Concordance of DNA Mismatch Repair Proteins in Primary Esophagogastric Adenocarcinomas and Distant Metastases
Article Information
Juliana Knief1*, Ekaterina Petrova2, Pamela Lazar-Karsten3, Ulrich Wellner2, Richard Hummel2, Christoph Thorns1
1Department of Pathology, Marienkrankenhaus Hamburg, Hamburg, Germany
2Department of Surgery, University Hospital of Schleswig-Holstein, Campus Luebeck, Luebeck, Germany
3Department of Pathology, University Hospital of Schleswig-Holstein, Campus Luebeck, Luebeck, Germany
*Corresponding Author: Juliana Knief, Department of Pathology, Marienkrankenhaus Hamburg, 22087 Hamburg, Germany
Received: 06 January 2020; Accepted: 14 January 2020; Published: 20 February 2020
Citation: Juliana Knief, Ekaterina Petrova, Pamela Lazar-Karsten, Ulrich Wellner, Richard Hummel, Christoph Thorns. Concordance of DNA Mismatch Repair Proteins in Primary Esophagogastric Adenocarcinomas and Distant Metastases. Journal of Surgery and Research 3 (2020): 013-019.
View / Download Pdf Share at FacebookAbstract
Introduction: Mismatch repair proteins (MMR) are commonly evaluated during routine workup in a variety of epithelial tumours. The incidence of deficiency in upper gastrointestinal carcinomas varies between 10-20%. In colorectal adenocarcinomas, mismatch repair protein expression shows high concordance between primary tumours and metastases but up to date no such data is available for esophagogastric adenocarcinomas.
Materials and Methods: 41 primary adenocarcinomas of the esophagogastric junction and matched distant metastases were analysed using immunohistochemistry with antibodies against MLH1 and MSH2. DNA mismatch repair status (deficient or proficient) was determined and correlated with clinical outcome.
Results: Concordance of mismatch repair status between primary tumours and metastases was relatively low (52.5%). No correlation with clinical features and no impact on overall survival could be demonstrated although mismatch repair protein proficiency showed a trend towards prolonged survival (p=0.097).
Conclusion: In contrast to colorectal carcinomas, concordance of MMR in esophagogastric carcinomas is low. Therefore, clinicians and pathologists alike should exercise caution when choosing tissue for immunohistochemical evaluation as these results might influence therapeutic procedures (i.e. the application of immune checkpoint inhibitors).
Keywords
Mismatch Repair Proteins, Metastases, Esophagogastric Adenocarcinoma, Biomarkers
Mismatch Repair Proteins articles, Metastases articles, Esophagogastric Adenocarcinoma articles, Biomarkers articles
Mismatch Repair Proteins articles Mismatch Repair Proteins Research articles Mismatch Repair Proteins review articles Mismatch Repair Proteins PubMed articles Mismatch Repair Proteins PubMed Central articles Mismatch Repair Proteins 2023 articles Mismatch Repair Proteins 2024 articles Mismatch Repair Proteins Scopus articles Mismatch Repair Proteins impact factor journals Mismatch Repair Proteins Scopus journals Mismatch Repair Proteins PubMed journals Mismatch Repair Proteins medical journals Mismatch Repair Proteins free journals Mismatch Repair Proteins best journals Mismatch Repair Proteins top journals Mismatch Repair Proteins free medical journals Mismatch Repair Proteins famous journals Mismatch Repair Proteins Google Scholar indexed journals Metastases articles Metastases Research articles Metastases review articles Metastases PubMed articles Metastases PubMed Central articles Metastases 2023 articles Metastases 2024 articles Metastases Scopus articles Metastases impact factor journals Metastases Scopus journals Metastases PubMed journals Metastases medical journals Metastases free journals Metastases best journals Metastases top journals Metastases free medical journals Metastases famous journals Metastases Google Scholar indexed journals Esophagogastric Adenocarcinoma articles Esophagogastric Adenocarcinoma Research articles Esophagogastric Adenocarcinoma review articles Esophagogastric Adenocarcinoma PubMed articles Esophagogastric Adenocarcinoma PubMed Central articles Esophagogastric Adenocarcinoma 2023 articles Esophagogastric Adenocarcinoma 2024 articles Esophagogastric Adenocarcinoma Scopus articles Esophagogastric Adenocarcinoma