Concurrent Versus Sequential Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer-A Comparative Study
Article Information
Aditi Paul Chowdhury1, Md. Ruhul Amin Bhuiyan2, Md. Ershadul Haque3, Abdul Mannan4, Abdullah Al Mamun5
1Junior Consultant, Department of Oncology, Ahsania Mission Cancer & General Hospital, Dhaka, Bangladesh
2Assistant Professor, Department of Oncology, North East Medical College Hospital, Sylhet, Bangladesh
3Medical Officer, Department of Radiotherapy, Rangpur Medical College Hospital, Rangpur, Bangladesh
4Junior Consultant, Labaid Cancer and Superspeciality Center, Dhaka, Bangladesh
5Specialist, Labaid Cancer and Superspeciality Center, Dhaka, Bangladesh
*Corresponding Author: Aditi Paul Chowdhury, Junior Consultant, Department of Oncology, Ahsania Mission Cancer & General Hospital Dhaka, Bangladesh
Received: 24 July 2023; Accepted: 09 August 2023; Published: 09 October 2023
Citation: Aditi Paul Chowdhury, Md. Ruhul Amin Bhuiyan, Md. Ershadul Haque, Abdul Mannan, Abdullah Al Mamun. Concurrent Versus Sequential Chemoradiotherapy in Stage III Non-Small Cell Lung Cancer-A Comparative Study. Journal of Cancer Science and Clinical Therapeutics. 7 (2023): 169-178.
View / Download Pdf Share at FacebookAbstract
Background: Non-small cell Lung carcinoma is the most common carcinoma in both worldwide and Bangladesh. Most of them presents with locally advanced disease. Though Concurrent Chemoradiation (CCRT) is the standard approach, Sequential chemoradiotherapy (SCRT) can also be considered in stage III NSCLC. This study’s aim is to compare the local control and toxicity of CCRT and SCRT in stage III NSCLC.
Materials and Methods: Quasi-experimental study was carried out in the Department of Oncology, Khwaja Yunus Ali Medical College and Hospital (KYAMCH), Sirajgonj during the period November 2018 to October 2019. Patients of stage III NSCLC who met the set inclusion criteria were included and distributed in Arm A and B. Treatment schedule for Arm A was Inj. Cisplatin 50 mg/m2 on days 1, 8, 29 and 36 and Inj. Etoposide 50 mg/m2 on days 1-5 and 29-33 concurrently with RT. In arm B, the sequential chemotherapy protocol consisted with Inj. Paclitaxel 200 mg/m2 day 1 and Inj. Carboplatin AUC-6 day 1 for 3 cycles followed by radiotherapy. RT dose was 60 Gy in 30 daily fractions over 6 weeks for both the arms. Every patient was evaluated during and after completion of treatment for response and toxicity. All the informations were recorded, analyzed statistically and results were compiled accordingly.
Results: During the study period a total of 60 patients of stage III NSCLC were included in this study and distributed in A and B. In Arm A 30 patients were enrolled but one patient discontinued treatment. After completion of RT, complete response was observed in 8(27.6%) patients, partial response was seen in 18(62.1%) in Arm A and in Arm B same number had complete response and 17(60.7%) had partial response. P value was 0.7503 which was statistically not significant. At 3rd follow up 5(17.2%) patients had complete response and 7(24.13%) had partial response. In SCRT arm 4(14.3%) had complete response and 8(28.6%) had partial response, P value was 0.161 which was statistically not significant. Various toxicities were observed in this study, most common were esophagitis and pneumonitis but all those were manageable.
Conclusion: It may be said that SCRT with paclitaxel and carboplatin regimen followed by external beam radiotherapy is equally effective like CCRT with cisplatin and etoposide regimen in locoregional control of stage III NSCLC with acceptable toxicity.
Keywords
<p>Non-small cell Lung carcinoma; Concurrent chemoradiationon; Sequential chemoradiotherapy (SCRT); Stage III NSCLC</p>
Non-small cell Lung carcinoma articles; Concurrent chemoradiationon articles; Sequential chemoradiotherapy (SCRT) articles; Stage III NSCLC articles.
Non-small cell Lung carcinoma articles Non-small cell Lung carcinoma Research articles Non-small cell Lung carcinoma review articles Non-small cell Lung carcinoma PubMed articles Non-small cell Lung carcinoma PubMed Central articles Non-small cell Lung carcinoma 2023 articles Non-small cell Lung carcinoma 2024 articles Non-small cell Lung carcinoma Scopus articles Non-small cell Lung carcinoma impact factor journals Non-small cell Lung carcinoma Scopus journals Non-small cell Lung carcinoma PubMed journals Non-small cell Lung carcinoma medical journals Non-small cell Lung carcinoma free journals Non-small cell Lung carcinoma best journals Non-small cell Lung carcinoma top journals Non-small cell Lung carcinoma free medical journals Non-small cell Lung carcinoma famous journals Non-small cell Lung carcinoma Google Scholar indexed journals Concurrent chemoradiationon articles Concurrent chemoradiationon Research articles Concurrent chemoradiationon review articles Concurrent chemoradiationon PubMed articles Concurrent chemoradiationon PubMed Central articles Concurrent chemoradiationon 2023 articles Concurrent chemoradiationon 2024 articles Concurrent chemoradiationon Scopus articles Concurrent chemoradiationon impact factor journals Concurrent chemoradiationon Scopus journals Concurrent chemoradiationon PubMed journals Concurrent chemoradiationon medical journals Concurrent chemoradiationon free journals Concurrent chemoradiationon best journals Concurrent chemoradiationon top journals Concurrent chemoradiationon free medical journals Concurrent chemoradiationon famous journals Concurrent chemoradiationon Google Scholar indexed journals Sequential chemoradiotherapy (SCRT) articles Sequential chemoradiotherapy (SCRT) Research articles Sequential chemoradiotherapy (SCRT) review articles Sequential chemoradiotherapy (SCRT) PubMed articles Sequential chemoradiotherapy (SCRT) PubMed Central articles Sequential chemoradiotherapy (SCRT) 2023 articles Sequential chemoradiotherapy (SCRT) 2024 articles Sequential chemoradiotherapy (SCRT) Scopus articles Sequential chemoradiotherapy (SCRT) impact factor journals Sequential chemoradiotherapy (SCRT) Scopus journals Sequential chemoradiotherapy (SCRT) PubMed journals Sequential chemoradiotherapy (SCRT) medical journals Sequential chemoradiotherapy (SCRT) free journals Sequential chemoradiotherapy (SCRT) best journals Sequential chemoradiotherapy (SCRT) top journals Sequential chemoradiotherapy (SCRT) free medical journals Sequential chemoradiotherapy (SCRT) famous journals Sequential chemoradiotherapy (SCRT) Google Scholar indexed journals Stage III NSCLC articles Stage III NSCLC Research articles Stage III NSCLC review articles Stage III NSCLC PubMed articles Stage III NSCLC PubMed Central articles Stage III NSCLC 2023 articles Stage III NSCLC 2024 articles Stage III NSCLC Scopus articles Stage III NSCLC impact factor journals Stage