Colitis Nucleomigrans: A Proposal for The Third Type of Microscopic Colitis
Article Information
Mitsuhiro Tachibana, MD1*, Yutaka Tsutsumi, MD1,2
1Department of Diagnostic Pathology, Shimada Municipal Hospital, Shimada, Shizuoka, Japan
2Diagnostic Pathology Clinic, Pathos Tsutsumi, Inazawa, Aichi, Japan
*Corresponding Author: Dr. Mitsuhiro Tachibana, Department of Diagnostic Pathology, Shimada Municipal Hospital, 1200-5 Noda, Shimada, Shizuoka 427-8502, Japan
Received: 12 March 2021; Accepted: 22 March 2021; Published: 31 March 2021
Citation: Mitsuhiro Tachibana, Yutaka Tsutsumi. Colitis Nucleomigrans: A Proposal for The Third Type of Microscopic Colitis. Archives of Clinical and Medical Case Reports 5 (2021): 342-354.
View / Download Pdf Share at FacebookAbstract
Recently, we proposed the third entity of microscopic colitis (MC), termed colitis nucleomigrans (CN). The present review describes clinicopathological features of CN. CN shares clinical and endoscopic features of MC with collagenous colitis and lymphocytic colitis. We analyzed endoscopic biopsy specimens of nonspecific colitis clinically manifesting chronic watery diarrhea or inflammatory bowel disease (IBD)-like symptoms, but with minor endoscopic abnormality. The histopathological criteria of CN are as follows: a) chained nuclear migration to the middle part of the surface-lining columnar epithelia, b) apoptotic nuclear debris scattered below the nuclei, and c) mild to moderate chronic inflammation in the lamina propria. Thirty-three patients (M: F=20:13, median 63 years; range 17-88) fulfilled the above criteria. Seven cases accompanied MC-like clinical/endoscopic features. Mucosal reddening with or without erosions/aphthae was endoscopically observed in the remaining 26 cases with IBD-like clinical features: occult/gross hematochezia (n=19), abdominal pain (n=2) and mucin secretion (n=2). Apoptotic debris immunoreactive for cleaved caspase-3 appeared more frequently in IBD-like CN than in MC-like CN. CD8-positive intraepithelial lymphocytes were comparable in both types. Proton pump inhibitors (PPIs) were administered in five (71%) CN cases with MC-like features, and the diarrhea improved after cessation of PPIs in three. In IBD-like CN cases, eight (31%) received PPIs. Altered apoptotic processes in the colorectal surface-lining epithelia, predominantly with a debris pattern of apoptosis, may be involved in the pathogenesis. Mechanisms of nuclear migration to the unusual position and the impairment of nuclear anchoring to the basal situation in the surface-lining epithelia remain to be established.
Keywords
Apoptosis; Colitis nucleomigrans; Colorectal diseases; Watery diarrhea; Intraepithelial lymphocytes; Microscopic colitis
Apoptosis articles; Colitis nucleomigrans articles; Colorectal diseases articles; Watery diarrhea articles; Intraepithelial lymphocytes articles; Microscopic colitis articles
Apoptosis articles Apoptosis Research articles Apoptosis review articles Apoptosis PubMed articles Apoptosis PubMed Central articles Apoptosis 2023 articles Apoptosis 2024 articles Apoptosis Scopus articles Apoptosis impact factor journals Apoptosis Scopus journals Apoptosis PubMed journals Apoptosis medical journals Apoptosis free journals Apoptosis best journals Apoptosis top journals Apoptosis free medical journals Apoptosis famous journals Apoptosis Google Scholar indexed journals Colitis nucleomigrans articles Colitis nucleomigrans Research articles Colitis nucleomigrans review articles Colitis nucleomigrans PubMed articles Colitis nucleomigrans PubMed Central articles Colitis nucleomigrans 2023 articles Colitis nucleomigrans 2024 articles Colitis nucleomigrans Scopus articles Colitis nucleomigrans impact factor journals Colitis nucleomigrans Scopus journals Colitis nucleomigrans PubMed journals Colitis nucleomigrans medical journals Colitis nucleomigrans free journals Colitis nucleomigrans best journals Colitis nucleomigrans top journals Colitis nucleomigrans free medical journals Colitis nucleomigrans famous journals Colitis nucleomigrans Google Scholar indexed journals Colorectal diseases articles Colorectal diseases Research articles Colorectal diseases review articles Colorectal diseases PubMed articles Colorectal diseases PubMed Central articles Colorectal diseases 2023 articles Colorectal diseases 2024 articles Colorectal diseases Scopus articles Colorectal diseases impact factor journals Colorectal diseases Scopus journals Colorectal diseases PubMed journals Colorectal diseases medical journals Colorectal diseases free journals Colorectal diseases best journals Colorectal diseases top