Comparison of Canola and Soybean Oils on Serum Lipid and Glucose Profiles and Anthropometric Parameters in overweight and Obese Type 2 Diabetes Mellitus Patients: A Randomized Clinical Trial
Article Information
Mansour Shahraki1, Sara Rahati2*, Mahmood Ali Keykhaei3, Nasim Niknejad4
1Department of Nutrition, Faculty of Medicine and Children and Adolescent Health Research Center, Resistant Tuberculosis Institute, Zahedan University of Medical Sciences, Zahedan, Iran.
2Department of Cellular- Molecular Nutrition, School of Nutrition Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
3Internal Seat, Ali-ibn-Abitaleb Hospital, Zahedan University of Medical Sciences, Zahedan, Iran.
4School of Population and Public Health, University of British Columbia, Vancouver, BC V6T 1Z3, Canada
*Corresponding Author: Sara Rahati. Department of Cellular- Molecular Nutrition, School of Nutrition Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran.
Received: 19 September 2022; Accepted: 27 September 2022; Published: 08 November 2022
Citation: Sara Rahati. Department of Cellular–Molecular Nutrition, School of Nutrition Sciences and Dietetics, Tehran University of Medical Sciences, Tehran, Iran
View / Download Pdf Share at FacebookAbstract
No earlier human study compared influences of canola and soybean oils on patients with type 2 diabetes (T2D). Current study aimed to investigate effects of canola and soya oils on blood and anthropometric parameters in overweight and obese Iranian diabetic (II) patients. A total of sixty-six T2D subjects were randomly allocated to three groups. Canola oil (CO; n 23, received 30g canola oil); Soya oil (SO; n 19, received 30g soya oil) and control group (n 24, their usual intake of dietary oils) for 8 weeks. Lipid and glycemic profiles as well as anthropometric indicators were evaluated before and after the intervention. Repeated-measures ANOVA was used to evaluate time×group interactions for the outcome variables followed by a t test (significance level, p < 0.05). After 8 weeks, serum total cholesterol (-21.3 and -36.4v. -2.2 mg/dl; P=0·007), low density lipoprotein (-6.6 and -15.9v. +3.0 mg/dl; P=0·013), fasting blood sugar (-39.6 and -30.5v. +11.7 mg/dl; P<0.001) significantly decreased and high density lipoprotein (+3.0 and +3.5v. +2.4 mg/dl; P=0.038) significantly increased in CO and SO groups compared with controls. Changes in lipid profiles were more considerable in the soybean oil group than the canola oil group. The mean changes of waist circumference (WC; −4.1 v. -1.4 and -1.3 cm; P=0·031) and weight (−3.1 v. -0.3 and +0.5 kg; P=0·048) significantly decreased in canola group comparing to the two other groups. Current study showed that daily consumption of canola and soybean oil for 8 weeks improved serum levels of fasting blood sugar, total cholesterol, low density lipoprotein and high density lipoprotein in T2D patients. Changes were more considerable in those consumed soybean oil. Canola oil decreased central obesity indices (waist circumference and weight) in T2D patients. Further studies are needed to shed light on this issue.
T
Keywords
Diabetes mellitus, Canola oil, Soya oil, Obesity
Diabetes mellitus articles; Canola oil articles; Soya oil articles; Obesity articles
Diabetes mellitus articles Diabetes mellitus Research articles Diabetes mellitus review articles Diabetes mellitus PubMed articles Diabetes mellitus PubMed Central articles Diabetes mellitus 2023 articles Diabetes mellitus 2024 articles Diabetes mellitus Scopus articles Diabetes mellitus impact factor journals Diabetes mellitus Scopus journals Diabetes mellitus PubMed journals Diabetes mellitus medical journals Diabetes mellitus free journals Diabetes mellitus best journals Diabetes mellitus top journals Diabetes mellitus free medical journals Diabetes mellitus famous journals Diabetes mellitus Google Scholar indexed journals Canola oil articles Canola oil Research articles Canola oil review articles Canola oil PubMed articles Canola oil PubMed Central articles Canola oil 2023 articles Canola oil 2024 articles Canola oil Scopus articles Canola oil impact factor journals Canola oil Scopus journals Canola oil PubMed journals Canola oil medical journals Canola oil free journals Canola oil best journals Canola oil top journals Canola oil free medical journals Canola oil famous journals Canola oil Google Scholar indexed journals Soya oil articles Soya oil Research articles Soya oil review articles Soya oil PubMed articles Soya oil PubMed Central articles Soya oil 2023 articles Soya oil 2024 articles Soya oil Scopus articles Soya oil impact factor journals Soya oil Scopus journals Soya oil PubMed journals Soya oil medical journals Soya oil free journals Soya oil best journals Soya oil top journals Soya oil free medical journals Soya oil famous journals Soya oil Google Scholar indexed journals Obesity articles Obesity Research articles Obesity review articles Obesity PubMed articles Obesity PubMed Central articles Obesity 2023 articles Obesity 2024 articles Obesity Scopus articles Obesity impact factor journals Obesity Scopus journals Obesity PubMed journals Obesity medical journals Obesity free journals Obesity best journals Obesity top journals Obesity free medical journals Obesity famous journals Obesity Google Scholar indexed journals diabetic patients articles diabetic patients Research articles diabetic patients review articles diabetic patients PubMed articles diabetic patients PubMed Central articles diabetic patients 2023 articles diabetic patients 2024 articles diabetic patients Scopus articles diabetic patients impact factor journals diabetic patients Scopus journals diabetic patients PubMed journals diabetic patients medical journals diabetic patients free journals diabetic patients best