III NSCLC Scopus journals Stage III NSCLC PubMed journals Stage III NSCLC medical journals Stage III NSCLC free journals Stage III NSCLC best journals Stage III NSCLC top journals Stage III NSCLC free medical journals Stage III NSCLC famous journals Stage III NSCLC Google Scholar indexed journals Oncology articles Oncology Research articles Oncology review articles Oncology PubMed articles Oncology PubMed Central articles Oncology 2023 articles Oncology 2024 articles Oncology Scopus articles Oncology impact factor journals Oncology Scopus journals Oncology PubMed journals Oncology medical journals Oncology free journals Oncology best journals Oncology top journals Oncology free medical journals Oncology famous journals Oncology Google Scholar indexed journals paclitaxel articles paclitaxel Research articles paclitaxel review articles paclitaxel PubMed articles paclitaxel PubMed Central articles paclitaxel 2023 articles paclitaxel 2024 articles paclitaxel Scopus articles paclitaxel impact factor journals paclitaxel Scopus journals paclitaxel PubMed journals paclitaxel medical journals paclitaxel free journals paclitaxel best journals paclitaxel top journals paclitaxel free medical journals paclitaxel famous journals paclitaxel Google Scholar indexed journals carboplatin regimen articles carboplatin regimen Research articles carboplatin regimen review articles carboplatin regimen PubMed articles carboplatin regimen PubMed Central articles carboplatin regimen 2023 articles carboplatin regimen 2024 articles carboplatin regimen Scopus articles carboplatin regimen impact factor journals carboplatin regimen Scopus journals carboplatin regimen PubMed journals carboplatin regimen medical journals carboplatin regimen free journals carboplatin regimen best journals carboplatin regimen top journals carboplatin regimen free medical journals carboplatin regimen famous journals carboplatin regimen Google Scholar indexed journals etoposide regimen articles etoposide regimen Research articles etoposide regimen review articles etoposide regimen PubMed articles etoposide regimen PubMed Central articles etoposide regimen 2023 articles etoposide regimen 2024 articles etoposide regimen Scopus articles etoposide regimen impact factor journals etoposide regimen Scopus journals etoposide regimen PubMed journals etoposide regimen medical journals etoposide regimen free journals etoposide regimen best journals etoposide regimen top journals etoposide regimen free medical journals etoposide regimen famous journals etoposide regimen Google Scholar indexed journals esophagitis articles esophagitis Research articles esophagitis review articles esophagitis PubMed articles esophagitis PubMed Central articles esophagitis 2023 articles esophagitis 2024 articles esophagitis Scopus articles esophagitis impact factor journals esophagitis Scopus journals esophagitis PubMed journals esophagitis medical journals esophagitis free journals esophagitis best journals esophagitis top journals esophagitis free medical journals esophagitis famous journals esophagitis Google Scholar indexed journals pneumonitis articles pneumonitis Research articles pneumonitis review articles pneumonitis PubMed articles pneumonitis PubMed Central articles pneumonitis 2023 articles pneumonitis 2024 articles pneumonitis Scopus articles pneumonitis impact factor journals pneumonitis Scopus journals pneumonitis PubMed journals pneumonitis medical journals pneumonitis free journals pneumonitis best journals pneumonitis top journals pneumonitis free medical journals pneumonitis famous journals pneumonitis Google Scholar indexed journals
Article Details
1. Introduction
Cancer is the second leading cause of death worldwide. Globally 1 in 6 deaths is due to cancer. GLOBOCAN 2018 report estimated 9.6 million deaths due to cancer. Lung cancer imposes major cancer burden among all cancers. More than 2 million new cases were diagnosed in 2018. Cancer is one of the major causes of morbidity and mortality among the non-communicable disease in Bangladesh. Cancer is the sixth cause of mortality in Bangladesh and more than half of the cancer patients die within five years of diagnosis. In Bangladesh there is no any national statistics available for cancer cases, but according to the Cancer Registry Report 2014 published in December 2015 by National Institute of Cancer Research and Hospital (NICRH 2014) [1], lung was the main leading site of cancers in both sexes which about 17.9%. Lung cancer happened to be the first in number in male patients, about 27.5% and fourth in female patients, about 6.0 %. Mean age group was 58.7 yrs. Lung cancer comprises a group of malignant epithelial tumors arising from cells lining of the lower respiratory tract. There are two main subtypes of lung cancer, Small cell lung cancer and Non- Small cell lung cancer. Of the two main types of lung cancer, NSCLC, is the most frequent and represents about 70% to 80% of the cases [2]. According to WHO 2015 classification major types of epithelial carcinomas are 1. Adenocarcinoma, 2. Squamous cell carcinoma, 3. Neuroendocrine tumors, 4. Large cell carcinoma, 5. Adenosquamous carcinoma and others like carcinoma with pleomorphic, sarcomatoid, or sarcomatous elements, Carcinosarcoma, Carcinoid tumor, Carcinomas of salivary gland type and unclassified carcinoma respectively. Annual report of NICRH reveals that the incidence of Squamous cell carcinoma is 44% whereas Adenocarcinoma is 27.4%. But this histologic spectrum of lung cancer has changed worldwide. Squamous cell Carcinoma has decreased from 25% to less than 15%. Cigarette smoking was associated with a 70% increase in the age-specific death rates of men and a lesser increase in the death rates of women. Cigarette smoking was causally related to lung cancer in men. The magnitude of the effect of cigarette smoking far outweighed all other factors leading to lung cancer. The risk for lung cancer increased with the duration of smoking and the number of cigarettes smoked per day. The report estimated that the average male smoker had an approximately 9 fold to 10-fold risk for lung cancer, whereas heavy smokers had at least a 20fold risk. Cigarette smoking is believed to be more important than occupational exposures in the causation of lung cancer in the general population [3]. There is a genetic component to the pathogenesis of lung cancer, whether it relates to host susceptibility to lung cancer, with or without exposure to cigarette smoke to the development of certain types of lung cancer, or to an individual’s responsiveness to biologic therapies. A lung cancer risk prediction analysis developed by Spitz et al. (2007) [4] incorporated multiple variables, such as smoking history, exposure to environmental tobacco smoke, occupational exposures to dusts and to