Complete and Durable Response in Metastatic Uterine Leiomyosarcoma with Multimodal Therapy: A Case Report
Article Information
Ramin Ajami MD1*, Reza Moghareabed MD2, Golsa Shekarkhar MD3, Amir Hosein Mehrtash4
1Department of Oncology, Royal Free Hospital, London, UK
2Department of Radiation Oncology, Isfahan University of Medical Sciences
3Department of Molecular Pathology, Deghat Private Lab,
4Department of Molecular pathology, Omid genetics, UK
*Corresponding Author: Ramin Ajami, Medical Oncologist, Department of Oncology, Royal Free Hospital, London, UK.
Received: 29 September 2025; Accepted: 13 October 2025; Published: 27 October 2025
Citation: Ramin Ajami, Reza Moghareabed, Golsa Shekarkhar, Amir Hosein Mehrtash. Complete and Durable Response in Metastatic Uterine Leiomyosarcoma with Multimodal Therapy: A Case Report. Archives of Clinical and Medical Case Reports. 9 (2025): 217-224.
View / Download Pdf Share at FacebookAbstract
Background: Uterine leiomyosarcoma (LMS) is a rare, aggressive malignancy (1-2% of uterine cancers) [1] often initially misdiagnosed as benign fibroids. There is no reliable preoperative method to distinguish LMS from leiomyoma, and the diagnosis is typically confirmed post-hysterectomy on pathology [1]. LMS frequently metastasises hematogenously (commonly to the lungs, liver, and peritoneum) [1,10], and the prognosis of advanced disease is poor (5-year survival rate is ~15- 20%) [1,10]. The standard treatment is total hysterectomy; the benefit of adjuvant therapy remains unclear, although chemotherapy and radiation are often attempted in high-risk cases [7,8]. LMS can express hormone receptors, suggesting a potential benefit of endocrine therapy in select cases [2,5].
Case: We present the case of a 54-year-old woman with a history of a rapidly enlarging “fibroid” who underwent hysterectomy and was found to have high-grade LMS with metastatic spread. The tumour was strongly positive for oestrogen, progesterone, and androgen receptor. Postoperatively, she received pelvic radiation, combined ovarian suppression (goserelin) plus antiandrogen (bicalutamide) hormonal therapy, and targeted kinase inhibitor pazopanib. This regimen was chosen in lieu of conventional chemotherapy, given the tumour’s receptor profile.
Outcomes: The patient achieved complete metabolic remission on PET-CT three months after treatment. She has remained disease-free on maintenance therapy with goserelin, bicalutamide, and pazopanib for over 24 months of follow-up, with no evidence of recurrence.
Conclusion: This exceptional response in patients with metastatic LMS highlights the potential of an individualised multimodal approach. Exploiting hormone receptor positivity with endocrine therapy and incorporating targeted anti-angiogenic therapy (pazopanib) achieved an outcome rarely observed in advanced LMS. Ongoing surveillance is paramount. This case underscores the importance of multidisciplinary, personalised strategies and encourages further research on hormonetargeted and innovative therapies for uterine LMS.
Keywords
Uterine leiomyosarcoma; Metastatic uterine cancer; Hormone receptor-positive sarcoma; Androgen receptor antagonists; Gonadotropinreleasing hormone agonist; Pazopanib; Multimodal therapy; Complete remission
Article Details
1. Background
Uterine leiomyosarcoma (LMS) is a rare and aggressive smooth muscle tumour that accounts for only 1-2% of uterine cancers [1]. Distinguishing an LMS from benign uterine fibroids (leiomyomas) preoperatively is exceptionally challenging, and there is no definitive preoperative test to reliably differentiate a uterine sarcoma from a fibroid [1]. Both can present with abnormal bleeding, pelvic pain, or an enlarged uterine mass. However, a rapidly growing “fibroid” in peri- or postmenopausal women should raise the suspicion of an underlying sarcoma [2,3]. In most cases, the accurate diagnosis of LMS is only made after histopathological examination of a hysterectomy or myomectomy specimen initially presumed to be a benign fibroid [1]. The incidence of unexpected LMS in women undergoing surgery for presumed fibroids is low (~0.1-0.3%) [1], but this possibility has significant implications.
