Complete Response after Long-Term 2nd Line Treatment with Trabectedin in a Hemodialyzed Patient with Metastatic High-Grade Ovarian Sarcoma, Case Report and Short Review
Article Information
Efthymiadis Konstantinos, Adamidis Christos, Koukoutzeli Chrysanthi, Tzelepi Vassiliki, Timotheadou Eleni*
Department of Medical Oncology, Aristotle University of Thessaloniki, “Papageorgiou” General Hospital, Nea Efkarpia, Thessaloniki, Greece
*Corresponding Author: Dr. Timotheadou Eleni, Department of Medical Oncology, Aristotle University of Thessaloniki, “Papageorgiou” General Hospital, Nea Efkarpia, Thessaloniki, Greece
Received: 18 August 2019; Accepted: 07 September 2019; Published: 26 November 2019
Citation: Efthymiadis Konstantinos, Adamidis Christos, Koukoutzeli Chrysanthi, Tzelepi Vassiliki, Timotheadou Eleni. Complete Response after Long-Term 2nd Line Treatment with Trabectedin in a Hemodialyzed Patient with Metastatic High-Grade Ovarian Sarcoma, Case Report and Short Review. Archives of Clinical and Medical Case Reports 3 (2019): 591-599.
View / Download Pdf Share at FacebookAbstract
Primary ovarian sarcomas are very rare tumours and are characterized by poor prognosis. Surgical treatment is mainly based on epithelial ovarian cancer debulking approaches, while chemotherapy is based on data extrapolation from studies in sarcomas of other primary sites. Trabectedin has been established as a standard of care for the treatment of soft-tissue sarcomas in the 2nd line setting. So far, no data from randomized clinical trials support specific maximum number of cycles of trabectedin for any gynaecological sarcoma. Moreover, limited evidence in the literature supports the feasibility and safety of trabectedin administration in haemodialyzed patients.
This report presents the case of a 48-year old female diagnosed with metastatic high-grade undifferentiated sarcoma of the right ovary. She underwent primary surgery followed by 1st line chemotherapy with doxorubicin plus ifosfamide, and had a partial response after cycle 3. However, treatment tolerance was very poor with grade 3 and 4 hematologic and non-hematologic toxicities, including the development of chronic kidney failure that required permanent haemodialysis. Treatment was changed to trabectedin, leading to a complete response that is ongoing and lasts for over 2 years. Our case supports safety of long-term trabectedin administration in a hemodialyzed patient with the achievement of durable complete disease response on the metastatic setting.
Ovarian sarcoma articles, Trabectedin articles, Second-line chemotherapy articles, Complete response articles, Chronic kidney failure articles, Hemodialysis articles
Trabectedin articles Trabectedin Research articles Trabectedin review articles Trabectedin PubMed articles Trabectedin PubMed Central articles Trabectedin 2023 articles Trabectedin 2024 articles Trabectedin Scopus articles Trabectedin impact factor journals Trabectedin Scopus journals Trabectedin PubMed journals Trabectedin medical journals Trabectedin free journals Trabectedin best journals Trabectedin top journals Trabectedin free medical journals Trabectedin famous journals Trabectedin Google Scholar indexed journals health articles health Research articles health review articles health PubMed articles health PubMed Central articles health 2023 articles health 2024 articles health Scopus articles health impact factor journals health Scopus journals health PubMed journals health medical journals health free journals health best journals health top journals health free medical journals health famous journals health Google Scholar indexed journals Hemodialyzed articles Hemodialyzed Research articles Hemodialyzed review articles Hemodialyzed PubMed articles Hemodialyzed PubMed Central articles Hemodialyzed 2023 articles Hemodialyzed 2024 articles Hemodialyzed Scopus articles Hemodialyzed impact factor journals Hemodialyzed Scopus journals Hemodialyzed PubMed journals Hemodialyzed medical journals Hemodialyzed free journals Hemodialyzed best journals Hemodialyzed top journals Hemodialyzed free medical journals Hemodialyzed famous journals Hemodialyzed Google Scholar indexed journals Patient articles Patient Research articles