journals diabetic patients top journals diabetic patients free medical journals diabetic patients famous journals diabetic patients Google Scholar indexed journals low density lipoprotein articles low density lipoprotein Research articles low density lipoprotein review articles low density lipoprotein PubMed articles low density lipoprotein PubMed Central articles low density lipoprotein 2023 articles low density lipoprotein 2024 articles low density lipoprotein Scopus articles low density lipoprotein impact factor journals low density lipoprotein Scopus journals low density lipoprotein PubMed journals low density lipoprotein medical journals low density lipoprotein free journals low density lipoprotein best journals low density lipoprotein top journals low density lipoprotein free medical journals low density lipoprotein famous journals low density lipoprotein Google Scholar indexed journals high density lipoprotein articles high density lipoprotein Research articles high density lipoprotein review articles high density lipoprotein PubMed articles high density lipoprotein PubMed Central articles high density lipoprotein 2023 articles high density lipoprotein 2024 articles high density lipoprotein Scopus articles high density lipoprotein impact factor journals high density lipoprotein Scopus journals high density lipoprotein PubMed journals high density lipoprotein medical journals high density lipoprotein free journals high density lipoprotein best journals high density lipoprotein top journals high density lipoprotein free medical journals high density lipoprotein famous journals high density lipoprotein Google Scholar indexed journals fasting blood glucose articles fasting blood glucose Research articles fasting blood glucose review articles fasting blood glucose PubMed articles fasting blood glucose PubMed Central articles fasting blood glucose 2023 articles fasting blood glucose 2024 articles fasting blood glucose Scopus articles fasting blood glucose impact factor journals fasting blood glucose Scopus journals fasting blood glucose PubMed journals fasting blood glucose medical journals fasting blood glucose free journals fasting blood glucose best journals fasting blood glucose top journals fasting blood glucose free medical journals fasting blood glucose famous journals fasting blood glucose Google Scholar indexed journals
Article Details
Introduction
Today, global prevalence of type 2 diabetes is increasing continuously, along with the increased prevalence of overweight and obesity [1]. Fat accumulation in the visceral area is a major risk factor of diabetes mellitus [2,3]. Chronic diseases, such as obesity and type 2 diabetes mellitus (T2D), are the main health concerns in the current century. They have put a great burden to health systems all over the world [4,5]. The global prevalence of T2D in 2000 was about 150 million people, which is estimated to increase to 300 million in 2025 [6]. In addition, more than four million Iranian adults are suffering from T2D, which has been increased by 35% over the past seven years [7].
Diabetes is a chronic disease that requires continual medical and self -care training. Nutrition therapy is an integral part of diabetes management, playing an essential role in the treatment of the disease [8]. It seems that the control of cholesterol, triglycerides and glucose in diabetic patients plays an effective role in the prevention and treatment of atherosclerosis [9]. High consumption of saturated fatty acids and carbohydrates causes several diabetes complications [10]. The majority of cooking oils used in Iran are hydrogenated and semi-hydrogenated oils [10]. Researches show that substitution of this type of oil with liquid vegetable oils significantly increases serum high density lipoprotein (HDL-) and decreases serum low density lipoprotein (LDL) [11]. Therefore, more consumption of polyunsaturated fatty acids (PUFA) (having more than a single double bond) instead of saturated fatty acids (SFA) is commonly recommended in T2D patients [12]. In addition, higher intake of omega-3 fatty acids has been associated to better serum lipid profile and decreased cardiovascular risks [13]. Moreover, studies suggest that adherence to a diet containing high amounts of MUFA (Mono Unsaturated Fatty Acids), having one double bond, is a good choice for patients with T2D [14]. Canola oil (rapeseed oil) contains the lowest amount of saturated fatty acids (6.7% of the oil's total fatty acids), in comparison to other common types of cooking oils. In addition, it holds 18.7% PUFA (n-6), 65.3% MUFA, and almost 11% alpha-linolenic acid (essential n-3 fatty acids), which is the highest rate in comparison to other types of cooking oils [15,16]. Soybean oil is primarily comprised of PUFA (55 to 58%), 12 to 15% saturated fat, and 22 to 30% MUFA (oleic acid) [17]. Although, rare clinical trials have been done on the effects of these two oils on metabolic profiles and anthropometric measures in patients with type 2 diabetes mellitus, findings are controversial. A systematic review and meta-analysis in 2019 showed that canola oil consumption had a modest effect only on body weight, no on other obesity indices [18]. Moreover, most previous studies about the effects of canola and soybean oil on lipid and glucose profiles were done on animal models. Therefore, more human studies are needed. Furthermore, these two types of edible oils are commonly considered to be healthy, however, no comparison has been done between them to find which is healthier among T2D patients. Therefore, current randomized clinical trials aimed to compare effects of canola and soybean oils on lipid and glucose profiles and anthropometric measures among a group of Iranian patients with type 2 diabetes mellitus.