LMS generally progresses aggressively with a high risk of recurrence and early metastatic spread (commonly to the lungs, liver, or peritoneum) [1,10]. Even when diagnosed in the early stages, uterine LMS has a propensity for hematogenous dissemination, and the prognosis is typically poor once the disease advances. Five-year survival rates for stage IV uterine LMS is < 20% [1,10]. The standard treatment involves surgical resection (total hysterectomy with removal of the ovaries) whenever possible [1]. The benefit of routine adjuvant therapy (chemotherapy or radiotherapy) remains uncertain; studies have not shown a clear improvement in overall survival with the addition of adjuvant treatment in uterine LMS [7,8]. Nonetheless, for high-risk or advanced cases, a multidisciplinary therapeutic approach is often adopted for high-risk or advanced cases.
Anthracycline-based chemotherapy (e.g., doxorubicin ± ifosfamide, or combination regimens such as gemcitabine/docetaxel) is commonly used for metastatic LMS, but objective response rates are modest (approximately 20%) [7]. Targeted therapy with anti-angiogenic agents such as pazopanib is an approved option after chemotherapy failure. In the phase III PALETTE trial, pazopanib significantly prolonged progression-free survival in metastatic soft-tissue sarcomas, with similar efficacy observed in uterine LMS as in other sarcoma subtypes [6]. Having said that, there is no definitive treatment for metastatic LMS with a meaningful and sustainable response.
Notably, a substantial subset of uterine LMS expresses hormone receptors. Approximately 40-60% of cases have oestrogen receptor (ER) and/or progesterone receptor (PR) expression [4], and approximately 20-30% express the androgen receptor (AR) [5]. While hormonal therapy is the standard treatment for low-grade endometrial stromal sarcomas, its role in high-grade LMS is not well established. Small retrospective studies have reported some clinical benefits from endocrine therapy (such as aromatase inhibitors) in ER/PR-positive metastatic LMS, for example, partial response rates of approximately 9-12% and disease stabilisation in approximately 30-60% of patients in selected series [2]. Accordingly, expert guidelines suggest that endocrine therapy (e.g., ovarian suppression or aromatase inhibitors) may be considered for advanced uterine LMS if the tumour is strongly hormone receptor-positive, especially when the disease is indolent or of low volume [2]. AR expression in LMS has been associated with more favourable outcomes; one study found that co-expression of AR along with ER/PR was linked to significantly improved survival (100% 5-year survival in AR-positive cases vs. ~64% in AR-negative cases) [5]. These observations raise the possibility of targeting hormonal pathways in select patients with LMS. Here, we report a unique case of an advanced uterine LMS that was “triple-positive” (ER, PR, and AR positive) and achieved complete remission with an individualised multimodal treatment approach including surgery, radiotherapy, hormonal therapy, and targeted therapy. This case highlights the potential benefit of leveraging the tumour hormone receptor status and targeted agents in the management of metastatic LMS.
2. Case Presentation
A 54-year-old woman (gravida 3, para 2) presented in April 2023 after undergoing a hysterectomy with bilateral salpingo-oophorectomy as adjuvant therapy. She had a history of pelvic pain and intermittent vaginal bleeding for three years and was diagnosed with uterine fibromatosis one year prior through transvaginal sonography, which showed mixed echo masses measuring 67×64 mm and 65×78 mm, respectively. The initial management plan was conservative, given her proximity to menopause, and the gynaecologist recommended observation, assuming that fibroid-related symptoms might decrease after menopause. The patient was treated symptomatically with tranexamic acid to manage the menorrhagia.