Patient review articles Patient PubMed articles Patient PubMed Central articles Patient 2023 articles Patient 2024 articles Patient Scopus articles Patient impact factor journals Patient Scopus journals Patient PubMed journals Patient medical journals Patient free journals Patient best journals Patient top journals Patient free medical journals Patient famous journals Patient Google Scholar indexed journals Metastatic articles Metastatic Research articles Metastatic review articles Metastatic PubMed articles Metastatic PubMed Central articles Metastatic 2023 articles Metastatic 2024 articles Metastatic Scopus articles Metastatic impact factor journals Metastatic Scopus journals Metastatic PubMed journals Metastatic medical journals Metastatic free journals Metastatic best journals Metastatic top journals Metastatic free medical journals Metastatic famous journals Metastatic Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals Ovarian Sarcoma articles Ovarian Sarcoma Research articles Ovarian Sarcoma review articles Ovarian Sarcoma PubMed articles Ovarian Sarcoma PubMed Central articles Ovarian Sarcoma 2023 articles Ovarian Sarcoma 2024 articles Ovarian Sarcoma Scopus articles Ovarian Sarcoma impact factor journals Ovarian Sarcoma Scopus journals Ovarian Sarcoma PubMed journals Ovarian Sarcoma medical journals Ovarian Sarcoma free journals Ovarian Sarcoma best journals Ovarian Sarcoma top journals Ovarian Sarcoma free medical journals Ovarian Sarcoma famous journals Ovarian Sarcoma Google Scholar indexed journals Sarcoma articles Sarcoma Research articles Sarcoma review articles Sarcoma PubMed articles Sarcoma PubMed Central articles Sarcoma 2023 articles Sarcoma 2024 articles Sarcoma Scopus articles Sarcoma impact factor journals Sarcoma Scopus journals Sarcoma PubMed journals Sarcoma medical journals Sarcoma free journals Sarcoma best journals Sarcoma top journals Sarcoma free medical journals Sarcoma famous journals Sarcoma Google Scholar indexed journals infusion articles infusion Research articles infusion review articles infusion PubMed articles infusion PubMed Central articles infusion 2023 articles infusion 2024 articles infusion Scopus articles infusion impact factor journals infusion Scopus journals infusion PubMed journals infusion medical journals infusion free journals infusion best journals infusion top journals infusion free medical journals infusion famous journals infusion Google Scholar indexed journals patient articles patient Research articles patient review articles patient PubMed articles patient PubMed Central articles patient 2023 articles patient 2024 articles patient Scopus articles patient impact factor journals patient Scopus journals patient PubMed journals patient medical journals patient free journals patient best journals patient top journals patient free medical journals patient famous journals patient Google Scholar indexed journals
Article Details
Abbreviations:
A & E: accident & emergency; AUC: area under the curve; CR: complete response; CrCl: creatinine clearance; D & C: dilation & curettage; DCR: disease control rate; ESS: endometrial stromal sarcoma; L-STS: leiomyosarcoma, liposarcoma); LMS: leiomyosarcoma; LN: lymph node; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; POLMS: primary ovarian leiomyosarcoma; PR: partial response; PRBC: packed red blood cell; RLL: right lower lobe; RML: right median lobe; RMS: rhabdomyosarcoma; STS: soft-tissue sarcoma
1. Introduction
1.1 Primary ovarian sarcomas
Primary ovarian sarcomas are very rare malignant solid tumors of mesenchymal histology, characterized by limited response to platinum-based chemotherapy and lower progression-free and overall survival compared to epithelial ovarian cancers [1]. Based on the latest WHO classification they are divided in:
- malignant mesenchymal ovarian tumors which include low and high-grade Endometrial Stromal Sarcoma (ESS)
- mixed mesenchymal and epithelial malignant tumors including adenosarcomas and carcinosarcomas (ex -malignant mixed mullerian tumors) [2].
Other primary ovarian sarcomas, like leiomyosarcomas and rhabodmyosarcomas can be classified either as their primary uterine counterparts or as abdominal Soft Tissue Sarcoma (STS) [3].