Methods
2.1 Study design
This was an 8-week parallel double-blind randomized clinical trial (RCT). This RCT was conducted between mid-July 2012 to mid-January 2013 in one hospital in Zahedan, Iran. All study participants received informations regarding the study design and objectives and then they signed a written consent. This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving patients were approved scientifically and ethically by the Zahedan University of Medical Sciences (ZUMS) (identification: IR.ZAUMS.REC.1393.2160). The trial registration number at irct.ir is IRCT2012062510110N1.
2.2 Study participants
Participants were recruited from the Ali-Asqar Diabetes Clinic in Zahedan, Iran. All patients with type 2 diabetes mellitus who met following criteria and was agreed to participate in the current trial, were recruited: (a) were overweight or obese (25≤BMI≤39.9), (b) aged 30-65 years old, (c) and had a mean Fasting Blood Sugar (FBS) of ≥126mg/dl. Subjects with the following criteria were not included: (a) patients with hyperglycemia because of other diseases rather than type 2 diabetes mellitus, (b) patients with liver/ kidney/thyroid diseases, (c) patients who received insulin therapy, (d) patients who received cholesterol-lowering agents or beta blockers, and (e) patients who drank alcohol. We also excluded patients with active and intense infectious diseases during blood collection as well as patients who were hospitalized over the plan implementation.
2.3 Study protocol
Before intervention, all patients entered a run-in period for 2 weeks through which they instructed to have a weight maintenance diet according to the American Diabetes Association guidelines [19]. All patients were also asked to not use canola and soya oil for two weeks prior to the study. Instead, they were allowed to use only corn oil for cooking. Then, patients were divided into 3 groups through using computer-generated random numbers: 1) CO (receivers of 30 g canola oil; n 23); 2) SO (receivers of 30 g soya oil; n 19); and 3) control group who continued their usual diet; n 24. Randomization was done by a third investigator out the current study. Therefore, all participants and study directors were blinded. The two first groups were asked to: a) replace their usual oil with canola and soya oil and to not use any other types of solid or liquid oils; b) prepare and cook their foods separately from the other family members; and c) use only 30 grams of the allowed oil in their daily diet. The control group was asked to continue its usual diet. Bottles of canola or soybean oils were given to the participants for 4 weeks. The intervention period was 8 weeks. We assessed dietary intakes of participants by employing three 24 h recalls questionnaires (including a weekend day) in the beginning of study, which was repeated in the end of the intervention period. Data on demographic, medical history, physical activity, and duration of diabetes were gathered using a general questionnaire by the face-to-face interview. After 4 weeks, all subjects were visited to evaluate their compliance and to receive new bottles of the oils for another 4 week (for the canola and soybean oil groups). Compliance was evaluated by counting the empty bottles [20].
2.4 Anthropometrics measurements
Anthropometric measurements were done at the study beginning and end of the 8th week. Weight was measured with light clothing and without shoes using a digital scale (Seca 808) to the nearest of 0·1 kg. Height was measured without shoes using a stadiometer (Seca) to the nearest of 0·1 cm. Waist circumferences (WC) and hip circumferences (HC) were measured by a tape to the nearest of 0·1 cm. BMI was calculated using the equation BMI (weight (kg)/height2 (m2)). WHpR (waist hip ratio) was calculated using the equation WHpR (waist (cm)/hip (cm)). Mid arm circumference (MAC) was measured midway between olecranon and acromion [21]. Calf circumference (CC) was measured on the left leg in a sitting position, with the knee and ankle at a right angle and feet resting on the floor, at the point of greatest circumference [21]. We used non-elastic tape to measure MAC and CC.
2.5 Biochemical measurements
At the study beginning and the end of trials, participants were invited to attend the diabetes clinic laboratory while they were fasting for 12–14 h. Then, 10 cc blood was collected from each patient. Blood samples were divided into tubes without the anticoagulant (EDTA). FBS was measured using enzymatic method by using commercial kits (Pars Azmoon) and an auto-analyzer system (Selectra E; Vitalab). Glycosylated hemoglobin (HbA1c) was measured using colorimetric method after an initial chromatographic separation (BioSystems) [20]. TG (Triglycerides), TC (Total Cholesterol), LDL (Low density lipoprotein) and HDL (High density lipoprotein) were measured by the enzymatic method (Pars Azmoon kit). ESR (Erythrocyte Sedimentation Rate) was measured by the Westergren method, and CBC (Cell Blood Counter) was measured by a cell counter. In addition, CRP (C- reactive protein) was measure using a qualitative method (OMEGA). Finally, serum creatinine (Cr) and urea were assayed by the JAFFE and UV-test, respectively (Pars Azmoon).
2.6 Statistical analysis
The sample size was defined according to type I error of α = 0.05 and type II error of β = 80 %. Initially, participants were classified into three groups: control, canola oil and soya oil. Normal distribution of data was checked using the Kolmogrov–Smirnov. Comparison of quantitative variables between the CO, SO and control groups was performed using Independent Samples T tests (for normally distributed variables) or Mann–Whitney U tests (for non-normally distributed variables) and analysis of covariance (ANCOVA), that adjusted for energy intake. Repeated-measures ANOVA was used to evaluate time×group interactions for the outcome variables, with time and group as factors. In case of significant time–group interaction, between-group comparison of changes at week 8 was carried out using ANOVA followed by Tukey’s post hoc analysis with polynomial contrast analysis for trend when indicated. When time effect was significant, the within-group comparison of values was performed by paired sample t tests. All statistical analyses were carried out using the Statistical Package for Social Sciences (SPSS) version 18. P values <0·05 were considered as statistically significant.