asbestos, and family history of cancer. They showed the influence of a family history of cancer on the risk for lung cancer in never smokers, former smokers, and current some lung cancer surpassed breast cancer as the leading cause of cancer deaths in women in the late 1980s, and now almost twice as many women die of lung cancer than breast cancer. Since 1950 there has been more than a 600% increase in the lung cancer mortality rate in women. In the United States, the cigarette smoking rate for women increased during the period from 1930 to 1960, and this increase was followed two decades later by an increase in lung cancer in women starting in 1960 [5]. It has been suggested that diet is responsible for approximately 30% of all cancers [6]. Many reports suggest that dietary factors contribute to the risk for lung cancers [7]. For example, low serum concentrations of antioxidants, such as vitamins A, C, and E, have been associated with the development of lung cancet [8]. In 2000, it was estimated that 10% of lung cancer deaths among men and 5% among women worldwide could be attributable to exposure to eight occupational lung carcinogens, namely asbestos, arsenic, beryllium, cadmium, chromium, nickel, silica, and diesel fumes [9]. Most of patients are symptomatic on presentation. Central tumors produce symptoms of cough, pain and hemoptysis. Obstructive infective symptoms or lobar collapse may cause dyspnoea. If the mediastinum is directly invaded or mediastinal glands are present, hoarseness of voice, dysphagia, superior vena cava obstruction and pericardial irritation or effusion develops. Peripheral tumors may grow to large size before causing symptoms. Dyspnea may be the presenting sign of a pleural effusion. Direct extension into the rib or brachial plexus causes Pan Coast’s syndrome. If sympathetic plexus, which lies on carotid artery, is affected Horner’s syndrome is also seen. Invasion of the lymphatic system often occurs early. Diagnostic work up should include careful clinical history taking of the history of any respiratory change or complain and clinical examination including performance status and weight loss. History of smoking should also be included in detail. Patients should do a chest X-ray (P/A view) first. A lateral view may be helpful. A Contrast enhanced computed tomography of chest and upper abdomen is recommended before bronchoscopy as peripheral tumors are not be reached by bronchoscopy and, in these cases, a Computed tomography (CT)-guided FNAC is required for histological diagnosis. Mediastinoscopy or endobronchial ultrasound can be used to obtain biopsy samples for mediastinal nodes. MRI of brain can be done for evaluation of CNS and Positron enhanced tomography (PET- CT) scan can be done to evaluate the mediastinal lymph node and also the distant metastases. Also immunohistochemistry and molecular marker can be performed from those tissues. Complete staging work up should be done before starting the treatment of lung cancer. The latest staging work up can be done based on American Joint Committee on Cancer (AJCC) staging system, 8th edition. Most of the NSCLC are diagnosed at a locally advanced stage in our country. There is no recent national data that how much patient we are getting at stage III. The treatment depends on stage, performance status and comorbidity of patients. In Stage I-II operable tumor lobectomy is preferred over pneumonectomy if anatomically feasible. Wedge resection is done if physiologically compromised. LN sampling or dissection generally indicated. For, resected (T12, N1; T2N0 >4 cm and T3N0) adjuvant chemotherapy should be given and for close/+ margin, resect or consider post-op RT. In Stage I-II inoperable tumor, T1-2 N0 definitive SBRT not 3DCRT is considered, if T2N0, tumor size >4cm adjuvant chemotherapy should be considered, for T3N0 definitive chemo-RT or hypo fractionated RT or SBRT and for T1-2N1 definitive chemo-RT to 60-66Gy is considered [10]. In stage III NSCLC, there is invasion of adjacent structures and/or lymph node metastases and this stage is not amenable for potentially curative resection [11]. In NSCLC, the overall survival is poor. Five-year survivals in surgical stage IIIA is 9-25%. Regarding inoperable stage III NSCLC, the median survival duration with radiotherapy alone varies between 9-11 months with a 2-year survival of 10-20% and a 3-year survival of 5-10% [12]. Current practice guidelines recommend that these cases be treated with a combination of chemotherapy and thoracic radiation. The two used methods of combining these two modalities are CCRT, defined as chemotherapy administered on the same day as radiotherapy, and SCRT, usually administered as two to four cycles of chemotherapy prior to radiotherapy [13]. N randomized clinical trials of inoperable patients with stage III NSCLC, CCRT seems to be superior in terms of overall survival compared with sequential SCRT, producing an absolute overall survival benefit of 5.7% and 4.5% in 3 and 5 years, respectively [14]. This benefit is probably the result of improved loco regional control and its radio sensitizing effect. Therefore, CCRT is considered the standard treatment regimen for inoperable patients. However, CCRT has been associated with more toxic adverse events, particularly treatmentrelated mortality and acute esophagitis [15]. SCRT is proposed for patients who are considered unfit to receive CCRT or when the volume to be irradiated is considered too large [16]. As CCRT is associated with a higher risk of toxicity, this treatment is usually given to relatively good performance status patients who are generally younger, with little or no comorbidities and a good performance status [17]. Conversely, stage III NSCLC patients are typically elderly with comorbidities, a group poorly represented in clinical trials, which may influence the choice for CCRT. Moreover, CCRT treatment usually requires a well-managed multidisciplinary infrastructure, which may be difficult to deliver in certain hospitals without a radiotherapy facility. Consequently, this may result in a variation of treatment policies across hospital [18] [19]. As there is a considerable treatment variation across different hospitals, this fact incites us to do a study regarding this topic.
2. Objective
2.1 General Objective:
To compare the local tumor control and toxicities of sequential chemoradiotherapy with paclitaxel and carboplatin and concurrent chemoradiotherapy with cisplatin and etoposide, in locally advanced non-small cell lung cancer.
2.2 Specific Objectives:
To measure and compare local tumor control of two modalities of treatment during treatment and regular follows up. To compare acute toxicities between baseline and follow up of two groups. To asses socio-demographic characteristics of both groups.