journals Colorectal diseases free medical journals Colorectal diseases famous journals Colorectal diseases Google Scholar indexed journals environment articles environment Research articles environment review articles environment PubMed articles environment PubMed Central articles environment 2023 articles environment 2024 articles environment Scopus articles environment impact factor journals environment Scopus journals environment PubMed journals environment medical journals environment free journals environment best journals environment top journals environment free medical journals environment famous journals environment Google Scholar indexed journals Watery diarrhea articles Watery diarrhea Research articles Watery diarrhea review articles Watery diarrhea PubMed articles Watery diarrhea PubMed Central articles Watery diarrhea 2023 articles Watery diarrhea 2024 articles Watery diarrhea Scopus articles Watery diarrhea impact factor journals Watery diarrhea Scopus journals Watery diarrhea PubMed journals Watery diarrhea medical journals Watery diarrhea free journals Watery diarrhea best journals Watery diarrhea top journals Watery diarrhea free medical journals Watery diarrhea famous journals Watery diarrhea Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals CT articles CT Research articles CT review articles CT PubMed articles CT PubMed Central articles CT 2023 articles CT 2024 articles CT Scopus articles CT impact factor journals CT Scopus journals CT PubMed journals CT medical journals CT free journals CT best journals CT top journals CT free medical journals CT famous journals CT Google Scholar indexed journals Intraepithelial lymphocytes articles Intraepithelial lymphocytes Research articles Intraepithelial lymphocytes review articles Intraepithelial lymphocytes PubMed articles Intraepithelial lymphocytes PubMed Central articles Intraepithelial lymphocytes 2023 articles Intraepithelial lymphocytes 2024 articles Intraepithelial lymphocytes Scopus articles Intraepithelial lymphocytes impact factor journals Intraepithelial lymphocytes Scopus journals Intraepithelial lymphocytes PubMed journals Intraepithelial lymphocytes medical journals Intraepithelial lymphocytes free journals Intraepithelial lymphocytes best journals Intraepithelial lymphocytes top journals Intraepithelial lymphocytes free medical journals Intraepithelial lymphocytes famous journals Intraepithelial lymphocytes Google Scholar indexed journals Colon Cancer articles Colon Cancer Research articles Colon Cancer review articles Colon Cancer PubMed articles Colon Cancer PubMed Central articles Colon Cancer 2023 articles Colon Cancer 2024 articles Colon Cancer Scopus articles Colon Cancer impact factor journals Colon Cancer Scopus journals Colon Cancer PubMed journals Colon Cancer medical journals Colon Cancer free journals Colon Cancer best journals Colon Cancer top journals Colon Cancer free medical journals Colon Cancer famous journals Colon Cancer Google Scholar indexed journals Microscopic colitis articles Microscopic colitis Research articles Microscopic colitis review articles Microscopic colitis PubMed articles Microscopic colitis PubMed Central articles Microscopic colitis 2023 articles Microscopic colitis 2024 articles Microscopic colitis Scopus articles Microscopic colitis impact factor journals Microscopic colitis Scopus journals Microscopic colitis PubMed journals Microscopic colitis medical journals Microscopic colitis free journals Microscopic colitis best journals Microscopic colitis top journals Microscopic colitis free medical journals Microscopic colitis famous journals Microscopic colitis Google Scholar indexed journals
Article Details
Abbreviations:
CN- Colitis nucleomigrans; CC- Collagenous colitis; IBD- Inflammatory bowel disease; LC- Lymphocytic colitis; MC- Microscopic colitis; NSAIDs- Non-steroid anti-inflammatory drugs; PPI- Proton pump inhibitor
1. Introduction
Chronic colitis can be divided into the following categories; i.e. inflammatory bowel disease (IBD), treatment-related colitis (pseudomembranous colitis, chemical colitis, chemotherapy-induced colitis, and radiation colitis), infectious colitis, ischemic colitis, and microscopic colitis (MC) [1]. The appropriate microscopic diagnosis of colitis is made by diagnostic pathologists who give routine pathology services. MC, predominantly seen in middle-aged women, is characterized by chronic non-bloody watery diarrhea and an endoscopically normal but microscopically altered specific appearance of the colonic mucosa [2,3]. Other common symptoms include nocturnal diarrhea, abdominal pain and weight loss [2, 3].