impact factor journals Esophagogastric Adenocarcinoma Scopus journals Esophagogastric Adenocarcinoma PubMed journals Esophagogastric Adenocarcinoma medical journals Esophagogastric Adenocarcinoma free journals Esophagogastric Adenocarcinoma best journals Esophagogastric Adenocarcinoma top journals Esophagogastric Adenocarcinoma free medical journals Esophagogastric Adenocarcinoma famous journals Esophagogastric Adenocarcinoma Google Scholar indexed journals Biomarkers articles Biomarkers Research articles Biomarkers review articles Biomarkers PubMed articles Biomarkers PubMed Central articles Biomarkers 2023 articles Biomarkers 2024 articles Biomarkers Scopus articles Biomarkers impact factor journals Biomarkers Scopus journals Biomarkers PubMed journals Biomarkers medical journals Biomarkers free journals Biomarkers best journals Biomarkers top journals Biomarkers free medical journals Biomarkers famous journals Biomarkers Google Scholar indexed journals prolonged survival articles prolonged survival Research articles prolonged survival review articles prolonged survival PubMed articles prolonged survival PubMed Central articles prolonged survival 2023 articles prolonged survival 2024 articles prolonged survival Scopus articles prolonged survival impact factor journals prolonged survival Scopus journals prolonged survival PubMed journals prolonged survival medical journals prolonged survival free journals prolonged survival best journals prolonged survival top journals prolonged survival free medical journals prolonged survival famous journals prolonged survival Google Scholar indexed journals epithelial tumours articles epithelial tumours Research articles epithelial tumours review articles epithelial tumours PubMed articles epithelial tumours PubMed Central articles epithelial tumours 2023 articles epithelial tumours 2024 articles epithelial tumours Scopus articles epithelial tumours impact factor journals epithelial tumours Scopus journals epithelial tumours PubMed journals epithelial tumours medical journals epithelial tumours free journals epithelial tumours best journals epithelial tumours top journals epithelial tumours free medical journals epithelial tumours famous journals epithelial tumours Google Scholar indexed journals adenocarcinomas articles adenocarcinomas Research articles adenocarcinomas review articles adenocarcinomas PubMed articles adenocarcinomas PubMed Central articles adenocarcinomas 2023 articles adenocarcinomas 2024 articles adenocarcinomas Scopus articles adenocarcinomas impact factor journals adenocarcinomas Scopus journals adenocarcinomas PubMed journals adenocarcinomas medical journals adenocarcinomas free journals adenocarcinomas best journals adenocarcinomas top journals adenocarcinomas free medical journals adenocarcinomas famous journals adenocarcinomas Google Scholar indexed journals primary tumours articles primary tumours Research articles primary tumours review articles primary tumours PubMed articles primary tumours PubMed Central articles primary tumours 2023 articles primary tumours 2024 articles primary tumours Scopus articles primary tumours impact factor journals primary tumours Scopus journals primary tumours PubMed journals primary tumours medical journals primary tumours free journals primary tumours best journals primary tumours top journals primary tumours free medical journals primary tumours famous journals primary tumours Google Scholar indexed journals immune checkpoint inhibition therapy articles immune checkpoint inhibition therapy Research articles immune checkpoint inhibition therapy review articles immune checkpoint inhibition therapy PubMed articles immune checkpoint inhibition therapy PubMed Central articles immune checkpoint inhibition therapy 2023 articles immune checkpoint inhibition therapy 2024 articles immune checkpoint inhibition therapy Scopus articles immune checkpoint inhibition therapy impact factor journals immune checkpoint inhibition therapy Scopus journals immune checkpoint inhibition therapy PubMed journals immune checkpoint inhibition therapy medical journals immune checkpoint inhibition therapy free journals immune checkpoint inhibition therapy best journals immune checkpoint inhibition therapy top journals immune checkpoint