Over the following months, the patient’s symptoms persisted and gradually worsened. Despite approaching menopause, she reported increasing pelvic pain, heavier, irregular bleeding, and noticeable abdominal distension. By late 2023, during re-evaluation, the pelvic mass had enlarged, raising concerns about a possible sarcomatous transformation in a fibroid (considering the unusual rapid growth in a 54-year-old patient) [2,3]. After counselling, the patient opted for definitive surgery. In November 2023, she underwent a total abdominal hysterectomy with bilateral salpingo-oophorectomy (TAH-BSO). The surgery was uncomplicated. Intraoperatively, the uterus was enlarged by a bulky mass; grossly, the tumour appeared somewhat atypical (fleshy, soft, with a yellow-tan cut surface, and areas of necrosis). The uterine tumour and ovaries were removed en-bloc. No obvious extrauterine disease or metastases were observed during surgery.
3. Investigations and Imaging
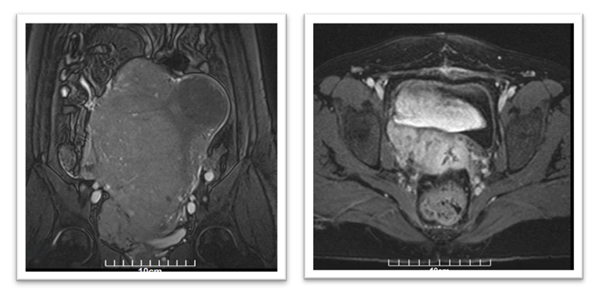
Postoperatively, the patient’s immediate recovery was unremarkable. However, approximately four weeks after surgery, she developed right-sided pelvic discomfort. MRI of the abdomen and pelvis was performed in May 2023 to assess any postoperative complications or residual disease. This imaging revealed a sizable mass in the right pelvic cavity (~8×6×4 cm) in the region of the hysterectomy bed, raising concerns for either local residual tumour or early recurrence. Further evaluation using chest imaging identified multiple bilateral lung nodules (up to 1-2 cm in diameter) that were suspicious for metastatic lesions (Figure 1).
No liver lesions or other distant metastases were seen on abdominal imaging. Based on these findings, the patient was diagnosed with metastatic uterine sarcoma (Stage IV) approximately one month after her initial surgery. To achieve a more accurate assessment of the tumour and expand therapeutic options, ER, PR, AR, Her-2 (Human epidermal growth factor receptor-2), PD-L1 (Programmed Death Ligand-1), and Ki-67 were analysed in the resected tissues.
To achieve a more accurate assessment of the tumour and expand therapeutic options, ER, PR, AR, Her-2 (Human epidermal growth factor receptor-2), PD-L1 (Programmed Death Ligand-1), and Ki-67 were analysed in the resected tissues.
4. Histopathology and Diagnosis
The surgical plan included transabdominal hysterectomy, right salpingo-oophorectomy, and left salpingectomy. Gross examination revealed that the uterus was bulky and deformed. Serial sections of the uterus revealed two intramural, relatively ill-defined masses with a soft consistency and a fleshy appearance. The largest mass measured 13.5 cm at its greatest dimension. Cut sections of this larger mass showed haemorrhagic and cystic areas. Representative sections were taken from each centimetre of the masses, as previously described.
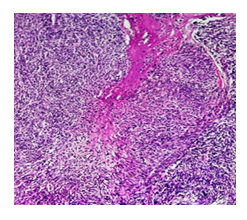
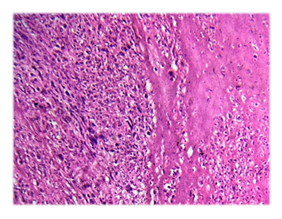
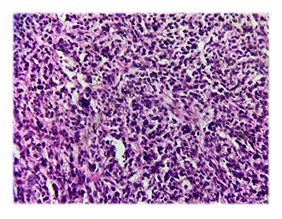
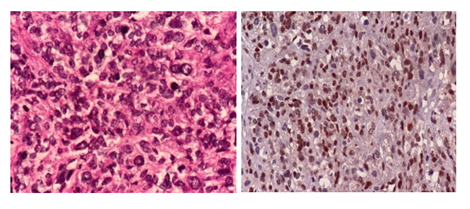
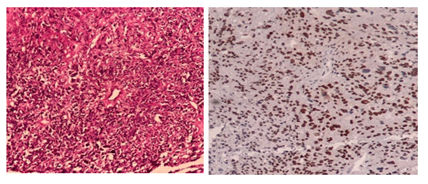
Microscopic examination of the H&E-stained slides showed a highly cellular tumour composed of long fascicles with an infiltrative border. The individual spindle cells exhibited hyperchromatic nuclei, moderate nuclear pleomorphism, and eosinophilic cytoplasm. Frequent mitoses, including atypical forms, were observed (> 25 per 10 high-power fields). Small areas of tumour cell necrosis with an abrupt transition from viable tumour cells were also present (Figures. 1 and 2). Based on histological findings, the main differential diagnosis was conventional leiomyosarcoma.