3. Results
3.1 Study population characteristics
Figure 1 shows the flowchart of study procedure. Study participants were 31 males and 35 females with an average age of 46.59 years (SD = 7.8). The three groups were not significantly different in terms of mean age (p=0.18), mean years lasted from first diagnosis of the disease (p=0.32), and gender (p=0.23) (Table 1).
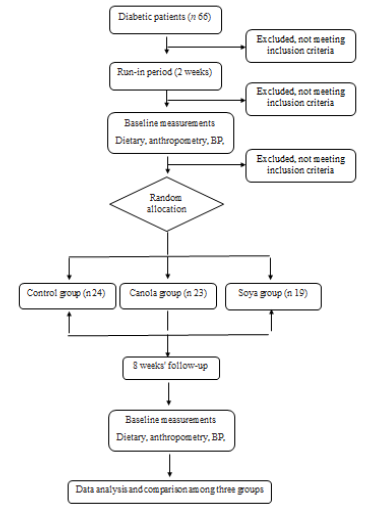
Figure 1: Study flow diagram (CONSORT 2010). BP, blood pressure
|
Variables |
Control (n= 24) |
Canola oil (n= 23) |
Soya oil (n=19) |
||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
P-value |
|
|
Age (year) |
48.38 |
6.33 |
46.43 |
7.46 |
44.53 |
9.67 |
0.18 |
|
Sex (male/ female) |
Nov-13 |
Oct-13 |
10-Sep |
0.23 |
|||
|
Diabetes duration (year) |
6.2 |
4.8 |
6.4 |
4.9 |
6.8 |
5.1 |
0.32 |
|
Physical activity (%) |
45.5 |
37.6 |
46.1 |
0.41 |
|||
|
Hight (cm) |
159.7 |
5.5 |
158.2 |
5.5 |
158 |
8.5 |
0.11 |
(RCT) study.
SD: Standard deviations. Variables are presented as mean ±SD for continuous variables and percent (%) for categorical variables. P from t test for continuous variables and chi-square test for categorical variables. P value < 0.05.
Table 1: Some selected individual characteristics of the subjects enrolled in the randomized clinical trial
3.2 Dietary intake of study population according to groups
Dietary intakes of participants has been compared before and after the intervention between the three groups (Table 2). No significant differences were seen between three groups in terms of energy intake, carbohydrates, proteins or fats (p > 0.05). The results of the comparison showed that mean linoleic and linolenic were higher in CO and SO compared with control group, even after adjusting for energy intake (p < 0.05).
|
Variables |
Time |
Canola (n=23) |
Soya (n=19) |
Control (n=24) |
|||||
|
Mean |
SD |
Mean |
SD |
Mean |
SD |
p-value |
*p-value |
||
|
Energy (kcal/day) |
Before |
2700.6 |
439.1 |
2721.1 |
396.9 |
2684.9 |
321.9 |
0.29 |
- |
|
After |
2712.1 |
398.5 |
2693.6 |
311.8 |
2712.1 |
371.1 |
0.53 |
- |
|
|
Carbohydrate (g/day) |
Before |
315.2 |
76.9 |
312.3 |
62.2 |
301.4 |
57.3 |
0.42 |
0.91 |
|
After |
303.5 |
51.3 |
301.9 |
48.1 |
309.2 |
41.7 |
0.82 |
0.9 |
|
|
Protein (g/day) |
Before |
91.7 |
19.3 |
96.9 |
16.7 |
92.3 |
21.1 |
0.57 |
0.5 |
|
After |
87.8 |
29.1 |
91.7 |
19.6 |
90.7 |
19.4 |
0.19 |
0.21 |
|
|
Total fat (g/day) |
Before |
54.2 |
14.2 |
51.8 |
14.2 |
53.9 |
13.2 |
0.5 |
0.59 |
|
After |
53.1 |
11.4 |
59.7 |
15.6 |
55.7 |
15.4 |
0.09 |
0.11 |
|
|
Trans. Fat (g/day) |
Before |
0.0007 |
0.001 |
0.0008 |
0.002 |
0.0007 |
0.002 |
0.85 |
0.92 |
|
After |
0.0006 |
0.002 |
0.0008 |
0.001 |
0.0006 |
0.001 |
0.81 |
0.9 |
|
|
Cholesterol (g/day) |
Before |
270.11 |
111.01 |
211.91 |
110.03 |
254.23 |
118.09 |
0.38 |
0.2 |
|
After |
271.98 |
121.98 |
209.23 |
112.56 |
229.65 |
115.54 |
0.2 |
0.18 |
|
|
SFA (g/day) |
Before |
27.31 |
12.67 |
28.11 |
12.98 |
28.01 |
10.05 |
0.55 |
0.61 |
|
After |
26.98 |
12.01 |
27.01 |
10.02 |
28.98 |
11.02 |
0.45 |
0.38 |
|
|
PUFA (g/day) |
Before |
19.66 |
8.23 |
20.21 |
7.65 |
20.11 |
8.18 |
0.51 |
0.73 |
|
After |
23.01 |
9.17 |
25.13 |
8.25 |
20.98 |
7.09 |
0.09 |
0.19 |
|
|
Linoleic (g/day) |
Before |
17.22 |
9.32 |
17.19 |
8.21 |
17.43 |
9.11 |
0.48 |
0.54 |
|
After |
20.54 |
8.45 |
23.78 |
8.76 |
18.02 |
8.23 |
0.05 |
0.04 |
|
|
Linolenic (g/day) |
Before |
1.23 |
0.61 |
1.28 |
0.75 |
1.32 |
0.11 |
0.92 |
0.78 |
|
After |
2.1 |
0.71 |
1.77 |
0.63 |
1.17 |
0.28 |
0.04 |
0.03 |
|
SFA, saturated fatty acid; PUFA, poly unsaturated fatty acid. P-values from independent samples t test. *P -value reported after adjusting kcal with ANCOVA. Significant items with a P value < 0.05 are bolded.