inhibition therapy free medical journals immune checkpoint inhibition therapy famous journals immune checkpoint inhibition therapy Google Scholar indexed journals surgical resection articles surgical resection Research articles surgical resection review articles surgical resection PubMed articles surgical resection PubMed Central articles surgical resection 2023 articles surgical resection 2024 articles surgical resection Scopus articles surgical resection impact factor journals surgical resection Scopus journals surgical resection PubMed journals surgical resection medical journals surgical resection free journals surgical resection best journals surgical resection top journals surgical resection free medical journals surgical resection famous journals surgical resection Google Scholar indexed journals
Article Details
1. Introduction
Mismatch repair proteins (MMR) have been extensively investigated, especially in colorectal carcinomas, with a reported incidence of mismatch repair protein deficiency (MMRd) between 10%-20% [1]. Additionally, deficient cases are commonly associated with improved clinical outcome although this effect cannot be demonstrated in all studies [2, 3]. Comparison between primary tumours in the colon and metastases has been attempted with most studies reporting high concordance rates of approximately 95% [4-6]. Additionally, MMRd tumours have been shown to be responsive to immune checkpoint therapies, thus providing a potential novel therapeutic strategy for those patients [7]. In upper gastrointestinal carcinomas data is scarce but the incidence of MMRd is comparable - recently we could show that 27.2% of analysed adenocarcinomas of the esophagogastric junction showed loss of MLH1 and/or MSH2 although no association with patients’ survival was identifiable [8, 9]. No studies addressing the possible differences between mismatch repair protein status in primary tumours and metastases exist for this entity. We therefore aimed to determine whether mismatch repair protein expression showed high concordance between primary tumours and metastases and whether MMR status was prognostic in metastatic disease. Additionally, our analyses might provide a rationale to identify patients who benefit from immune checkpoint inhibition therapy.
2. Materials and Methods
2.1 Selection of cases
Samples (formalin-fixed, paraffin-embedded tissue, FFPE) of 41 adenocarcinomas of the esophagogastric junction and matched distant metastases which had been surgically removed at the University Hospital Schleswig-Holstein, Campus Luebeck during 1992-2014 were analysed. The study was approved by the local Ethics Committee beforehand (reference 14-242A), follow-up data was available for all patients.
2.2 Construction of tissue Microarrays (TMAs)
In accordance with the manufacturer’s instructions, tissue microarrays were constructed using the manual QuickRay® kit (Unitma, Seoul, Korea). One representative core per primary tumour and metastasis (each 2mm in diameter) was dissected. Appropriate positive and negative controls were included.
2.3 Immunohistochemistry and its evaluation
Immunohistochemical studies were performed using a standard, three-step immunoperoxidase technique and the automated Menarini Bond Max System (Menarini Diagnostics, Berlin, Germany) with the following antibodies: MLH1 (Cell Marque, clone G168-728, dilution 1:50) and MSH2 (Cell Marque, Rocklin, California, USA, clone G219-1129, dilution 1:100). Nuclear staining was evaluated as being either negative/absent or positive/retained. DNA mismatch repair status was then calculated using both markers, showing mismatch repair proficiency (MMRp) when expression of both markers was retained or mismatch repair deficiency (MMRd) when expression of one or both markers was lost.
2.4 Statistics
Statistical analysis was conducted using SPSS Statistics version 22 (IBM, Ehringen, Germany). Survival differences, outcome and overall survival were analysed via Kaplan-Meier estimate (including Log rank-test). Correlation of expression differences in primary tumours and metastases was determined with Fisher’s exact-test, correlation with clinical parameters was assessed using Chi-square-test. A p-value < 0.05 was considered to be statistically significant.