Immunohistochemistry demonstrated strong, diffuse positivity for smooth muscle markers, including desmin and h-caldesmon. Tumour cells also showed strong nuclear expression of oestrogen receptor (ER), progesterone receptor (PR), and androgen receptor. Endometrial stromal sarcoma was ruled out as the primary differential diagnosis based on histomorphological features and strong positivity for smooth muscle markers.
Given the presence of gross residual disease in the pelvis (8 cm pelvic mass on imaging) and documented pulmonary metastases, the case was discussed at a multidisciplinary tumour board. The consensus was to pursue aggressive adjuvant therapy with curative intent, taking into account the extensive spread of the tumour as well as its hormone receptor positivity.
5. Treatment
In May 2023, the patient was referred to the medical and radiation oncology department for postsurgical therapy. The treatment plan was multimodal, incorporating both locoregional and systemic approaches.
5.1 Radiation therapy:
Owing to the large unresected tumour mass in the pelvis, adjuvant radiotherapy was administered for local disease control. The patient received external beam radiotherapy (EBRT) to the pelvic lymph nodes with a total dose of 50.4 Gy in two fractions, followed by 70 Gy in two additional fractions to the residual area, as determined by MRI. (Although adjuvant pelvic radiotherapy has not demonstrated a clear overall survival benefit in prior trials for uterine LMS [8], it was considered justified in this scenario to attempt to control the bulky residual disease locally.) The patient tolerated the pelvic radiation well and experienced only mild gastrointestinal side effects.
5.2 Systemic Therapy:
- • Goserelin 3.6 mg), a gonadotropin-releasing hormone (GnRH) analogue, was subcutaneously administered every 28 days to suppress ovarian oestrogen production (chemically inducing menopause).
- • Bicalutamide 50 mg orally once daily: a non-steroidal antiandrogen that blocks the androgen receptor.
A Systemic treatment was initiated simultaneously. Instead of conventional cytotoxic chemotherapy, the oncology team opted for a hormone-targeted therapy approach given the tumour’s strong hormone receptor expression. From May 2023, the treatment was started with:
This dual hormonal blockade was designed to counter any growth drive from oestrogen and androgens, analogous to therapies used for hormone-sensitive breast and prostate cancer. While data on hormonal therapy in high-grade LMS are limited, there is evidence that ovarian suppression or aromatase inhibitors can stabilise the disease in ER-positive LMS [2], and AR-targeted therapy has a sound biological rationale given the tumour’s AR positivity (AR expression in LMS is associated with more indolent behaviour) [5].
In addition to hormonal therapy, pazopanib 800 mg daily was started. Pazopanib is an oral multi-tyrosine kinase inhibitor (targeting VEGFR, PDGFR, and c-kit) with anti-angiogenic effects. It is an approved targeted therapy for advanced soft tissue sarcomas, including uterine LMS, following chemotherapy failure.[6] Given the patient’s metastatic disease and good performance status, pazopanib was added to synergise with hormonal therapy by attacking a complementary pathway (tumour angiogenesis). No immediate cytotoxic chemotherapy was administered in favour of this targeted multimodal regimen.
The patient completed the combined therapy by August 2023, which included a full course of pelvic EBRT (completed in July 2023) and approximately three months of systemic treatment with goserelin, bicalutamide, and pazopanib. She was closely monitored during therapy with periodic laboratory tests (including blood counts and liver function tests, given the hepatic toxicity risk of pazopanib). The treatment was reasonably well tolerated, and she experienced expected side effects such as grade 1-2 fatigue and hot flashes from hormonal therapy and mild hepatotoxicity from pazopanib (transient elevation of ALT/AST to approximately twice the upper limit of normal, which improved with dose adjustment). She did not develop significant bone marrow suppression, and overall, she maintained a good quality of life throughout treatment.