3. Methodology
This was a quasi-experimental study. The patients were selected by convenient and purposive sampling method. A total of 60 patients were selected in this study, 30 patients in each arm. This study was conducted on November 2018 to October 2019 and conducted in the Department of Oncology, Khwaja Yunus Ali Medical College and Hospital, Enayetpur, Sirajgonj. Bangladesh
3.1 Inclusion Criteria:
Histologically or cytologically proven NSCLC. Locally advanced NSCLC (Stage III A, B, C).
3.2 Exclusion Criteria:
- Patients ECOG performance scores more than grade 2.
- Age below 18 years and above 75 years.
- Patients with history of prior chemotherapy or radiotherapy to lung region.
- Serious concomitant medical illness including severe heart disease, uncontrolled diabetes mellitus or hypertension.
- Life expectancy <6 months.
- Patient with uncontrolled infection.
- Psychiatric illness Pregnant or lactating woman.
3.3 Data analysis procedure
The information gathered during the study period was interpreted and conclusion and recommendation were drawn, in order to address the objectives of the study. The factors which could cause possibly bias in the study were acknowledged and limited as much as possible. The data were tabulated in separate tables for both Arm-A and B. They were checked, edited and coded manually. Data analysis was done according to the objectives of the study by using the SPSS software program for windows, version 24.0. The statistical data were analyzed by Chi-square test. The P value less than 0.05 were taken as significant.
3.4 Ethical Consideration
In this study the following criteria were set to ensure maintaining the ethical values. Permission was taken from IRB of KYAMCH.
4. Results
A total of 60 patients were enrolled in this study to compare the tumor response and toxicity of two different sequential chemotherapy followed by radiotherapy in locally advanced NSCLC. Among 60 patients, 30 patients were treated CCRT with cisplatin and etoposide in Arm A 30 were treated with paclitaxel and carboplatin followed by radiotherapy in Arm B. Patients were evaluated during and after completion of treatment according to follow up schedule. The statistical data was analyzed by Chi- square test. The P value less than 0.05 were taken as significant. Observations and results of this study are shown in following tables and graphs.
Table 1 showed maximum and minimum ages found in both age group. The mean age of patients in Arm A was 58.5±8.1 and Arm B was 61.2±7.4 respectively in both arms.
Table 1: Distribution of patients according to their age characteristics of both arms (N=60)
|
Arm A |
Arm B |
|
|
(n=30) |
(n=30) |
|
|
Age range Minimum age and Maximum age |
34-70 |
43-75 |
|
34 |
43 |
|
|
70 |
75 |
|
|
Mean age |
58.5±8.1 |
61.2±7.4 |
Figure 1 showed percentage of the age of the patients in both arms. Mean age of arm A is 58.5±8.1 and mean age of arm B is 61.2±7.4. Most of the patients were in 56-65 age group 55.9%. Second most common age group was 66-75, having 20 % patients in each. Age group 34- 45 had least patients, 6.7 % only.
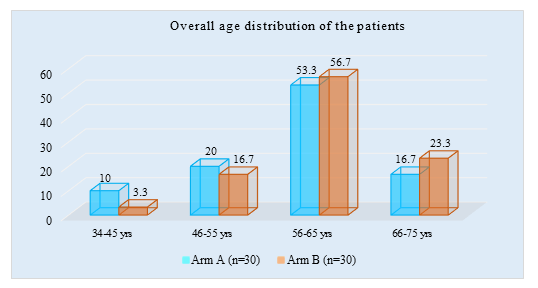
Figure 1: Bar chart showed Overall age distribution of patients among both arms (N=60)
Figure 2 showed majority of patients belongs to male sex. In both arm 54(90%) patients were male and 6(10%) patients were female. In arm A 28(93.3%) patients were male and 2(6.7%) patients were female. In arm B 26(86.7%) patients were male and 4(13.3%) patients were female.
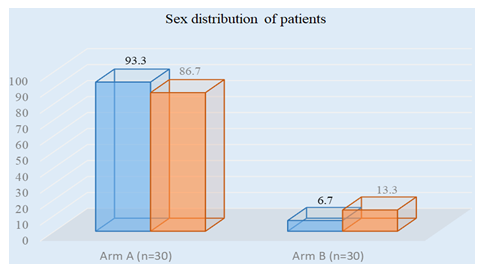
Figure 2: Bar chart showed Overall sex distribution of patients among both the arms. (N=60)
Figure 3 showed the smokers percentage in study population. Pie chart showed 70% patients were smokers and 30 % were nonsmokers. The percentage applicable for both Arm A and Arm B.
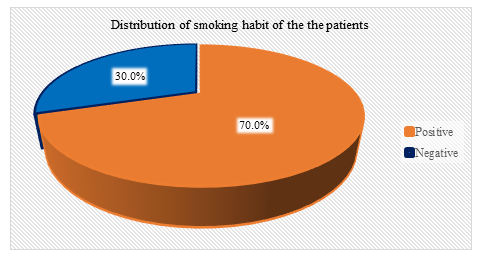
Figure 3: pie chart showed distribution of patients according to their smoking habit. (N=60)
Table 2 showed the staging of the patient at the time of presentation in both arms. In Arm A 15(50%) patients were Stage III A, 13(43.3%) patients were Stage III B, 2(6.6%) patients were stage IIIC and in Arm B 12(40%) patients were Stage III A, 16 (40%) patients were in Stage IIIB and 2(6.7%) patients were stage IIIC.
Table 2: Distribution of patients according to the stage of disease at diagnosis (N=60)
|
Stage at diagnosis |
Arm A |
Arm B |
Total |
|
(n=30) |
(n=30) |
(N=60) |
|
|
Stage IIIA |
15(50%) |
12(40%) |
27(45%) |
|
Stage IIIB |
13(43.4%) |
16(53.3%) |
29(49.2%) |
|
Stage IIIC |
2(6.6%) |
2(6.7%) |
4(6.7%) |
Table 3 showed that histologically both adenocarcinoma and Squamous cell carcinoma is equally prevalent in both arms. In Arm A 14(46.66%) patients had squamous cell carcinoma, 14(46.66%) had adenocarcinoma, 2(6.66%) had large cell carcinoma and in Arm B 14(46.66%) patients, had squamous cell carcinoma, 15(50%) had adenocarcinoma and 1(3.33%) had large cell carcinoma.