The most frequent ovarian sarcoma is mixed mullerian tumor/ carcinosarcoma accounting for 1-4% of primary ovarian cancer with an incidence of 0.19/ 100,000 women [4]. Prognosis is poorer than epithelial ovarian cancer with a median survival of 18 months [5]. Reports of patients surviving over 46 months underline the importance of optimal debulking followed by ifosfamide/ cisplatin or platinum-based chemotherapy [6]. Other ovarian sarcoma types are considered very rare, accounting for approximately 1% of primary ovarian cancer [7] and include angiosarcoma, rhabdomyosarcoma, endometrial stromal sarcoma and fibrosarcoma.
1.2 Trabectedin
Trabectedin is an antineoplastic agent that was initially extracted from the marine tunicate species Esteinascidia turbinata. Its mechanism of action involves direct interference with the minor DNA groove, induction of apoptosis and inhibition of the expression of FUS-CHOP, EWS-CHOP and other transcriptional factors that act as oncogene products and immunomodulatory and anti-inflammatory activity [8-10]. Currently, trabectedin is used as 2nd line therapy and beyond for advanced STS. Initial approval was granted in 2007 in Europe, for administration in advanced STS patients progressing on/ or being ineligible for anthracycline and ifosfamide therapy. Phase 2 clinical trial results showed a median duration of survival of 9.2 months and comparable PFS to standard chemotherapy regimens in pretreated patients [11]. Subsequently, approval was granted in the USA in 2015 for patients with advanced pretreated L-STS, including uterine LMS.
In pretreated patients with advanced/ metastatic L-STS, treatment with trabectedin showed notable efficacy and favorable toxicity profile at a dose of 1.5 mg/m2 through 24-hour continuous intravenous infusion q3w achieving superior disease control compared to a dose of 0.58 mg/m2 3-hour intravenous infusion d1, d8, d15 q4w [12]. Subsequently, the dose of 1.5 mg/m2 through 24-hour continuous infusion q3w exhibited significantly longer PFS [4.2 versus 1.5 months, (hazard ratio, 0.55; P<0.001)] compared to iv. dacarbazine 1000mg/m2 q3w in patients with L-STS [13].
2. Case Presentation
A 48year-old female was admitted in the A & E department with abdominal pain reflecting to the right iliac and inguinal region. Lab workup indicated acute kidney injury and abdominal & pelvic imaging with ultrasonography, CT and MRI revealed a 9cm pelvic mass, causing right ureter obstruction and a LN block encasing right iliac vessels and para-aortic LNs (Figure 1). Medical history included recurrent vaginal bleeding for which she had three dilatation and curretage procedures (all negative for malignancy) within ten years and eventually a TAH that was performed ten months before present diagnosis.
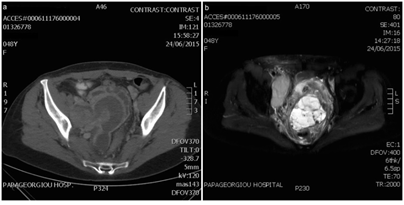
Figure 1: Diagnostic imaging of the primary right ovary lesion; a: CT scan, b: MRI scan, T2 weighted sequence with SPAIR (SPectral Attenuated Inversion Recovery) fat suppression.
Right percutaneous nephrostomy was placed and the patient underwent primary debulking surgery, including BSO, omentectomy, douglasectomy, bilateral cremasterectomy and pelvic, paraortic and small bowel mesenteric LN dissection. Pathology reported a high-grade undifferentiated sarcoma of the right ovary. Specimen stained positive for vimentin, desmin, S100, CD10, CD117, p53, with IHC being negative for AE1/AE3, HMW, LMW, Ck7, Ck20, EMA, LCA, inhibin A, CD99, calretinin, SMA, caldesmon, and ER. Stain for PR was focally positive. The omentum, all para-aortic LNs (0/22), small bowel mesenteric lymph nodes (0/3) and left pelvic LNs (0/17) were clear. Metastatic disease was detected in one mesenteric nodule, one left paracolic nodule, 13 of 16 right pelvic LNs and in Douglas’ pouch specimen.