Table 2: Comparison of the initial and final values of the energy and macronutrients intake
3.3 Comparison of biochemical parameters in study groups
At baseline, no significant differences were seen between the three groups in terms of serum concentrations of the study main outcomes (Table 3). However, a significant effect of time was observed in serum FBS (p< 0.001), TG (p< 0.001), TC (p< 0.001) and LDL (p=0.028) concentrations from week 0 to week 8 (Table 3). A significant reduction of TG, TC, and LDL was observed in the CO group (p = 0.01 for TG, p = 0.01 for TC, p = 0.04 for LDL) and in the SO group (p = 0.002 for TG, p = 0.001 for TC, p = 0.01 for LDL) after 8 weeks, which was not significant in the control group (p = 0.6 for TG, p = 0.7 for TC, p = 0.3 for LDL). HDL increased in CO and SO groups (p = 0.001, p = 0.001, respectively), with no significant changes in control group (p =0.3) (Table 3). Serum Cr level significantly increased only in the CO group, and no significant changes were observed in other groups (SO and control) (p = 0.063 and p = 0.06, respectively). Significant time × group interaction was observed in the study groups in terms of serum concentrations of TC (p=0.007), LDL (p=0.013), HDL (p=0.038) and FBS (p< 0.001) (Table 3). Tukey’s post hoc test showed significant greater reduction of serum concentration of TC in SO and CO groups than the control group after the intervention (p= 0.009 and p= 0.034 respectively). Moreover, reduction in TC concentration in SO group was significantly greater than CO group (p= 0.049). LDL levels significantly decreased in SO (p= 0.01) and CO (p= 0.04) groups. Tukey’s post hoc test showed significant differences between SO and control groups (p = 0.01), CO and control (p = 0.023) groups, and reduction in LDL concentration in SO group was significantly greater than CO group (p = 0.051). Significant differences in HDL concentrations were found between CO and control group (p = 0.039) and also between SO and control group (p = 0.034); however, no significant differences were observed between CO and SO groups (p = 0.97). Moreover, significant differences were seen between CO and control (p < 0.001) groups and SO and control (p = 0.001) groups in terms of FBS, while no significant difference was observed between CO and SO groups (p = 0.47) (Table 3).
TG, Triglyceride; TC, Total cholesterol; LDL, Low density lipoprotein; HDL, High density lipoprotein; FBS, Fasting blood sugar; HgA1c, Hemoglobin A1c; ESR, Erythrocyte sedimentation rate; CRP, C reactive protein; Cr, Creatinine; WBC, White blood cell; RBC, Red blood cell; PLT, Platelet. All data are means ± standard deviations; P, before and after study in each group; P1, probability level by repeated-measures ANOVA for difference in time course; P2, probability level by repeated-measures ANOVA for difference between types of group; P3, probability level by repeated-measures ANOVA for interaction between time course and type of group. Significant items with a P value < 0.05 are bolded.
Table 3: Comparison of the initial and final values of the biochemical variables under study in the randomized clinical trial (RCT)
3.4 Comparison of anthropometric parameters in study groups
Effects of dietary interventions on anthropometric measures have been presented in Table 4. No significant differences in anthropometric measures were seen between three groups at the study beginning. The effect of time on body weight, HC, WC, and WHpR from week 0 to week 8 was significant in CO group (p= 0.02, 0.02, 0.0001, and 0.03, respectively), with significant reduction of weight, WC and WHpR and significant increase in HC. However, there was no significant effect of time on these variable in SO (p = 0.48, 0.09, 0.19, and 0.82, respectively) and control (p = 0.44, 0.72, 0.1, and 0.18, respectively) group (Table 4). A significant time × group interaction was observed in the study groups in terms of weight and WC (Table 4). Tukey’s post hoc test showed that after 8 weeks, CO group had significantly greater weight loss than the control and SO groups (p = 0.001 and p = 0.049), respectively. However, no significant differences were observed between SO and control (p = 0.54) groups (Table 4). Moreover, a significant difference
was observed between the CO and SO groups (p = 0.041) and the CO and control (p < 0.001) groups in terms of WC, while no significant difference was observed between SO and control groups (p = 0.63) (Table 4).