6. Outcome and Follow-Up
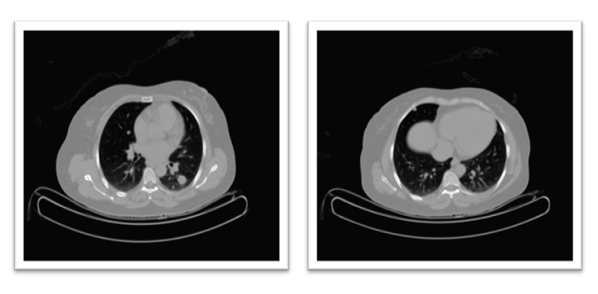
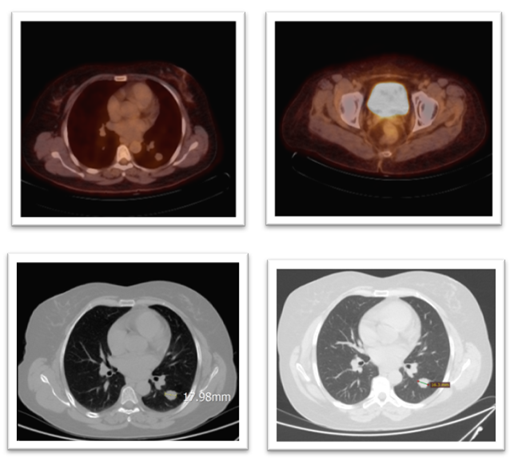
Restaging imaging was performed three months after the completion of radiotherapy and initial systemic therapy. In October 2023, a whole-body PET-CT scan demonstrated a complete metabolic response to treatment. The previously noted pelvic mass showed no discernible FDG uptake or residual tumour on PET, and multiple lung metastases were inactive and stable in size. Essentially, there was no visible evidence of active disease on the post-therapy scans. Complete remission was achieved approximately one year after the patient’s initial presentation. (Figure 8) Clinically, the patient’s symptoms resolved, her pelvic pain disappeared, and she had no respiratory symptoms or other complaints.
Figure 8: Top R&L: PET scan shows no metabolic activity of the previously seen avid lesions in the lung and pelvis, particularly the previously reported Right femoral head. Bottom Left: CT chest in May 2024 compared to bottom R CT in April 2025, showing a reduction in size of the target lesion in the left lung from 17.98mm to 16.3 mm.
Given this remarkable response, a decision was made to continue maintenance therapy to reduce the risk of relapse. From late 2023 onwards, the patient has remained on goserelin (3.6 mg monthly) to maintain a postmenopausal hormonal state, bicalutamide 50 mg daily, and pazopanib 800 mg daily. The patient continued on this maintenance regimen with ongoing surveillance.
Regular follow-up imaging was performed every 3-4 months. As of her last assessment in Aug 2025, approximately > 24 months after completing the initial therapy, the patient remains in complete metabolic remission. Serial computed tomography (CT) scans of the chest, abdomen, and pelvis showed no evidence of recurrent or new disease. Clinically, the patient was doing well and resumed normal activities. She will continue on the combined hormone-targeted maintenance for now, with periodic re-evaluation and imaging. Long-term surveillance is planned, as uterine LMS carries a significant risk of recurrence, even after sustained remission.