Postoperative CT revealed bilateral metastatic lung nodules and residual disease both in the primary site and in the pelvic nodes (Figure 2). She received 1st line chemotherapy with iv. doxorubicin 25 mg/m2 d1, ifosfamide 3000 mg/m2 d1-d3 q3w plus uromitexan 3000 mg/m2 d1- d4 and sc. filgrastim 48mU d5-d12. CTs after 3 cycles showed partial response in the thorax and abdomen (Figure 2).

Figure 2: Staging CT scans; a1, a2: post-operative chest and abdominal/pelvis CT; b1, b2: CT restaging after 3 cycles of 1st line treatment with doxorubicin/ ifosfamide; c1, c2: CT restaging after 3 cycles of 2nd line treatment with trabectedin.
Treatment tolerance was poor with serious adverse events. Twice she developed febrile neutropenia following cycles 2 and 3 requiring hospitalization and treatment with piperacilln/ tazobactam and meropenem/ teicoplanin respectively. She also developed grade IV anemia (Hgb=6,1 g/dL) following cycle 1 and grade 4 thrombocytopenia (PLT count=15,000/mm3) after cycle 3, despite dose modifications. Additionally, the patient developed grade 3 ifosfamide-induced encephalopathy during cycle 3, that fully resolved with discontinuation of the drug, without medication.
Renal function gradually deteriorated to grade 4 chronic renal failure, two weeks after cycle 3 (Cr=5.25 mg/dL, CrCl=11.16 mL/min/1.73 m2). The patient required long hospitalization due to complications including urinary tract infection. Obstructive renal disease was excluded and she was started on regular hemodialysis. Colimycin and tigecycline were administered for a multi-drug resistant strain of Pseudomonas aeruginosa in the urine. At that time, a right kidney biopsy indicated infectious pyelonephritis.
Treatment with doxorubucin/ ifosfamide was permanently discontinued and was replaced with iv. trabectedin 1.5 mg/2 d1 q3w, with d1 given at the same day with hemodialysis. First CT restaging after 3 cycles revealed thoracic and abdominal PR (Figure 3) that was maintained in the subsequent scans. However, new lung nodules of 5mm appeared in the RML and RLL at the CT restaging after cycle 14 (11 months of treatment) (Figure 3). Considering the small volume of progressive disease and the patient’s excellent performance status, treatment continuation was decided, leading to gradual partial remission and finally to complete thoracic disease remission and no evidence of abdominal disease after cycle 25 (Figure 3). The patient has so far received 47 cycles of trabectedin for 3 years and remains disease-free by conventional imaging for over one year, while completing 3 years on hemodialysis. Treatment has been overall well tolerated with mild hematological toxicity managed with a 25% dose reduction plus filgrastim-48mU, S:1 × 1 for 5 days after recurrent uncomplicated grade 3 neutropenia.

Figure 3: Staging CT scans; a1, a2: CT restaging after 3 cycles of 2nd line treatment with trabectedin; b1, b2: CT restaging after 11months of treatment with trabectedin revealing new lung nodules; c1, c2: CT restaging after 25 cycles of trabectedin revealing complete response with no evidence of disease.
3. Discussion
Real-life data of trabectedin in STS treatment are indicative of similar or even better outcomes than the original clinical trials that led to drug approval in terms of PFS and OS. These findings vary in efficacy with ORR of 37.8%, maximum DCR of 81.8%, median PFS of 11.6 months and OS of 22.3 months. Regarding safety, the most frequently reported grade 3/4 side effects are neutropenia and liver toxicity and toxicity-related treatment discontinuation rates reach 18% [14]. Buonnadonna et al. evaluated trabectedin treatment outcomes and toxicity in 218 patients from 41 centers in Europe mainly diagnosed with leiomyosarcomas, liposarcomas and synovial sarcomas. ORR was 26.6%, median PFS was 5.9 months, and median OS was 21.3 months. Most common grade 3/4 adverse effects were febrile neutropenia (2.3%), neutropenia (1.4%), nausea (1.4%), and pneumonia (1.4%). Approximately half of the patients received at least 7 cycles [15].