The category of MC has encompassed two distinct entities such as collagenous colitis (CC) and lymphocytic colitis (LC). They share clinical manifestations and are occasionally associated with autoimmune diseases [3-5]. Histologically, CC is featured by the deposition of subepithelial collagen fibers “collagen bands” in the uppermost part of the lamina propria mucosae, while LC characteristically reveals marked increase of CD8-positive intraepithelial lymphocytes (IELs). Overlapped lesions between CC and LC have been reported [3-5].
In daily diagnostic pathology practice, we often encounter biopsy specimens that display microscopic features of chronic nonspecific colitis without collagen bands or increased IELs. Recently, we proposed the third type of MC, colitis nucleomigrans (CN) [6,7]. Histologically, the nuclei of surface-lined colonic columnar cells migrate in chains to the middle part of the cells, and fragmented debris of apoptotic nuclei is scattered beneath the migrated nuclei. A total of 33 cases were analyzed clinicopathologically. The patients revealed chronic non-bloody watery diarrhea but with normal endoscopic appearance (MC-like symptoms) (n=7) or microscopic/gross bloody stools with endoscopic mucosal redness, abdominal pain and/or mucin secretion (IBD-like symptoms) (n=26).
2. Clinical and endoscopic features of CN
Thirty-three patients satisfied the microscopic criteria of CN [6]: a) chained nuclear migration to the middle part of the surface-lining columnar epithelium, b) apoptotic nuclear debris scattered below the migrated nuclei, and c) mild to moderate infiltration of lymphocytes and plasma cells in the lamina propria. The frequency of CN among colorectal biopsy specimens was 33/1287 (2.6%). Twenty men and 13 women were enrolled, and the age ranged from 17 to 88 years with the mean 58.4 and the median 63. Patients manifested chronic non-bloody watery diarrhea (MC-like symptoms) with normal endoscopic appearance (n=7; Figure 1) or occult (n=9)/gross (n=10) haematochezia, abdominal pain (n=2) or mucin secretion (n=2) (IBD-like symptoms), endoscopically showing mucosal reddening with or without focal erosions/aphthae (n=26; Figure 2). One patient in the MC-like group exceptionally accompanied fecal occult blood, while normal-looking endoscopic findings were recorded in three patients in the IBD-like group.
PPIs were regularly administered in 13 patients; five (71%) with MC-like features and eight (31%) with IBD-like features. In three in the MC-like group, diarrhea improved following the discontinuation of medication. H2-blockers and non-steroidal anti-inflammatory drugs (NSAIDs) were used in two patients each in the IBD-like group. Hypertension was documented in eight patients. Two patients were suspected of Behçet disease, but without intestinal ulceration. One patient suffered from IgA nephropathy, and one patient suffered Sjögren syndrome. Four patients underwent chemotherapy against plasma cell myeloma, nodal diffuse large B-cell lymphoma, splenic diffuse large B-cell lymphoma, or colonic mucinous adenocarcinoma. The periods between the completion of chemotherapy and the diagnosis of CN were 0, 5, 6 and 53 months, respectively.