BMI, body mass index; HC, hip circumference; WC, waist circumference; WHpR, waist to hip ratio; MAC, Mid-arm circumference; CC, Calf Circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure. All data are means ± standard deviations; P, before and after study in each group; P1, probability level by repeated-measures ANOVA for difference in time course; P2, probability level by repeated-measures ANOVA for difference between types of group; P3, probability level by repeated-measures ANOVA for interaction between time course and type of group. Significant items with a P value < 0.05 are bolded
Table 4: Comparison of the initial and final values of the anthropometric variables under study in the randomized clinical trial (RCT)
Discussion
Current study showed that consumption of 30 g/day of both canola or soybean oil in comparison to control significantly decreased fasting blood glucose and increased proinsulin to insulin ratio in overweight and obese patients with T2D. Consumption of these two oils also significantly reduced serum levels of TC and LDL and increased HDL concentrations, which was more considerable among those who consumed soybean oil. Body weight and waist circumference significantly decreased only in canola oil group. No significant changes were seen in serum levels of TG and HbA1C, and serum levels of inflammatory factors (ESR and CRP), urea and Cr, and in white blood cell (WBC), red blood cell (RBC), and platelet (PLT) levels.
Consumption of either soybean or canola oil resulted in significant reduction of FBS. Soybean and canola oil consumption also was associated to increased proinsulin to insulin ratio. Increased proinsulin to insulin ratio is a common manifest of T2D associated to insulin resistance or impaired conversion of proinsulin to insulin [22]. Significant improvement in blood glucose without changes in HbA1C and significant elevation of proinsulin to insulin ratio shows that consumption of these two oils had no significant effect on insulin resistance, as the major complication of T2D mellitus. Consumption of non-fried soya, but not its oil, resulted in significant reduction of insulin resistance in Japanese adults in a cross-sectional study [23]. Consumption of canola oil in patients with T2D reduced insulin resistance and serum levels of insulin in a randomized clinical trial [24]. It should be noted that most studies in this area have been done on animal models and further human studies are needed to reach a clear conclusion. Our study also found significant reduction of anthropometric measures in T2D patients following consumption of canola oil. Due to significant beneficial changes in anthropometric measures, it might be suggested that consumption of soybean or canola oil for a longer time influences more considerably insulin resistance in patients with T2D.
This study also showed that consumption of 30 g/day of soybean and canola oil has beneficial effects on lipid profiles, including TC, LDL, and HDL in patients with type 2 diabetes mellitus (T2D). These effects were more pronounced in the patients who consumed soybean oil that those at CO group. Earlier studies reported inconsistence findings for the association of soybean and canola oil with serum lipid profiles. Gulesserian et al. examined seventeen 4-19-year-old children and adolescents with baseline TC of 233±35 mg/dl who received canola oil for 5 months (15 gr/day in the first two months and 22 gr/day in the next three months). Serum levels of TG, TC and LDL-c decreased following canola oil consumption in that study, with no significant changes in serum HDL-c concentrations [25]. Negele et al. used lipid-lowering diets with canola and sunflower oils for 3 weeks in 95 hyperlipidemic subjects. In both regimes LDL, TC, HDL and TG decreased [26]. In another study by Bierenbaum et al., 30 ml/day of canola oil rather than other edible oils in daily diet of 46 patients with hyperlipidemia (baseline LDL level was 173±9 mg/dl) for 4 months decreased LDL-c, but had no significant influence on TC and HDL levels [27]. It seems that baseline level of lipid profiles is a crucial confounder for the effect of consumed oils on serum levels of these variables.
Canola and soybean oil are dietary sources of phytosterols [28]. Phytosterols (plant sterols) are structural analogs of cholesterol [29]. Several studies have shown that consumption of phytosterols reduces blood cholesterol, through which they reduce risk of cardiovascular diseases in diabetic and non-diabetic patients [30,31]. In addition, soybean oil is a major source of linoleic acid and linolenic acid [32]. Consumption of linoleic acid has been associated to reduced risk of cardiovascular diseases by modulation of serum lipids [33]. Moreover, canola oil (11%) and soybean oil (7%) are two major sources of dietary alpha-Linolenic acid (ALA) [10,15,17]. Several studies have indicated that consumption of ALA can improve function of the adipose tissue and inhibit fat accumulation through the activated protein kinase-related activities [34]. It has been generally accepted that the effects of n-3PUFA can be due to peroxisome proliferator-activated receptors (PPARs), especially PPARα, such that n-3PUFA acts as a ligand for PPARs [35]. Some other studies have shown that long-chain fatty acids can regulate carnitine palmitoyltransferase I (CPT1), by which they restrict rate of mitochondrial fatty acid oxidation [36, 37]. In addition, n-3PUFA stimulates 5'AMP-activated protein kinase in the adipose tissue [38]. AMP-activated protein kinase (AMPK) is an enzyme that plays an important role in the energy homeostasis in adipose tissues [39]. These mentioned mechanisms might help to explain effects of CO and SO on the anthropometric measures.
Small sample size and short duration of intervention were among the main limitations of the current study. In addition, because participants were at an age range of 45-49, the results cannot be generalized to young and elderly populations. It is necessary to done similar studies on other individuals and groups in order to investigate effects of canola and soybean oils on biochemical and anthropometric indices in patients with diabetes mellitus.