The present report is the first supporting the safety and effectiveness of long-term Trabectedin administration as 2nd line treatment for ovarian sarcoma in a hemodialyzed patient. Patient has received so far 47 cycles of Trabectedin and has been on hemodialysis for 3 years, having achieved an ongoing CR that lasts for over one year. Duration of treatment exceeds those in previous case reports and case series (Table 1, refs 16-21), and also the ones that have been reported in the prospective study of Buonadonna et al. (longest time on treatment 44.2 months, and maximum number of cycles 44, for which data on sarcoma location and histology is not reported).
|
Reference |
Sarcoma pathology |
Initial & later surgery |
Adjuvant therapy |
Trabectedin |
Best response |
|
|
Line |
Cycles |
|||||
|
Bongiovanni et al., 2015 [37] |
Uterine LMS |
TAH, post-recurrence oophorectomy, lung metastasectomy |
No |
4th |
22 |
PR |
|
Haslbauer, 2018 [38] |
Inguinal LMS |
Tumor resection, |
R/T (60Gy) C/T (Doxorubicin x6 cycles) |
2nd |
27 |
PR |
|
Maruzzo et al., 2015 [39] |
Pelvic epithelioid LMS |
TAH-BSO |
Radiotherapy |
1st |
25 |
SD (RECIST) PR (Choi) |
|
Nteli et al., 2018 [40] |
Uterine LMS |
TAH-BSO |
No |
2nd |
>9 |
CR thorax PR abdomen |
|
Tavella et al., 2017 [41] |
Uterine LMS |
TAH-BSO, secondary surgery |
C/T (Epirubicin, |
2nd |
>30 |
PR |
|
Tewari et al., 2006 [42] |
Uterine LMS |
TAH |
No |
5th |
>12 |
PR |
Table 1: Case reports of long-term metastatic sarcoma treatment with Trabectedin. Abbreviations: BSO: bilateral salpingo-oophorectomy, C/T: chemotherapy, CR: complete response, ESS: endometrial stromal sarcoma, LMS: leiomyosarcoma, TAH: total abdominal hysterectomy, SD: stable disease, PR: partial response, R/T: radiotherapy.
Lack of data on trabectedin administration in hemodialyzed patients is noteworthy. Thariat et al. [22] present an interesting case of an individualized pharmacokinetic study in a single hemodialyzed patient treated with trabectedin for a retroperitoneal myxoid liposarcoma that recurred following immunosuppressive therapy for kidney transplantation. Cmax and AUC were higher, while clearance, terminal half-life, and volume of distribution were lower in the patient compared to mean values of normal kidney function patients. The patient was reportedly on 5th month of treatment before presenting with disease progression. This is the only report in the literature supporting the feasibility and safety of trabectedin administration during hemodialysis for end-stage kidney disease.
Similarly, Moe et al. [23] report the case of a patient on peritoneal dialysis receiving treatment with trabectedin after end-stage kidney disease caused by ifosfamide therapy for a metastatic high-grade spindle-cell sarcoma. Trabectedin was not detectable in the peritoneal dialysates and no serious adverse effects were observed. The patient received 6 cycles before presenting with disease progression. In both cases, low dialysis clearance, as well as the absence of detectable trabectedin in the dialysate samples, implies that trabectedin is not efficiently cleared either by hemodialysis, or by peritoneal dialysis.
The present case indicates that durable complete responses can be achieved with trabectedin therapy in such patients. In this regard, more pharmacokinetic studies are needed, as cancer incidence and recurrence are high in end-stage kidney disease patients and trabectedin appears to be an attractive option for sarcoma treatment in this group.
Acknowledgements
The authors thank the Radiology Department of Papageorgiou General Hospital for kindly providing CT and MRI scan images.
Conflict of Interest
There are no conflicts of interest to declare.
References
- Magné N, Pacaut C, Auberdiac P, et al. Sarcoma of vulva, vagina and ovary. Best Pract Res Cl Ob 25 (2011): 797-801.
- Kurman RJ, Carcangiu ML, Herrington CS, et al. WHO Classification of Tumours of Female Reproductive Organs. Fourth Edition. 2014. WHO Classification of Tumours IARC 6 (2014).