Clinical and endoscopic features of CN with MC-like features (n=7) and CN with IBD-like features (n=26) are summarized in Table 1.
Abbreviations: M, male; F, female; yrs, years; watery diarrhea score: 0, negative; 1+, mild; 2+, moderate; 3+, severe IBD, inflammatory bowel disease; C-caspase 3, cleaved-caspase 3; IELs, intraepithelial lymphocytes; FOB, fecal occult blood; NSAIDs, non-steroidal anti-inflammatory drugs; FIV, focal indistinct vascular pattern; MR, mucosal roughening; * diarrhoea improved after cessation of PPIs.
Table 1: Summary of cases of colitis nucleomigrans.
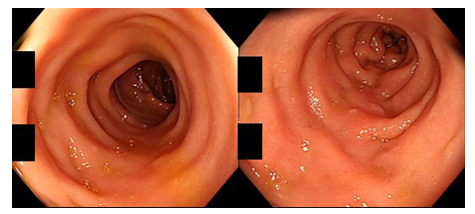
Figure 1: Colonoscopic findings in a patient of CN with MC-like symptoms (80-year-old man; left: transverse colon, right: sigmoid colon). The colorectal mucosa appears normal without mucosal edema, reddening, erosion or ulceration.
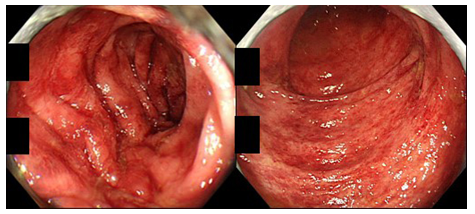
Figure 2: Colonoscopic findings in a patient of CN with IBD-like symptoms (82-year-old man; left: sigmoid colon, right: rectum). The colonic mucosa displays multiple patchy reddening.
3. Histopathological features of CN
The total number of biopsy pieces with microscopic features of CN sampled from 33 cases reached 65. The microscopic features of CN were observed in any part of the large intestine (cecum 3, ascending colon 7, transverse colon 10, descending colon 8, sigmoid colon 21 and rectum 16). The chained nuclear migration was never experienced in the crypt epithelial cells. Collagen bands and increased IELs (>20 IELs among 100 epithelial cells) failed to be noted in the specimens. Representative microscopic appearance of CN is demonstrated in Figure 3.
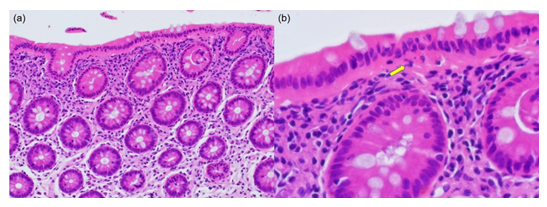
Figure 3: Microscopic findings of CN with IBD-like symptoms (54-year-old man; HE). (a) The nuclei of the surface-lining columnar cells are migrated in chain to the middle part of the cells. Eosinophilic cytoplasm is evident under the chained nuclei. The colonic crypts are arranged in parallel without features of cryptitis or nuclear migration. The lamina propria mucosae reveals moderate infiltration of lymphocytes and plasma cells. (b) A high-powered view shows the migrated chained nuclei in the surface-lining columnar epithelial cells. Under the nuclei, apoptotic bodies (clustered fragments of nuclear debris) are seen (arrow). Subepithelial collagen bands and increased IELs are not recognized.