Conclusion
In conclusion, results of our study showed that daily intake of canola and soybean oil for 8 weeks improved serum FBS, TC, LDL and HDL in T2D subjects. Consumption of canola oil decreased central obesity indices (WC and weight) in T2D subjects. Given the high prevalence of diabetes and obesity, there is a growing need for further studies about effects of canola and soybean oil instead of other edible oils on patients with T2D and other chronic diseases.
Abbreviations
ALA: Alpha-Linolenic acid; AMPK: AMP-activated protein kinase; ANCOVA: Analysis of covariance; EDTA: Ethylenediaminetetraacetic acid; CC: Calf circumference; CO: Canola oil; CPT1: Carnitine palmitoyltransferase I; CBC: Cell Blood Counter; Cr: Creatinine; CRP: C- reactive protein; ESR: Erythrocyte Sedimentation Rate; FBS: Fasting blood sugar; HbA1c: Glycosylated hemoglobin; HDL: High density lipoprotein; HC: Hip circumference; LDL: Low density lipoprotein; MAC: Mid arm circumference; MUFA: Mono unsaturated fatty acids; PPARs: Peroxisome proliferator-activated receptors; PLT: Platelet; PUFA: Polyunsaturated fatty acids; RCT: Randomized clinical trial; RBC: Red blood cell; SFA: Saturated fatty acids; SO: Soya oil; TC: Total cholesterol; TG: Triglyceride; T2D: Type 2 diabetes; WBC: White blood cell; WC: Waist circumference; WHpR: Waist hip ratio.
Funding
This research has been supported by Zahedan University of Medical Sciences. Also, this study was approved by the ethics committee at Zahedan University of Medical Sciences (IR.ZAUMS.REC.1393.2160).
Acknowledgments
The authors thank the Ali-Asqar Diabetes Clinic staffs for their collaborations. The authors sincerely appreciate all the subjects for their participation in this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Fiorentino TV, Marini MA, Andreozzi F, et al. One-hour postload hyperglycemia is a stronger predictor of type 2 diabetes than impaired fasting glucose. The Journal of Clinical Endocrinology & Metabolism 12 (2015): 3744-3751.
- Gómez-Hernández A, Beneit N, Díaz-Castroverde S, et al. Differential role of adipose tissues in obesity and related metabolic and vascular complications. International journal of endocrinology 11 (2016): 356-362.
- Randle PJ. Regulatory interactions between lipids and carbohydrates: the glucose fatty acid cycle after 35 years. Diabetes/metabolism reviews 23 (1998): 263-83.
- Snijder MB, Dekker JM, Visser M, et al. Associations of hip and thigh circumferences independent of waist circumference with the incidence of type 2 diabetes: the Hoorn Study. The American journal of clinical nutrition 11 (2003): 1192-1197.
- Seidell JC, Pérusse L, Després JP, et al. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. The American journal of clinical nutrition 5 (2001): 315-21.
- Dilzer A, Park Y. Implication of conjugated linoleic acid (CLA) in human health. Critical reviews in food science and nutrition 8 (2012): 488-513.
- Esteghamati A, Etemad K, Koohpayehzadeh J, et al. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005-2011. Diabetes research and clinical practice. 14 (2014): 319-327.
- Adepu R, Rasheed A, Nagavi B. Effect of patient counseling on quality of life in type-2 diabetes mellitus patients in two selected South Indian community pharmacies: a study. Indian Journal of Pharmaceutical Sciences 11 (2007): 519.
- Schwedes U, Siebolds M, Mertes G. Meal-related structured self-monitoring of blood glucose: effect on diabetes control in non-insulin-treated type 2 diabetic patients. Diabetes care 9 (2002): 1928-1932.
- Milajerdi A, Maghsoudi Z, Ghiasvand R. Different consumed oils and metabolic parameters in type 2 diabetes patients in diabetes society of Natanz. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 17 (2016): S11-S5.
- Bahrami G, Rahi H. The removal effect of hydrogenated shortening on serum levels of triglycerids, total cholesterol andhdl-cholesterol in normal subjects 7 (2000): 69356.
- Firoozrai M, Ghahramanpour F, Karani M, et al. A Comparative Study of Saturated, Trans and Cis Fatty Acids Measured in Serum and Adipose Tissue between Patients with Type 2 Diabetes and Normal Subjects. Razi Journal of Medical Sciences 10 (2007): 119-128.
- Shidfar F, Yar AS, Jalali M, et al. Effects of purified omega- 3 fatty acids in postmenopausal women with type 2 diabetes 13 (2007): 512-523.
- Rodriguez-Villar C, Perez-Heras A, Mercade I, et al. Comparison of a high-carbohydrate and a high-monounsaturated fat, olive oil-rich diet on the susceptibility of LDL to oxidative modification in subjects with Type 2 diabetes mellitus. Diabetic medicine 17 (2004): 142-149.
- Johnson GH, Keast DR, Kris-Etherton PM. Dietary modeling shows that the substitution of canola oil for fats commonly used in the United States would increase compliance with dietary recommendations for fatty acids. Journal of the American Dietetic Association 7 (2007): 1726-1734.