Table 3: Distribution of patients according to the histological type of NSCLC (N=60)
|
Histology |
Arm A |
Arm B |
Total |
|
(n=30) |
(n=30) |
(N=60) |
|
|
Adenocarcinoma |
14(46.66%) |
14(46.66%) |
28(46.66%) |
|
SCC |
14(46.66%) |
15(50%) |
29(48.33%) |
|
Large cell carcinoma |
2(6.68%) |
1(3.34%) |
29(48.33%) |
Figure 4 showed in arm A 5(16.6%) patients had grade I histology, 16(53.33%) patients had grade II and 9(30%) patients had grade III histology. In arm B 7(23.3%) had grade I histology,12(40%) had grade II and 11(36.7%) had grade III histology.
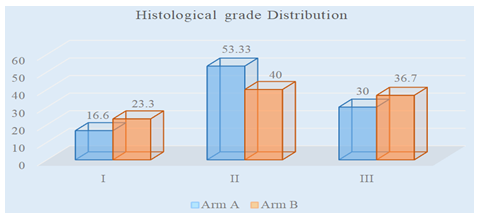
Figure 4: Distribution of patients according to the histological grade in both arms
Figure 5 showed the response of patients after three cycles of chemotherapy in arm B. About 21(70%) patients had a partial response, 2(7%) has a complete response, the same percentage of patients had progressive disease and 7(16%) patients had stable disease.
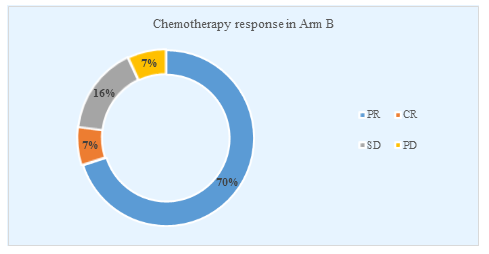
Figure 5: Distribution of patients according to their chemotherapy response in Arm B
Table 4 showed that in both arms most of the patients had ECOG performance status 1. In Arm A 20(66.6%) and in Arm B 16 (53.3%) patients had a score of 1.
Table 4: Distribution of patients according to their pretreatment performance status (N=60)
|
ECOG Score |
Arm A |
Arm B |
Total |
|
(n=30) |
(n=30) |
(N=60) |
|
|
1 |
20(66.66%) |
16(53.3%) |
36(60.00%) |
|
2 |
10(34.5%) |
14(46.7%) |
24(40.7%) |
Table 5 showed post treatment ECOG performance status. After completion of radiotherapy 8(26.6%) had ECOG score 3 and in Arm B 5(16.7%) had ECOG score 3.
Table 5: Distribution of patients according to their post treatment performance status (N=60)
|
ECOG Score |
Arm A |
Arm B |
Total |
|
(n=30) |
(n=30) |
(N=60) |
|
|
1 |
7(23.3%) |
7(23.3%) |
14(23.33%) |
|
2 |
15(50%) |
18(60%) |
33(55.9%) |
|
3 |
8(26.66%) |
5(16.7%) |
13(22%) |
Table 6 showed the local tumor control according to the radiological findings at 6th week after completion of treatment as 1st follow up. In Arm A 8(26.7%) patients had complete response, 18(60%) had partial response, 4(13.3%) had stable disease and none had progressive disease. In Arm B 8(26.7%) patients had complete response, 17(56.7%) had partial response, 2(6.2%) had stable disease and 3(10%) had progressive disease. P-Value is 0.296 which is statistically not significant.
Table 6: Local tumor control evaluation at 6th week after completion of treatment in both Arm A and Arm B (N=60)
|
Total tumor control |
Group |
χ2 |
p value |
|
|
Arm A |
Arm B |
3.695 |
0. 296 |
|
|
(n=30) |
(n=30) |
|||
|
Complete response |
8(26.7%) |
8(26.7%) |
||
|
Partial response |
18(60%) |
17(56.6%) |
||
|
Stable disease |
4(13.3%) |
2(6.7%) |
||
|
Progressive disease |
0(0%) |
3(10%) |
||
Table 7 showed the local tumor control according to the radiological findings at 12th week after completion of treatment as 2nd follow up. In Arm A 8(26.7%) patients had complete response, 13(43.3%) had partial response, 4(13.3%) had stable disease and had 5 (16.7%) progressive disease. In Arm B 7(23.3%) patients had complete response, 17(56.7%) had partial response, 2(6.7%) had stable disease and 4(13.3%) had progressive disease. P-Value is 0.711 which is statistically not significant.
Table 7: Local tumor control evaluation by at 12th week after completion of treatment in both Arm A and Arm B (N=60)
|
Total tumor control |
Group |
Chi square value |
p value |
|
|
Arm A |
Arm B |
1.378 |
0. 711 |
|
|
(n=30) |
(n=30) |
|||
|
Complete response |
8(26.7%) |
7(23.3%) |
||
|
Partial response |
13(43.3%) |
17(56.7%) |
||
|
Stable disease |
4(13.3%) |
2(6.7%) |
||
|
Progressive disease |
5(16.7%) |
4(13.3%) |
||
Table 8 showed the local tumor control according to the X-ray findings at 22nd week after completion of treatment as 3rd follow up. In Arm A 5(16.7) patients had complete response, 7(23.3%) had partial response, 6(20%) had stable disease and 12(40%) had progressive disease. In Arm B 4(13.3%) had complete response, 8(26.7%) had partial response, 8(26.7%) had stable disease and 10 (33.3%) had progressive disease. P- Value is .886 which is statistically not significant.
Table 8: Local tumor control evaluation at 24th week after completion of treatment in both Arm A and Arm B (N=60)
|
Group |
Chi square value |
p value |
||
|
Arm A |
Arm B |
0.645 |
0.886 |
|
|
(n=30) |
(n=30) |
|||
|
Complete response |
5(16.7) |
4(13.3%) |
||
|
Partial response |
7(23.3%) |
8(26.7%) |
||
|
Stable disease |
6(20%) |
8(26.7%) |
||
|
Progressive disease |
12(40%) |
10(33.3%) |
||
Table 9 showed After 3rd cycle chemotherapy grade 2 anemia observed in 4(13.3) % patients. Grade 3 neutropenia was in 7 (23.3%) patients and grade 2 thrombocytopenia was observed in 1(3.3%) patients.
