra samples.
4. Discuss
The reconstitution index [RI] of the oven-dried okra samples stored on the shelf was unaffected by the long duration of storage. The long term preservation of the value of the reconstitution index during storage is pertinent in the formation of quality mucilage texture by increasing its ability to absorb water and swell. Also, the enhanced gelation properties of the mucilage enables the chemical components of the okra samples to readily bind with water molecules, conferring a smooth slurry texture to the mucilage. This observation was in line with the research findings of Adebowale et al. [11] who reiterated that the reconstitution index play a key role in the texture and digestibility as well as the usefulness of starch based food products. The water absorption capacity (WAC) and the water solubility index (WSI) were also unaffected by the long term storage regardless of the storage technique applied. WAC and WSI are important functional properties that helps increase the water binding potentials of the molecules of the okra mucilage, conferring good product quality, better gel formation and also, help to preserve the medicinal, nutritional, mineral and vitamin contents of the stored okra powder. This research was in harmony with the findings of Yousf et al. [10] who stated that WSI measures the amount of soluble components released from the starch after extrusion, and that a very high WSI is an indicator of good starch digestibility as it implies the extent of gelatinization and the level of dextrin readily released by the food product. Also, Kohn et al. [9] described the water absorption capacity (WAC) as an indispensable functional property that basically consists in the method for quantifying the water retained by food substances after adding water or an aqueous solution to it. He further stated that this property is economically important because it affects the yield and quality of cooked hams, sausages and other meat products. The Kubewa okra powder was more resilient in retaining the standard of its bulk density all through the experiment. Bulk density is an important physical component of the okra mucilage that plays a key role in the texture, quality and particle fluidity of the okra mucilage. This was in agreement with the research work of Senadeera [12] who studied the effects of drying methods on bulk densities of several food crops, and came to the conclusion that bulk density is very important in food storage and packaging because it is a measure of the food particles dimensions and component’s compactness.
5. Conclusion
The current research was able to determine suitable storage method for processed okra powder in places where there are power insurgences, poor urban amenities or rural areas with poor market facilities. The local methods described in this research are very useful in preserving the functional properties of the processed okra powder, which can also indirectly serve as a forum for the conservation of other beneficial properties of the okra powder.
References
- Wankhade P K, Sapkal R S, Sapkal V S. Drying characteristics of okra slices using different drying methods by comparative evaluation. Proceedings of the World Congress on Engineering and Computer Science. San Francisco, USA 2 (2012): 1-4.
- Roy A, Shrivastava S L, Mandal S M. Functional properties of Okra [Abelmoschus esculentus L. (Moench)]: traditional claims and scientific evidences. Plant Science Today 1 (2014): 121-130.
- Mujumdar A S. Handbook of industrial drying (second edition). New York: Marcel Dekker (1995): 24.
- Deogade U M, Deshmukh V N, Sakarkar D M. Natural gums and gum’s in NDDS: Applications and recent approaches. International Journal of Pharmaceutical and Technological Research 4 (2012): 799-814.
- Mirhosseini H, Amid B T. A review study on chemical composition and molecular structure of newly plant gum exudates and seed gums. Food Research International 46 (2012): 387-398.
- Etaware P M, Etaware E U. The effects of food processing techniques on nutrient composition of okra (Abelmoschus esculentus L. Moench). International Journal of Innovative Research and Advance Studies 6 (2019): 90-94.
- Kulkarni K D, Kulkarni D N, Ingle U M. Sorghum malted and soya bean weaning food formulations: preparation, functional properties and nutritive value. Food Nutrition Bulletin 13 (1991): 322-327.
- A O A C. Official methods of Analysis of the Association of Official Analytical Chemists 15th Edition. Kenneth Helrich, Washington DC 2 (2005): 125- 576
- Köhn C R, Fontoura A M, Kempka A P, Demiate I M, Kubota E H, Prestes R C. Assessment of different methods for determining the capacity of water absorption of ingredients and additives used in the meat industry. International Food Research Journal 22 (2015): 356-362.
- Yousf N, Nazir F, Salim R, Ahsan H, Sirwal A. Water solubility index and water absorption index of extruded product from rice and carrot blend. Journal of Pharmacognosy and Phytochemistry 6 (2017): 2165-2168.
- Adebowale A A, Adegoke M T, Sanni S A, Agegunwa M O, Fetuga G O. Functional properties and biscuit making potentials of Sorghum-Wheat flour composite. American Journal of Food Technology 7(2012): 372-379.
- Senadeera W. Density variation of different shaped food particulates in fluid bed drying: Empirical models. Journal Agricultural and Marine Sciences 14 (2009): 27-34.