In the period of the present study, we experienced only five cases of CC and no case of LC. The colorectal mucosa in a remission state of UC (n=20), as well as normal colorectal mucosa sampled from surgical specimens of colorectal cancer (n=5), were also evaluated. The CN-type microscopic features were not seen in CC and control normal colorectal mucosa, but observed in twelve (60%) lesions in UC in a remission state. IELs were immunoreactive for both CD3 and CD8. IELs were scattered among the CN lesion (but without significant increase). The average numbers of CD8-positive IELs were 7.0 per 100 surface epithelial cells (median: 3.6, range: 2.3-17.4) in CN with MC-like features, and 7.9 (median: 7.9, range: 1.6-17.2) in CN with IBD-like features. In the control group, the mean numbers of IELs were 18.1 (median: 7.2, range: 4.0-64.9) in CC, 7.6 (median: 6.0, range: 0-18.3) in UC in a remission state, and 4.4 (median: 4.2, range: 1.3-7.1) in surgically resected normal colorectal mucosa. Apoptotic bodies with a ‘debris pattern’ (clustered fragments of nuclear debris) beneath the migrated nuclei in CN were clearly immunoreactive for cleaved caspase-3 (Figure 4a and 4b). In the MC-like CN lesions, cleaved caspase-3-positive apoptotic bodies of debris type counted 10.3 per 100 surface-lining epithelial cells on average (median: 9.9, range: 1.4-22.6), while the average number in the IBD-like CN lesions was higher: 28.2 (median: 20.5, range: 1.8-64.8).
In the surgically resected normal colorectal mucosa, cleaved caspase-3-immunoreactivity was seen mainly as non-fragmented apoptotic nuclei along the luminal space on the surface-lining cells, representing an ‘extrusion pattern’ of apoptosis (Figure 4c and 4d). Apoptosis of debris type was infrequently observed in normal colorectal mucosa. Apoptosis of extrusion type was occasionally seen also in the CN lesions. The number of cleaved caspase 3-immunoreactive apoptotic bodies of extrusion type in the normal colorectal mucosa ranged from 1.0 to 5.4 (mean 3.4, median: 3.5) per 100 epithelial cells. The apoptotic bodies in CC (n=5) were predominantly categorized in the debris type and their number ranged from 6.1 to 23.4 (mean: 14.3, median: 13.5). In the lesions in UC in a remission state, epithelial apoptosis was identified in mixed debris and extrusion patterns: The number of apoptosis of the debris type ranged from 6.2 to 63.2 (mean: 29.1, median 28.2). CD68-positive macrophages located in the upper part of the lamina propria phagocytized cleaved caspase 3-immunoreactive apoptotic bodies (fragmented nuclear debris) in the cytoplasm. Such a pattern was observed in any type of the colorectal lesions and in normal colorectal mucosa, but their number was increased in the lesion of CN (Figure 4e and 4f). The CD68-positive macrophages in CN showed the plump and ameboid cytoplasm, while those in the normal colorectal mucosa tended to be round and smaller in size. The number of apoptotic debris-phagocytizing CD68-positive macrophages in CN with IBD-like features was comparable with that in CN with MC-like features.
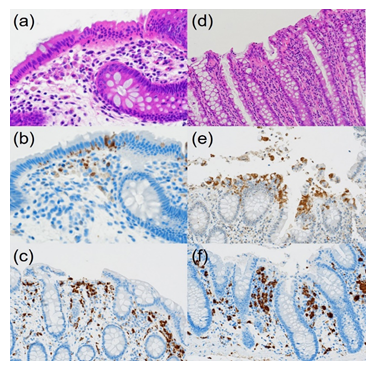
Figure 4: Comparative immunohistochemical features of CN (a-c, 40-year-old woman) and control normal colon (d-f, 82-year-old man): HE (a&d), cleaved caspase-3 (b&e) and CD68 (c&f). In CN, fragmented apoptotic bodies, clustered under the chained migrated nuclei, are immunoreactive for cleaved caspase-3. In contrast, in the surgically removed normal colorectal mucosa, cleaved caspase-3-immunoreactive rounded nuclei are extruded onto the apical surface. CD68-positive macrophages located in the upper part of the lamina propria mucosae actively phagocytize apoptotic debris in the plump and ameboid cytoplasm, while macrophages in the normal mucosa are round in shape and smaller in size.