- Fletcher CDM, Bridge JA, Hogendoorn P, et al. WHO Classification of Tumours of Soft Tissue and Bone. Fourth Edition. 2013. WHO Classification of Tumours IARC 5 (2013).
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Population), National Cancer Institute, Bethesda (2012).
- del Carmen MG, Birrer M, Schorge JO. Carcinosarcoma of the ovary: a review of the literature. Gynecol Oncol 125 (2012): 271-277.
- Mok JE, Kim YM, Jung MH, al. Malignant mixed mullerian tumors of the ovary: experience with cytoreductive surgery and platinum-based combination chemotherapy. Int J Gynecol Cancer 16 (2006): 101-
- DiSaia PJ, Pecorelli S. Gynecological sarcomas. Semin Surg Oncol 10 (1994): 369-
- D’Incalci M, Galmarini CM. A review of trabectedin (ET-743): a unique mechanism of action. Mol Cancer Ther 9 (2010): 2157-
- Guirouilh-Barbat J, Redon C, Pommier Y. Transcription-coupled DNA double-strand breaks are mediated via the nucleotide excision repair and the Mre11-Rad50-Nbs1 complex. Mol Biol Cell 19 (2008): 3969-
- Allavena P, Signorelli M, Chieppa M, et al. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res 65 (2005): 2964-2971.
- Le Cesne A, Blay JY, Judson I, et al. Phase II study of ET-743 in advanced soft tissue sarcomas: a European Organisation for the Research and Treatment of Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin Oncol 23 (2005): 576-5
- Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol 27 (2009): 4188-
- Demetri GD, von Mehren M, Jones RL, et al. Efficacy and safety of trabectedin or dacarbazine for metastatic liposarcoma or leiomyosarcoma after failure of conventional chemotherapy: results of a Phase III Randomized Multicenter Clinical Trial. J Clin Oncol 34 (2016): 786-
- Assi T, Kattan J, El Rassy E, et al. A comprehensive review of the current evidence for trabectedin in advanced myxoid liposarcoma. Cancer Treat Rev 72 (2019): 37-44.
- Buonadonna A, Benson C, Casanova J, et al. A noninterventional, multicenter, prospective phase IV study of trabectedin in patients with advanced soft tissue sarcoma. Anticancer Drugs 28 (2017): 1157-1165.
- Bongiovanni A, Riva N, Ricci M, et al. Long-lasting activity of trabectedin in refractory uterine leiomyosarcoma: a case report. BMC Cancer 15 (2015): 998.
- Haslbauer F. Long-Term Progression-Free Survival in a Patient with Metastatic Leiomyosarcoma of the Inguinal Region Treated with Trabectedin. Case Rep Oncol 11 (2018): 246-251.
- Maruzzo M, Brunello A, Diminutto A, et al. Long-term response to first-line trabectedin in an elderly female patient with a metastatic leiomyosarcoma unfit for anthracycline. Anticancer Drugs 27 (2016): 264-267.
- Nteli VA, Knauf W, Janton-Klein A, et al. Long-Lasting Response to Trabectedin in a Patient with Metastatic Uterine Leiomyosarcoma: A Case Report. Case Rep Oncol 11 (2018): 81-89.
- Tavella K, Villanucci A, Vannini L, et al. Stable disease in a patient with metastatic leiomyosarcoma treated with trabectedin. Anticancer Drugs 28 (2017): 465-468.
- Tewari D, Saffari B, Cowan C, et al. Activity of trabectedin (ET-743, Yondelis) in metastatic uterine leiomyosarcoma. Gynecol Oncol 102 (2006): 421-424.
- Thariat J, Etienne-Grimaldi MC, Launay-Vacher V, et al. Pharmacokinetics of trabectedin on hemodialysis: an application for the management of cancer patients with end-stage renal disease. Cancer Chemother Pharmacol 68 (2011): 1363-136
- Moe MM, Fernandez Teruel C, Soto-Matos A. Experience with trabectedin in an advanced sarcoma patient on peritoneal dialysis for end-stage renal failure. Chemotherapy 59 (2013): 458-4