3. Results
3.1 Clinical characteristics of the study population
41 patients were included, 36 patients were male (87.8%), 5 female (12.2%) with a median age of 56 years (ranging from 27 to 72 years. Most tumours were locally advanced with the majority (34 cases 82.9%) presenting with infiltration beyond the muscularis propria (pT3 and pT4). Lymph node metastases were common (33 cases; 80.4%) as well as lymphovascular invasion (28 cases; 68.3%). The most common metastatic sites were non-regional lymph nodes (7 cases; 17.1%), liver (6 cases; 14.6%), soft tissue (5 cases; 12.2%), bone, peritoneum and chest wall (4 cases each; 9.8%). Characteristics of the study group are summarized in Table 1.
3.2 MMR status in primary tumours and metastases
14 primary tumours (34.1%) showed loss of expression of either MLH1 or MSH2 (MMRd) while 27 cases showed nuclear staining for both markers (MMRp). Loss of MLH1 was observed in 8 carcinomas, while the remaining 6 tumours showed loss of both immunohistochemical markers. None of the cases were MSH2 negative alone. 15 metastases (36.6%) showed loss of one or both markers, being classified as MMRd, 25 showed retained expression (MMRp), 1 case was not evaluable. MLH1 expression alone was lost in 3 cases while 7 tumours showed absence of MSH2 staining. In the remaining 5 carcinomas expression of both markers was lost. Overall, 11 metastases showed a shift from MMRp to MMRd, while 8 showed MMR proficiency with the primary tumour classified as MMRd. In 21 cases MMR status was stable between primary tumour and metastasis (concordance rate 52.5%).
3.3 Correlation with clinical characteristics
Statistical analysis showed no significant correlation between mismatch repair protein status and various clinical features such as gender, pT- and pN-stage, lymphovascular invasion, perineural invasion and completeness of surgical resection (p-values 0.138-0.975). Characteristics of the study population are summarized in Table I.
3.4 Correlation with overall survival
Considering MMR status in primary tumours, overall survival was not different between MMRd and MMRp groups (p=0.826) with median survival times of 44.62 months +/- 17.56 months for MMRd tumors and 41.52 months +/- 9.97 months for MMRp tumors. Using MMR status of metastatic sites, overall survival was markedly better for MMRp cases, though not statistically significant (p=0.097). Median survival times were 21.92 months +/- 5.44 months for MMRd cases and 56.08 months +/- 13.82 months for MMRp cases. Appropriate survival curves are shown in Figure 1.
|
Characteristic |
All cases |
MMRp |
MMRd |
p-value |
|
Total n |
41 |
25 |
15 |
|
|
Gender |
||||
|
Male |
36 |
22 |
13 |
0.902 |
|
Female |
5 |
3 |
2 |
|
|
pT |
||||
|
(low) pT0 |
1 |
1 |
0 |
0.975 |
|
pT1a |
0 |
0 |
0 |
|
|
pT1b |
3 |
2 |
1 |
|
|
pT2 |
3 |
2 |
1 |
|
|
(high) pT3 |
29 |
17 |
11 |
|
|
pT4a |
3 |
2 |
1 |
|
|
pT4b |
2 |
1 |
1 |
|
|
pN |
||||
|
pN0 |
8 |
6 |
2 |
0.850 |
|
pN1 |
8 |
4 |
3 |
|
|
pN2 |
11 |
7 |
4 |
|
|
pN3 |
14 |
8 |
6 |
|
|
LVSI |
||||
|
Present |
28 |
17 |
11 |
0.722 |
|
Absent |
13 |
8 |
4 |
|
|
Perineural invasion |
||||
|
Present |
13 |
6 |
7 |
0.138 |
|
Absent |
28 |
19 |
8 |
|
|
Surgical resection |
||||
|
Complete |
33 |
21 |
11 |
0.414 |
|
Incomplete |
8 |
4 |
4 |
|
Table 1: Clinicopathologic characteristics of the study population according to MMR status in metastases.