4. Ultrastructural features of CN
Buffered formalin-fixed biopsy tissue of CN (n=2) was dug out of paraffin blocks for the ultrastructural analysis [7]. After deparaffinization, tissue blocks were prepared with the conventional sequence. Ultrathin sections were stained with uranyl acetate and lead citrate. It is of note that ultrastructural morphology was satisfactory even after paraffin embedding. As illustrated in Figure 5, apoptotic nuclear debris was localized within the cytoplasm beneath the migrated nuclei of the surface-lining columnar cells. Abnormality of cytoskeletal filaments was scarcely recognized in the epithelial cytoplasm. Macrophages located in the uppermost part of the lamina propria phagocytized electron-dense globular apoptotic materials. IELs with scattered dense bodies were scattered among the columnar cells.

Figure 5: Ultrastructural features of CN with IBD-like symptoms (54-year-old man). (a) Low-powered views of transmission electron microscopy for CN. (b–d) High-powered views of transmission electron microscopy for CN. (a) The nuclei of the surface-lining columnar cells are migrated in chain to the middle part of the cells. In the uppermost part of the lamina propria mucosae, macrophages phagocytizing electron-dense apoptotic bodies (arrowheads) are clustered, and lymphocytes are also moderately infiltrated (bar=10 mm). (b) An IEL is indicated by an arrowhead, and apoptotic debris (arrow) is seen among the epithelial cells. No aggregation or reorganization of cytoskeletal filaments is observed in the infranuclear cytoplasm (bar=5 mm). (c) A close view of a macrophage observed in the uppermost part of the lamina propria, actively phagocytizing electron-dense apoptotic nuclear debris (bar=5 mm). (d) The dense bodies are dispersed in the cytoplasm of the IEL among the surface-lining columnar cells (bar=2 mm).
5. Discussion
Recent epidemiological studies has suggested that MC is more common than initially expected: one study identified MC in 10% of colorectal biopsy specimens from patients with non-bloody diarrhea, and in >20% of such patients are older than 70 years [3,4]. Reportedly, MC occurs more frequently in women than in men, and usually affects patients in the sixth and seventh decades [3,4]. In our analysis, however, all the cases of CC were men. In 15% of MC cases, a range of mild endoscopic abnormality was reported, such as patchy reddening/inflammation, pancolitis and featureless mucosa [5]. The etiology of MC remains unclarified, but oral medication has been suggested as a potential trigger: The drugs include lansoprazole, simvastatin, flutamide, ranitidine, carbanazepin, vinburninem paroxetine, sertraline, penicillin V, pivmecillinam, orlistate, tardyferon and Cyclo 3 Fort [8]. Autoimmune disorders may be associated with MC, in a range of 20-60% for both CC and LC [8].
We proposed CN as the third-type MC, microscopically characterized by chained migration of the nucleus in the colorectal surface-lining epithelium, in association with accelerated apoptosis of debris type beneath the nuclei [6]. Patients with CN were categorized into two clinical/endoscopic subtypes. One group manifested MC-like symptoms (watery non-bloody diarrhea) with minimal endoscopic abnormality. Another group is featured by IBD-like symptoms (occult/gross hematochezia, abdominal pain and/or mucin secretion) with patchy reddening of the colorectal mucosa with or without erosions/aphthae.
When compared with conventional MC (CC and LC), CN showed a male predominance, with a wide age range from 17 to 88 years (median: 63 years). Some patients received medication of PPIs as a potential trigger, as is so in conventional MC. In three cases of CN with MC-like features, diarrhea improved after discontinuation of PPIs. In four patients (one in MC-like type, and three in IBD-like type), CN occurred during or several months after chemotherapy against hematopoietic malignancies or colonic adenocarcinoma. Regarding autoimmune disorders, two patients of CN were clinically suspected of Behçet disease without intestinal ulceration, and a total of two patients suffered from IgA nephropathy or Sjögren syndrome. CN-type microscopic features were also seen in a remission state of ulcerative colitis. The candidate etiologic factors for provoking CN should thus include the medication of PPIs or anti-cancer drugs, the association of immune-related disorders and the altered cell turnover secondary to the treatment of IBD.