7. Discussion
This case exemplifies a notable outcome of metastatic uterine leiomyosarcoma, highlighting several key aspects of its diagnosis and management. First, it demonstrated the diagnostic challenge of uterine LMS mimicking benign fibroid disease. Our patient’s tumour was initially presumed to be a leiomyoma, which is a common scenario, and most LMS cases were discovered incidentally, only after surgery for an enlarging fibroid [1]. In retrospect, there were warning signs: the rapid growth of the uterine mass and the patient’s perimenopausal status aligned with known indicators for potential sarcoma [2,3]. Unfortunately, no definitive noninvasive test exists; imaging studies (ultrasound or MRI) may sometimes suggest atypical features (e.g., irregular or heterogeneous fibroids), but they cannot conclusively differentiate LMS from a benign fibroid preoperatively [1]. Thus, the degree of diagnostic uncertainty often remains until the histopathological results are obtained. This case underscores the importance of maintaining a high index of suspicion and appropriate counselling for patients when presumed fibroids exhibit unusual behaviour. In hindsight, earlier surgical intervention might have been considered, given the continued tumour growth, which could potentially have addressed the malignancy at an earlier stage.
Second, this case illustrates an individualised multimodal treatment approach for advanced LMS. The standard of care for localised (Stage I) uterine LMS is surgical resection (hysterectomy with bilateral salpingo-oophorectomy) [1]. Our patient underwent surgery; however, microscopic metastatic disease was likely already present, as evidenced by pelvic and pulmonary recurrence within 1 month of hysterectomy. There is no established protocol for adjuvant therapy in uterine LMS because of the lack of a clear survival benefit in clinical trials [7,8]. Adjuvant chemotherapy is often considered for high-grade or advanced cases; however, uterine LMS has shown limited responsiveness to chemotherapy and no proven improvement in overall survival with routine postoperative chemotherapy [7]. Adjuvant radiotherapy can reduce local pelvic recurrence rates; however, in a randomised trial, it did not improve survival in patients with early-stage uterine LMS [8]. In our patient’s case, given a gross residual pelvic tumour, we employed postoperative radiotherapy aiming to achieve local control, a decision supported by retrospective data suggesting reduced local relapse with adjuvant RT in select high-risk sarcoma patients [8]. The pelvic radiation likely contributed to the eradication of the unresected mass, as evidenced by a clear PET scan after treatment.
The most novel aspect of management here was the use of hormonal therapy (GnRH agonist plus anti-androgen) combined with pazopanib instead of traditional cytotoxic chemotherapy. There is limited literature on hormonal treatments in high-grade LMS, but our rationale is centred on the “triple-positive” receptor status of the tumour. Oestrogen and progesterone receptor expression in LMS suggests a potential sensitivity to hormonal suppression. Small studies have indicated that aromatase inhibitors can induce disease stabilisation and occasional regression in ER/PR-positive LMS [2], and current guidelines recognise that endocrine therapy may be beneficial on a case-by-case basis for advanced LMS with hormone receptor positivity [2]. In this case, we employed goserelin to suppress oestrogen production (essentially ovarian ablation) and added bicalutamide to block AR signalling. While AR-targeted therapy is not a standard approach for sarcomas, emerging data suggest that AR is a meaningful biomarker in uterine LMS and may be associated with better outcomes [5]. The excellent response observed in our patient supports the hypothesis that multi-hormonal blockade can effectively control tumour growth in hormone receptor-positive LMS. It is possible that the combined anti-oestrogen and anti-androgen effects acted synergistically to induce tumour regression. Additionally, anecdotal evidence from other rare hormone-driven mesenchymal tumours (e.g., aggressive angiomyxoma) suggests that AR antagonism can achieve prolonged disease control in AR-positive cases [9], further indicating that AR-directed therapy may have tangible benefits in select patients.
Pazopanib was another key component of the treatment in this case. Pazopanib targets tumour angiogenesis and has shown activity in soft tissue sarcomas after chemotherapy [6]. Uterine LMS tends to be a highly vascular tumour, and anti-angiogenic therapy can help to slow its progression. In the PALETTE study (which included approximately 40% of uterine LMS patients), pazopanib significantly improved progression-free survival compared to placebo [6]. Although the increase in PFS seen in trials might seem modest, there are occasional “exceptional responders” who gain substantial benefits. Our patient’s complete metabolic remission following the addition of pazopanib was remarkable and could indicate an outsized tumour sensitivity to angiogenesis inhibition in a particular case. It is noteworthy that she achieved a complete response, an extremely rare outcome in metastatic LMS with any form of therapy. The synergistic effect of pazopanib combined with hormonal blockade likely played a key role in the patient’s outcome.