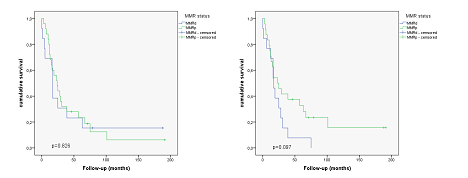
Figure 1: Kaplan Meier curves showing survival differences in primary tumors and metastases in relation to MMR status. Left: Assessment using MMR status in primary tumors (p=0.826). Right: Assessment in metastases (p= 0.097).
4. Conclusion
Reported incidences of mismatch repair protein deficiency vary but approximately 10-20% of colorectal carcinomas are MMRd-rates are comparable for upper gastrointestinal tract carcinomas [1, 3, 10]. In our study we could demonstrate incidence rates that were slightly higher, especially concerning tumours that metastasized: 34.1% of primary tumours and 36.6% of metastases showed loss of one or both mismatch repair proteins. Appropriately, in our cohort which consisted mainly of patients with advanced tumours (82.9% of patients initially presented with pT3- and pT4-stages) a clear, though not statistically significant, difference in overall survival could be seen when comparing MMRd and MMRp cases (p=0.097). Patients with retained expression of mismatch repair proteins had markedly prolonged survival with a median of 56.08 months while patients with MMR deficiency showed survival times of 21.92 months which contrasts reports stating that MMRd tumours often show a more favourable clinical course and prolonged survival [2, 3, 11]. However, when taking into consideration only primary tumours, survival times were almost equal between MMRd and MMRp cases (p=0.826). Correlation of MMR status and clinical features showed no statistically significant differences (p-values 0.138-0.975)-this is in line with prior studies concerning gastric carcinomas which demonstrated no association between MMR status and various clinicopathologic factors [11, 12].
Reported concordance rates concerning MMR status are almost always very high, in colorectal carcinomas concordance rates of approximately 95% are reported while no such data is available for gastric and esophagogastric carcinomas [5]. Interestingly, our study revealed a much lower concordance rate of 52.5% which is in stark contrast to earlier results in colon carcinomas. Nevertheless, a thorough literature research showed that for other biomarkers and other entities, reported discordance rates are much higher. Especially for gastric and esophagogastric carcinomas, several studies comparing primary tumours and metastases reported discordance rates of 20-50%, for instance analysing Her2, c-MET, p53 [13-16]. This is attributed to the fact that several large studies could demonstrate that esophagogastric adenocarcinomas are often heterogeneous in their biomarker expression between primary tumours and metastases and show discordances in up to 63% providing potential barriers to personalized medicine and precision therapies [17]. Therefore, our results underline the previously reported heterogeneity of biomarker expression in this entity and should caution clinicians and pathologists alike to select appropriate material for immunohistochemical testing.
In addition to its sometimes attributed prognostic value, assessment of mismatch repair protein expression in primary tumours and metastases carries therapeutic value as well as MMRd tumours show markedly better response to immune checkpoint therapies because of their high immunogenic potential with elevated levels of tumour-infiltrating lymphocytes and PD-L1 expression [18, 19]. Therefore, concerning esophagogastric carcinomas, it might be crucial to test not only tissue from primary tumours but also from metastatic sites to stratify patients according to their potential therapeutic response. Further analyses might elucidate the true biological value of changes in expression profiles between primary tumours and metastases and provide a rationale for testing in daily practice.
Conflicts of Interest
None.
Author Contributions
JK and PLK performed morphological and immunohistochemical studies, analyzed the data and wrote the manuscript. EP, UW and RH contributed cases and clinical data and revised the manuscript. CT designed the research, performed morphological studies and revised the manuscript. All authors read and approved the final version of the manuscript.