- Vega GL, Barlow CE, Grundy SM, Leonard D, DeFina LF. Triglyceride–to–high-density-lipoprotein-cholesterol ratio is an index of heart disease mortality and of incidence of type 2 diabetes mellitus in men. Journal of Investigative Medicine 6 (2014): 345-349.
- Zambiazi RC, Przybylski R, Zambiazi MW, et al. Fatty acid composition of vegetable oils and fats. Boletim do Centro de Pesquisa de Processamento de Alimentos (2007).
- Raeisi-Dehkordi H, Amiri M, Humphries KH, Salehi-Abargouei A. The effect of canola oil on body weight and composition: a systematic review and meta-analysis of randomized controlled clinical trials. Advances in Nutrition 8 (2019): 419-432.
- Prevention I. Standards of medical care in diabetes- 2011. Diabetes Care 11 (2011): S11.
- Shab-Bidar S, Neyestani TR, Djazayery A. Vitamin D receptor Cdx-2-dependent response of central obesity to vitamin D intake in the subjects with type 2 diabetes: a randomised clinical trial. British Journal of Nutrition 9 (2015): 1375-1384.
- Shidfar F, Darabkhani PB, Yazdanpanah L, et al. Assessment of nutritional status in patients with Parkinson’s disease and its relationship with severity of the disease. Medical journal of the Islamic Republic of Iran 11 (2016): 454.
- Zethelius Br, Byberg L, Hales CN, et al. Proinsulin is an independent predictor of coronary heart disease: report from a 27-year follow-up study. Circulation 15 (2002): 2153-2158.
- Nakamoto M, Uemura H, Sakai T, et al. Inverse association between soya food consumption and insulin resistance in Japanese adults. Public health nutrition 12 (2015): 2031-2040.
- Raeisi-Dehkordi H, Amiri M, Zimorovat A, et al. Canola oil compared with sesame and sesame-canola oil on glycaemic control and liver function in patients with type 2 diabetes: A three-way randomized triple-blind cross-over trial. Diabetes/metabolism research and reviews 19 (2021): e3399.
- Gulesserian T, Widhalm K. Effect of a rapeseed oil substituting diet on serum lipids and lipoproteins in children and adolescents with familial hypercholesterolemia. Journal of the American College of Nutrition. 11 (2002): 103-108.
- Negele L, Schneider B, Ristl R, et al. Effect of a low-fat diet enriched either with rapeseed oil or sunflower oil on plasma lipoproteins in children and adolescents with familial hypercholesterolaemia. Results of a pilot study. European journal of clinical nutrition 8 (2015): 337-343.
- Bierenbaum ML, Reichstein RP, Watkins TR, et al. Effects of canola oil on serum lipids in humans. Journal of the American College of Nutrition 5 (1991): 228-233.
- Yang R, Xue L, Zhang L, et al. Phytosterol contents of edible oils and their contributions to estimated phytosterol intake in the Chinese diet. Foods 11 (2019): 334.
- Calpe-Berdiel L, Escolà-Gil JC, Blanco-Vaca F. New insights into the molecular actions of plant sterols and stanols in cholesterol metabolism. Atherosclerosis 6 (2009): 18-31.
- Salamatdoustnobar R, Nazeradl K, Ayazi A, et al. Beneficial effects of canola oil on serum biochemical parameters of Iranian native turkeys. Journal of Animal and Veterinary Advances 18 (2009): 2206-2209.
- Santas J, Codony R, Rafecas M. Phytosterols: Beneficial Effects. Journal of Animal and Veterinary Advances 9 (2009): 9-113.
- Yan M, Frank EM, Cochran EW. Effects of vegetable oil composition on epoxidation kinetics and physical properties. Journal of the American Oil Chemists' Society 10 (2018): 209-216.
- Marangoni F, Agostoni C, Borghi C, et al. Dietary linoleic acid and human health: Focus on cardiovascular and cardiometabolic effects. Atherosclerosis 6 (2020): 90-98.
- Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends in Endocrinology & Metabolism 7 (2017): 545-560.
- Yao J, Lu Y, Zhi M, et al. Dietary n-3 polyunsaturated fatty acids ameliorate Crohn's disease in rats by modulating the expression of PPAR-γ/NFAT. Molecular medicine reports 9 (2017): 8315-8322.
- Louet JF, Chatelain F, Decaux JF, et al. Long-chain fatty acids regulate liver carnitine palmitoyltransferase I gene (L-CPT I) expression through a peroxisome-proliferator-activated receptor α (PPARα)-independent pathway. Biochemical Journal 12 (2001): 189-197.
- Le May Cd, Cauzac Ml, Diradourian C, et al. Fatty acids induce L-CPT I gene expression through a PPARα-independent mechanism in rat hepatoma cells. The Journal of nutrition 13 (2005): 2313-2319.
- Kopecky J, Rossmeisl M, Flachs P, et al. n-3 PUFA: bioavailability and modulation of adipose tissue function: Symposium on ‘Frontiers in adipose tissue biology’. Proceedings of the Nutrition Society 11 (2009):361-9.
- Ceddia R. The role of AMP-activated protein kinase in regulating white adipose tissue metabolism. Molecular and Cellular Endocrinology 4 (2013): 194-203.