Table 9: Distribution of patients according to Hematologic toxicity after 3rd cycle chemotherapy toxicity in Arm B
|
Toxicity |
Frequency |
Percentage |
|
(n) |
(%) |
|
|
Anemia |
||
|
Grade 1 |
10 |
33.30% |
|
Grade 2 |
4 |
13.30% |
|
Neutropenia |
||
|
Grade 1 |
5 |
16.70% |
|
Grade 2 |
3 |
10.00% |
|
Grade 3 |
7 |
23.30% |
|
Thrombocytopenia |
||
|
Grade 1 |
3 |
10.00% |
|
Grade 2 |
1 |
3.30% |
Table 10 showed After 3rd cycle chemotherapy 3(6.7%) patients had grade 3 vomiting. Most evident non hematological toxicity observed is peripheral neuropathy, 5(16.7%) patients had grade 2toxicity.
Table 10: Distribution of patients according to Non Hematologic after 3rd cycle chemotherapy toxicity in Arm B
|
Toxicity |
Frequency |
Percentage |
|
(n) |
(%) |
|
|
Nausea |
||
|
Grade 1 |
20 |
66.6 |
|
Grade 2 |
3 |
10% |
|
Grade 3 |
1 |
3.30% |
|
Vomiting |
||
|
Grade 1 |
8 |
26.70% |
|
Grade 2 |
5 |
16.70% |
|
Grade 3 |
3 |
10% |
|
Mucositis |
||
|
Grade 1 |
3 |
10% |
|
Grade 2 |
3 |
10% |
|
Grade 3 |
2 |
6.70% |
|
Diarrhea |
||
|
Grade 1 |
3 |
10% |
|
Grade 2 |
1 |
3.30% |
|
Grade 3 |
2 |
6.70% |
|
Peripheral neuropathy |
||
|
Grade 1 |
12 |
40% |
|
Grade 2 |
5 |
16.70% |
|
Grade 3 |
1 |
3.30% |
Table 11 showed 2 patients had progressive disease after chemotherapy in Arm B as regarding hematologic adverse events anemia was most common. Grade 2 anemia occurred in 4(13.8%) patients.
Table 11: Distribution of patients according to Hematologic toxicity after Radiotherapy (N=60)
|
Toxicity |
Arm A |
Arm B |
χ2test |
p value |
|
(n=30) |
(n=28) |
|||
|
Anemia |
||||
|
Grade 1 |
0(0.0%) |
2(7.1%) |
6.004 |
0 .4969 |
|
Grade 2 |
4(13.8%) |
0(0.0%) |
||
|
Grade 3 |
0(0.0%) |
0(0.0%) |
||
|
Neutropenia |
||||
|
Grade 1 |
1(3.4%) |
1(3.6%) |
0.983 |
`0.6117 |
|
Grade 3 |
1(3.4%) |
0(0.0%) |
||
Table 12 showed in arm A 6(20%) patients had grade 1, 10(34.7%) had grade2, 3(10.3%) had grade 3 mucositis and in arm B 3(10.7%) patients had grade 1, 2(7.1%) had grade 2 and 1(3.6%) had grade 3mucositis. Here p value is statistically significant. Result of toxicity, vomiting is not statistically significant.
Table 12: Distribution of patients according to Non-hematological toxicity after Radiotherapy (N=60)
|
Variables of Toxicity |
Arm A |
Arm B |
χ2 |
p value |
|
(n=30) |
(n=28) |
|||
|
Mucositis |
||||
|
Grade 1 |
6(20.0%) |
3(10.7%) |
12.162 |
0.007 |
|
Grade 2 |
10(33.3%) |
2(7.1%) |
||
|
Grade 3 |
3(10.0%) |
1(3.6%) |
||
|
Vomiting |
||||
|
Grade 1 |
18(62.1%) |
26(92.9%) |
8.323 |
0.156 |
|
Grade 2 |
8(27.6%) |
2(07.1%) |
||
Table 13 showed in arm A 3 (10.4%) patients had grade1, 15(50.0%) had grade 2 and 13(43.3%) had grade 3
Esophagitis and in arm B 7(75%) patients had grade 1, 18(64.3%) had grade 2 and 3.6 %, 2 (7.1%) had grade 3 esophagitis. Here p value is statistically significant. In arm A 3(10.0%) patients had grade 1, 24(80.3%) had grade 2, 3(10.0%) had grade 3 pneumonitis. In arm B 4(14.3%) patients had grade 1, 13(46.4%) had grade 2, 7(25%) had grade 3 pneumonitis. Here p value is statistically not significant.
Table 13: Distribution of patients according to Non-haematological toxicity after Radiotherapy (N=60)
|
Variables of Toxicity |
Arm A |
Arm B |
χ2 |
p value |
|
(n=30) |
(n=28) |
|||
|
Esophagitis |
||||
|
Grade 1 |
3(10.4%) |
7(75%) |
13.826 |
0.0032 |
|
Grade 2 |
15(50.0%) |
18(64.3%) |
||
|
Grade 3 |
13(43.3%) |
2(7.1%) |
||
|
Pneumonitis |
||||
|
Grade 1 |
3(10.0%) |
4(14.3%) |
7.82 |
0.05 |
|
Grade 2 |
24(80.3%) |
13(46.4%) |
||
|
Grade 3 |
3(10.0%) |
7(25%) |
||
Table 14 showed in Arm B 14(50%) patients had grade 1 and 10(35.7%) had grade 2 peripheral neuropathies. P value is 0.0001, which is statistically significant.