Overall, this case emphasises the importance of personalised therapy for uterine LMS. Standard treatments often achieve limited success in metastatic LMS (which is usually deemed incurable once it has become widespread) [1,10]; therefore, utilising specific tumour biology - in this case, hormone receptor status - is essential. Our patient outcomes may encourage clinicians to routinely assess ER/PR/AR status in LMS and to consider incorporating endocrine therapies or clinical trial options targeting these pathways for patients with receptor-positive tumours. Additionally, the role of pazopanib and other targeted agents in LMS should be further investigated, especially in combination with hormonal or chemotherapeutic modalities, to improve outcomes. Of course, this is a single case, and one must be cautious when generalising it. The patient will require long-term surveillance, as LMS has a high propensity for recurrence, even after a year or more of apparent dormancy [10,11]. The durability of remission after maintenance therapy remains uncertain over time.
Ultimately, this case highlights the significance of ongoing research and clinical trials on uterine sarcomas. Uterine LMS is rare, and evidence-based guidelines are difficult to establish; much of the treatment approach is extrapolated from studies on soft-tissue sarcomas in general or based on limited retrospective series [3]. Novel strategies, such as hormonal agents and targeted therapies, including tyrosine kinase inhibitors, or emerging immunotherapies, should be investigated in clinical trials to identify effective treatments. Multidisciplinary care is critical, and our patient’s case was managed by a coordinated team including gynaecologic oncology, medical oncology, radiation oncology, pathology, and pulmonology. Such collaboration is key to achieving an optimal outcome in a complex case.
In summary, this case demonstrates that even metastatic uterine LMS can occasionally achieve complete remission with aggressive and tailored therapy. Key factors contributing to this success include the early identification of recurrence, the exploitation of hormone receptor positivity with endocrine therapy, the use of advanced targeted therapy (pazopanib), and close monitoring. It provides hope that a subset of patients with this aggressive disease can attain durable responses, and encourages clinicians to think beyond conventional treatments when managing uterine LMS.
8. Conclusion
Metastatic uterine leiomyosarcoma usually has a very poor prognosis, but this case demonstrates that individualised multimodal treatment can yield an exceptional outcome. An ER/PR/AR-positive metastatic LMS in a 54-year-old woman was successfully treated to complete metabolic remission using a combination of surgery, radiotherapy, intensive hormonal therapy (GnRH agonist plus antiandrogen), and the targeted anti-angiogenic agent pazopanib. This approach, based on the unique biology of the tumour, may be considered in similar cases of hormone receptor-positive LMS. Close long-term follow-up is essential to monitor for relapse, given the high risk of recurrence associated with LMS. Overall, collaborative care and innovative biology-driven treatment strategies are crucial in managing rare and aggressive cancers.
Learning Points
- • Suspect LMS in Rapidly Growing “Fibroids”: Uterine leiomyosarcoma should be considered when a presumed fibroid grows rapidly or causes unusual symptoms in peri- or postmenopausal women [2,3]. Definitive diagnosis often requires postoperative pathology, as imaging and biopsies have a limited ability to distinguish LMS from benign leiomyomas preoperatively [1].
- • Multidisciplinary Management: A multidisciplinary approach is essential for high-risk or metastatic uterine LMS. Surgical resection remains the cornerstone of treatment for localised disease [1], but adjuvant therapies (radiation and systemic treatment) should be tailored to individual patient and tumour factors. In selected cases, adjuvant pelvic radiotherapy can improve the local control of residual disease, and systemic therapy is necessary to manage metastatic spread.
- • Role of Hormone Receptor-Targeted Treatment: Determining ER/PR/AR status in uterine LMS can uncover additional treatment options. In this case, aggressive hormonal blockade (GnRH agonist for oestrogen suppression and bicalutamide for AR inhibition) was associated with an excellent response. Endocrine therapy (ovarian suppression or aromatase inhibitors) may provide clinical benefits in ER/PR-positive LMS [2], and AR expression might indicate more favourable tumour biology [5], suggesting that personalised hormone-targeted strategies should be considered for hormone receptor-positive LMS.