References
- Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 100 (2009): 266-273.
- Hou JT, Zhao LN, Zhang DJ, et al. Prognostic Value of Mismatch Repair Genes for Patients With Colorectal Cancer: Meta-Analysis. Technol Cancer Res Treat 17 (2018).
- Lee HS, Choi SI, Lee HK, et al. Distinct clinical features and outcomes of gastric cancers with microsatellite instability. Mod Pathol 15 (2002): 632-640.
- He WZ, Hu WM, Wang F, et al. Comparison of Mismatch Repair Status Between Primary and Matched Metastatic Sites in Patients With Colorectal Cancer. J Natl Compr Canc Netw 17 (2019): 1174-1183.
- Jung J, Kang Y, Lee YJ, et al. Comparison of the Mismatch Repair System between Primary and Metastatic Colorectal Cancers Using Immunohistochemistry. J Pathol Transl Med 51 (2017): 129-136.
- Haraldsdottir S, Roth R, Pearlman R, et al. Mismatch repair deficiency concordance between primary colorectal cancer and corresponding metastasis. Fam Cancer 15 (2016): 253-260.
- Ryan E, Sheahan K, Creavin B, et al. The current value of determining the mismatch repair status of colorectal cancer: A rationale for routine testing. Crit Rev Oncol Hematol 116 (2017): 38-57.
- Knief J, Reddemann K, Petrova E, et al. Expression of cyclooxygenase-2 has no impact on survival in adenocarcinoma of the esophagogastric junction but is associated with favourable clinicopathologic features. Histol Histopathol 32 (2017): 735-741.
- Jin Z and Yoon HH. The promise of PD-1 inhibitors in gastro-esophageal cancers: microsatellite instability vs. PD-L1. J Gastrointest Oncol 7 (2016): 771-788.
- Huiping C, Kristjansdottir S, Bergthorsson JT, et al. High frequency of LOH, MSI and abnormal expression of FHIT in gastric cancer. Eur J Cancer 38 (2002): 728-735.
- Polom K, Böger C, Smyth E, et al. Synchronous metastatic gastric cancer-molecular background and clinical implications with special attention to mismatch repair deficiency. Eur J Surg Oncol 44 (2018): 626-631.
- Karpi?ska-Kaczmarczyk K, Lewandowska M, ?awniczak M, et al. Expression of Mismatch Repair Proteins in Early and Advanced Gastric Cancer in Poland. Med Sci Monit 22 (2016): 2886-2892.
- Amato M, Perrone G, Righi D, et al. HER2 Status in Gastric Cancer: Comparison between Primary and Distant Metastatic Disease. Pathol Oncol Res 23 (2017): 55-61.
- Wang B, Sun K, Zou Y. Comparison of a Panel of Biomarkers Between Gastric Primary Cancer and the Paired Krukenberg Tumor. Appl Immunohistochem Mol Morphol 25 (2017): 639-644.
- Stahl P, Seeschaaf C, Lebok P, et al. Heterogeneity of amplification of HER2, EGFR, CCND1 and MYC in gastric cancer. BMC Gastroenterol 15 (2015): 7.
- Peng Ye, Meizhuo Zhang, Shuqiong Fan, et al. Intra-Tumoral Heterogeneity of HER2, FGFR2, cMET and ATM in Gastric Cancer: Optimizing Personalized Healthcare through Innovative Pathological and Statistical Analysis. PLoS One 10 (2015): e0143207.
- Pectasides E, Stachler MD, Derks S, et al. Genomic Heterogeneity as a Barrier to Precision Medicine in Gastroesophageal Adenocarcinoma. Cancer Discov 8 (2018): 37-48.
- Viale G, Trapani D, Curigliano G. Mismatch Repair Deficiency as a Predictive Biomarker for Immunotherapy Efficacy. Biomed Res Int (2017): 4719194.
- Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 20 (2017): 407-415.