Table 14: Toxicities (Skin reaction, Neuropathy and Diarrhea) in both arms during and after radiotherapy (N=60)
|
Variables of Toxicity |
Arm A |
Arm B |
χ2 |
p value |
|
(n=30) |
(n=28) |
|||
|
Skin Reaction |
||||
|
Grade 1 |
16(53.2%) |
21(75%) |
11.09 |
0.01 |
|
Grade 2 |
11(36.7%) |
1(3.6%) |
||
|
Grade 3 |
0 |
1(3.6%) |
||
|
Neuropathy |
||||
|
Grade 1 |
0 |
14(50%) |
18.261 |
0.0001 |
|
Grade 2 |
0 |
10(35.7%) |
||
|
Diarrhea |
||||
|
Grade 1 |
3(10.3%) |
0(0.0% |
3.057 |
0.0803 |
5. Discussion
Lung cancer is the most commonly diagnosed cancer (11.6% of the total cases) and the leading cause of cancer death (18.4% of the total cancer deaths) worldwide. In Bangladesh lung was the main leading site of cancers in both sexes which about 17.9%. Lung cancer happened to be the first in number in male patients, about 27.5% and fourth in female patients, about 6.0 %. (GLOBACAN 2018) NSCLC is the predominant histology among lung cancers. This type of cancer needs multimodality treatment like radiotherapy and chemotherapy. The NSCLC Collaborative Group meta-analysis and the meta-analysis of cisplatin-based concomitant chemotherapy in NSCLC demonstrated that adding sequential or concomitant chemotherapy to radical radiotherapy improved survival in locally advanced NSCLC [11]. The patients enrolled in this study were histologically or cytologically proven NSCLC and were locally advanced Stage III. The tumor was at inoperable state and had not received any definitive oncologic treatment. The patients included for study were randomized in two different arms. Arm A was given CCRT with cisplatin and etoposide regimen and Arm B was given SCRT with paclitaxel and carboplatin. Each arm received RT 60 Gy in 30 fractions. The mean age of patients at diagnosis in Arm A was 58.5 years and in Arm B was 61.2 years. The patients according to the gender were distributed as follows in both arms. In between total number of 60 patients 89.8% were male and 10.2% were female. In Arm A 93.1% were male and 6.9%, were female. In Arm B 86.7% were male and 13.3% were female. This age group and gender percentage were similar to the Cancer Registry Report 2014 where Mean age group was 58.7 years. Histologically among all patients 28 (46.66%) had adenocarcinoma, 29(48.33%) patients had SCC and 3(5%) patients had large cell carcinoma. In a study by Zappa and Mousa (2016) [20] showed that adenocarcinoma is the most common histology, about 40 % of total study population, SCC comprises of 25-30% of study population and large cell carcinoma was present in 5% population in my study patients with adenocarcinoma and SCC having almost similar percentage. The patients enrolled in the study were related to various occupations. Included both arms, most of the patients were farmer 27 (45.8%). The educational status of the patients reflects that most of the patients were illiterate 23 (38.3%) and about half of the patient were farmer 27(45%). The majority of the patients in this study belong to lower middle class population group, where a total number of 23(38.3%) patients from lower middle class. These results to correlate the study of Parveen et al. (2018) [21], where most of the patients belongs to this group. In that study 80% of all cases belonged to poor and lower middle-class families with 54% illiterate and 26% had primary education. Smoking is the overwhelming cause for lung cancer in both men and women; 85 to 90% of patients with lung cancer are current or former tobacco smokers. In this study overall 71.2 % patients were smokers. In Arm A 14 (48.3%) patients were in Stage III A, 13 (44.8%) patients were in Stage III B and 2(6.9%) patients were in Stage III C. In Arm B 12 (40%) were in Stage III A and 12 (40%) were in Stage III B and in Stage III C 2(6.7%). This data is almost similar to a study conducted by Vinod et al. [18] on stage III NSCLC, there were 42% patients in III A and 48.1% patients in III B. In this study stage IIIC was also included due recent change in staging of AJCC 8th edition. The main objective of the study was to see the local tumor control with CCRT and SCRT. Based on the X-ray findings, 6th week after completion of treatment 1st follow up was done. In Arm A, 8(26.7%) patients had complete response, 18(60%) had partial response, 4(13.3%) had stable disease and none had progressive disease. In Arm B 8(26.7%) had complete response, 17(56.7%) had partial response, 2(7.1%) had stable disease and 3(10%) had progressive disease. Here P value is not statistically significant. The local tumor control according to the X-ray findings 12th week after completion of treatment 2nd follows up was done. In Arm A 8(26.7%) had complete response, 13(43.3%) had partial response, 4(13.3%) had stable disease and 5(17.2%) had progressive disease. In Arm B 7(23.3%) had complete response, 17(56.7%) had partial response, 2(7.1%) had stable disease and 4(13.3%) had progressive disease. Here p value is not statistically significant. The local tumor control according to the X-ray and CECT findings at 24th week after completion of treatment at 3rd follows up was done. In Arm A 5(16.7%) had complete response, 7(23.3%) had partial response, 6(20%) had stable disease and 12 (40%) had progressive disease. In Arm B 4(13.3%) had complete response, 8(26.7%) had partial response, 8(26.7%) had stable disease and 10 (33.3%) had progressive disease. Here p value is not statistically significant. There is no head to head comparison of these two regimens but two different studies which showed outcome of these regimens. In a study done by Belani et al. in 2005 [22], where paclitaxel and carboplatin was used as sequential chemotherapy followed by radiotherapy in locally advanced NSCLC. There is no data regarding the tumor response but median overall survival was mentioned which was 13 months. Another study was done by Reboul et al. in 1996, Phase II study was undertaken to determine the feasibility, toxicity, response rate, local control, and survival of concurrent chemotherapy with cisplatin-etoposide and radiotherapy in unresectable Stage III NSCLC. In that study response rate for that regimen was 84%, including 68% complete response. With a minimum follow-up of 23 months, overall survival was 70% at 1 year, 39.7% at 2 years, and 34.7% at 3 years. Median survival was 18 months. Different kinds of acute toxicities were observed in the patients of both arms during the course of treatment and subsequent follow ups. Nausea in Arm A was more than Arm B. Peripheral neuropathy was more is Arm B. All other toxicities among two arms showed no statistical significant differences. In Arm A grade 2 anemia occurred in 4(13.8%) grade 3 leukopenia in 1(3.4%), grade 1 and grade 2 radiation dermatitis in 16(55.2%) and10(34.5%), mucositis grade 1, grade 2 and grade 3 in 6(20.7%), 10(34.5%) grade 3 in3(10.3%),3 grade 2 vomiting in 3(10.3%), grade 2 and grade 3 esophagitis in 15(55.7%) and 13(44.8%).In Arm B grade 1 anemia occurred in 2(6.7%, grade 2 and grade 1 leukopenia in 1(3.3%) ,grade 1 and grade 2 radiation dermatitis in 23(76.7%) and 1(3.3%), mucositis grade 1 in 3(10%) 2(6.7%) and 1(3.3%), grade I nausea in 19(36.7%) and grade 1 vomiting in 2(6.7%) and 8(26%), grade 1, grade 2 and grade 3 esophagitis in 8(26.7%), 19(63.3%) and 3(6.7%), grade 1 , grade 2 and grade 3 pneumonitis was 4(13.3%) ,15(50%) and grade 3 in 7(23.3%). Grade 1 and grade 2 neurological toxicity in 10(33.3%), and 4(13.3%). The study conducted by Belani et al. had similar leukopenia as in Arm A but differ in other toxicities like anemia was less (4 % vs 26.67%), thrombocytopenia (4% vs 0%), nausea and vomiting (30% vs 7%), esophagitis (0 % vs 36%) and neurological toxicity (3% vs 36 %). A meta-analysis by Auperin et al (2010) showed that increased esophageal toxicity with CCRT compared to SCRT. CCRT Arm had grade 3, 4 toxicities about 4-18%. This study has almost similar result. The performance score, ECOG score of the patients present at the time of diagnosis of the patient were as follows. In Arm A 19(65.5%) patients had ECOG score 1 and 10(34.5%) patients had ECOG score 2. In Arm B 16(53.3%) patient had ECOG score 1 and 14(46.7%) patient had ECOG score 2. The performance score, ECOG score after the completion of treatment in subsequent follow up. In Arm A 14(46.7%) patients had ECOG score 1, 6(20.7%) patients had ECOG score 2(51.7%) patients had ECOG score 3 was 8(27.6%). In Arm B 16(53.3%) patients had ECOG score 1, 14(46.7%) patients had ECOG score 3. Post treatment ECOG in Arm B was score 1 in 7(23.3%), score 2 in 18(60%) and score 3 in 5(16.7%). There was improvement in ECOG score in many patients in both arms but 1(3.3%) patient in Arm A and 3(10%) patient in Arm B deteriorated in ECOG score and reached up to 3 after the completion of treatment during follow up. Though, the tumor response two arms were not very significantly different but considering the toxicities of CCRT arm SCRT it could be a good option for patients who has advanced disease with other comorbidities. In our country most of the patients presents with locally advanced stage with poor performance status. For them SCRT can be good curative option.