- • Pazopanib and Targeted Agents: The anti-angiogenic TKI, pazopanib, played a crucial role in this case, leading to complete remission. Pazopanib is an evidence-based option for refractory metastatic soft tissue sarcomas (including uterine LMS) [6] and should be considered after (or instead of) conventional chemotherapy in appropriate patients. Combining targeted therapy with other modalities (such as hormonal therapy or chemotherapy) may improve the efficacy of LMS and warrants further investigation.
- • Prognosis and Follow-up: Uterine LMS has a high propensity for recurrence and hematogenous metastasis (particularly to the lungs)[1]. Careful long-term follow-up with imaging is required even after achieving a complete response. While advanced LMS is generally aggressive and difficult to cure [10], this case exemplifies that durable remission is possible. This emphasises the need to tailor treatment based on tumour characteristics and promotes further investigation of novel therapies for uterine sarcomas [2].
Author's Contribution
Ramin Ajami, MD: Conceptualisation, Clinical data curation, writing - original draft, writing - review, and editing.
Reza Moghareabed, MD: Conceptualisation, Clinical data curation, writing - original draft, writing - review, and editing.
Golsa Shekarkhar, MD: Pathology investigation, Validation, Writing, review, and editing.
Amir Hosein Mehrtash: Histopathology, Genomic analysis, Methodology.
Faezeh Ghasemi: Histopathology, Genomic analysis, Methodology.
Hadis Mirzaei: Imaging investigation, Resources, Patient consent, Validation.
Funding Statement:
The study was not supported by any organisational funding.
Ethical Compliance:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee, as well as the 1964 Helsinki Declaration and its subsequent amendments or comparable ethical standards.
Conflict of Interest declaration:
The authors declare that they have no affiliations with or involvement in any organisation or entity with any financial interest in the subject matter or materials discussed in this manuscript.
References
- George S, Serrano C, Hensley ML, et al. Soft Tissue and Uterine Leiomyosarcoma. J Clin Oncol 36 (2018):144-150.
- Pérez-Fidalgo JA, Ortega E, Ponce J, et al. Uterine sarcomas: Clinical practice guidelines for diagnosis, treatment, and follow-up (Spanish Group for Research on Sarcomas - GEIS). Ther Adv Med Oncol 15 (2023): 17588359231157645.
- Prat J, Mbatani Y. Uterine sarcomas. Int J Gynaecol Obstet 131 (2015): S105-S110.
- Ioffe YJ, Li AJ, Walsh CS, et al. Hormone receptor expression in uterine sarcomas: prognostic and therapeutic roles. Gynecol Oncol 115 (2009): 466-471.
- Baek MH, Park JY, Park Y, et al. Androgen receptor as a prognostic biomarker and therapeutic target in uterine leiomyosarcoma. J Gynecol Oncol 29 (2018): e30.
- van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 379 (2012): 1879-1886.
- Hensley ML, Enserro D, Hatcher HM, et al. Adjuvant gemcitabine plus docetaxel followed by doxorubicin versus observation for high-grade uterine leiomyosarcoma: A phase III NRG Oncology/GOG study. J Clin Oncol 36 (2018): JCO1800454.
- Malouf GG, Duclos J, Rey A, et al. Adjuvant pelvic radiotherapy in uterine sarcoma: results of the French Sarcoma Group retrospective study. Ann Oncol 24 (2013): 1099-1104.
- Franza A, Gusmaroli L, Del Priore ML, et al. Long-term disease stability with bicalutamide in a man with aggressive angiomyxoma: case report and review of the literature. Front Oncol 13 (2024):1260668.
- Makhoul K, Miller D, Ilyas U, et al. Leiomyosarcoma: Lung Metastasis (17 cm lung metastasis one year post-hysterectomy). Cureus 15 (2023): e34373.
- Senol T, Kahramanoglu I, Muezzinoglu B, et al. Giant leiomyosarcoma of the uterus: a case report. Int J Surg Case Rep 19 (2016): 109-111.