Conclusions
CCRT with cisplatin and etoposide followed by radiotherapy and paclitaxel and carboplatin followed by same radiotherapy for locally advanced NSCLC with almost similar local tumor control. But there is increased acute toxicities in CCRT arm.
Limitation of the Study
Although optimum care had been tried by the researcher in every steps of the study, still some limitations exist: It was not a randomized controlled trial. Small sample size was a major limitation in getting accurate clinical outcome. The study was done in single institution in Bangladesh.
Recommendation of the Study
The SCRT is as effective as CCRT in respect to tumor control of locally advanced NSCLC. But Toxicities are increased while giving CCRT. So, SCRT can be used as popularly as CCRT in the treatment of locally advanced NSCLC. These regimens can be further studied on the basis of treatment cost evaluation to establish a cheaper but equally effective regimen. Multiple institutional based study can be done.
References
- Cancer Registry Report by National Institute of Cancer Research and Hospital (NICRH 2014) (2015).
- EL Sharouni S Y. ‘Sequential Versus concurrent chemoradiotherapy in inoperable stage III non-small cell lung cancer’, Anticancer Research 26 (2006): 495-505.
- Warner K E, and Mendez D. ‘Tobacco control policy in developed countries: yesterday, today, and tomorrow.’ Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco 12 (2010): 876-887.
- Spitz M R. ‘A risk model for prediction of lung cancer’, Journal of the National Cancer Institute 99 (2007): 715-726.
- Thomas L, Doyle L A, and Edelman M J. ‘Lung cancer in women: Emerging differences in epidemiology, biology, and therapy’, Chest. The American College of Chest Physicians 128 (2005): 370-381.
- Trichopoulos D. ‘Evidence-based nutrition’, Asia Pacific Journal of Clinical Nutrition 9 (2000): 4-9.
- Ruano-Ravina A, Figueiras A, and Barros-Dios J. ‘Diet and lung cancer: A new approach’, European Journal of Cancer Prevention, 9(2000): 395-400.
- Woodson K. ‘Serum alpha-tocopherol and subsequent risk of lung cancer among male smokers.’, Journal of the National Cancer Institute 91 (1999): 1738-43.
- Driscoll T, Nelson D I, Steenland K, et al. The global burden of disease due to occupational carcinogens. American journal of industrial medicine 48 (2005): 419-43
- Hansen and Roach, Handbook of Evidence Based Radiation Oncology 3rd Edition (2018).
- Curran Jr WJ, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non–small cell lung cancer: randomized phase III trial RTOG 9410.Journal of the National Cancer Institute 103 (2011): 1452-1460.
- Mountain C F. ‘Revision in the International System for Staging Lung Cancer’, Chest. The American College of Chest Physicians 111 (1997): 1710-1717.
- Song S Y, Das A K, and Minna J D. ‘Comparison between concurrent andsequential chemoradiation for non-small cell lung cancer in vitro’, Oncology Letters 7 (2014): 307-310.
- Auperin A, Le Pechoux C, Pignon JP, et al. Concomitant radio-chemotherapy based on platin compounds in patients with locally advanced non-small cell lung cancer (NSCLC): a meta-analysis of individual data from 1764 patients. Annals of oncology 17 (2006): 473-483.
- O’Rourke N and Macbeth F. ‘Is concurrent chemoradiation the standard of care for locally advanced non-small cell lung cancer? A review of guidelines and evidence’, Clinical Oncology. Elsevier Ltd 22 (2010): 347- 355.
- Vansteenkiste J, De Ruysscher D, Eberhardt WEE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology 24 (2013): 89-98.
- De Ruysscher D. ‘Eligibility for concurrent chemotherapy and radiotherapy of locally advanced lung cancer patients: a prospective, population-based study.’ Annals of oncology 20 (2009): 98-102.
- Vinod S K. ‘Stage III non-small-cell lung cancer: Populationbased patterns of treatment in British Columbia, Canada’, Journal of Thoracic Oncology. International Association for the Study of Lung Cancer 7 (2012): 1155-1163.
- Walraven I. ‘Long-term follow-up of patients with locally advanced non-small cell lung cancer receiving concurrent hypofractionated chemoradiotherapy with or without cetuximab’, Radiotherapy and Oncology. Elsevier Ireland Ltd 118 (2016): 442-446.
- Zappa C and Mousa S A. ‘Non-small cell lung cancer: current treatment and future advances’, Translational Lung Cancer Research 5 (2016): 288-283
- Parveen R. ‘38P Survival of lung cancer: Bangladesh perspective’, Journal of Thoracic Oncology. International Association for the Study of Lung Cancer 13 (2018): 21- 22.
- Belani CP, Choy H, Bonomi P, et al. Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non–small-cell lung cancer: A randomized phase II locally advanced multi-modality protocol. Journal of clinical oncology 23 (2005): 5883-5891.