The pathogenesis of CN may be linked to the abnormality of epithelial apoptosis of debris type in the colorectal mucosa [6,7]. In the intestinal mucosa, the microbiotas may induce apoptosis of epithelial cells in balance with adaptive immune homeostasis. Nakahashi-Oda et al. [8] recently documented that apoptotic epithelial cells negatively regulate the gut commensal bacteria-stimulated proliferation of regulatory T-cells, playing a central role in the maintenance of gut tissue homeostasis. Supposedly, apoptotic epithelial cells suppress the proliferation of regulatory T-cells for regulating mucosal homeostasis. Commensal enteric bacteria play an important role in autoimmune disorders [10]. In fact, dysbiosis of the gut normal flora has been observed in patients with autoimmune disorders, such as systemic lupus erythematosus [11]. In contrast, some microbiotas, especially Clostridium IV or XIV, may induce regulatory T-cells and inhibit the development of IBD [11]. The chained nuclear migration to the middle part of the surface-lining epithelial cells was quite unique and pathognomonic of CN, and easily recognizable in hematoxylin and eosin-stained biopsy specimens. Such microscopic appearance of CN was barely observed in CC and the control normal colorectal mucosa. The mechanisms of the nuclear migration remain uncertain. It is possible that abnormality of cytoskeletal proteins may impair localization of the nuclei that are normally anchored to the basal part of the columnar cells. However, negative electron microscopic findings were observed, as described above [7] (Figure 5). Under a low-powered magnification, pathologists may confuse CN with CC, because the eosinophilic cytoplasmic component beneath the migrated nuclei of the surface epithelial cells superficially looks like the collagen bands in CC. Therefore, in order to avoid inappropriate diagnosis, close-up microscopy under high magnification is inevitable.
The mode of apoptosis in normal gut mucosa has been analyzed microscopically. Sträter et al. [12] described two different patterns of enterocytic apoptosis: (a) apoptotic bodies (nuclear debris) were engulfed by adjacent epithelial cells, and (b) the apoptotic cells with only delicate morphological changes were extruded into the gut lumen. The engulfment pattern was observed predominantly in the crypt. The extrusion pattern was restricted to the luminal mucosal surface. Iwanaga et al. [13] showed unique features of apoptosis at the tip of small intestinal villi of the guinea pig. The apical cytoplasmic plates without containing nuclei were pinched off into the lumen, and the nuclei were engulfed by macrophages located in the upper part of the lamina propria mucosae. The fate of apoptotic nuclei of enterocytes may thus be dependent on the site in the mucosa and animal species. In the present study, the protrusion pattern of apoptosis was observed on the normal colorectal mucosa, just in accordance with the report by Sträter et al. [12]. CD68-positive macrophages actively phagocytizing apoptotic nuclear debris were more easily seen in CN than in the normal colorectal mucosa [6]. Abnormality of enterocytic apoptosis has so far been linked to a variety of intestinal disorders [14,15]. The chained nuclear migration in the surface-lining columnar cells, in association with fragmented nuclear debris beneath the nuclei, may represent an accelerated and altered apoptosis (switching from the extrusion type to debris type) in the diseased colorectal mucosa of CN [6].
IELs of CD8-positive cytotoxic T-cell type are known to promote epithelial apoptosis by secretion of cytotoxic molecules such as granzymes and perforin: Granzyme B accelerates apoptosis through both caspase-dependent and -independent pathways [16]. IELs also contribute to cryptal apoptosis and ulceration in active IBD [17]. In celiac disease, increase of IELs expressing Fas ligand and perforin stirs up mucosal damage and epithelial apoptosis [18,19]. In acute human immunodeficiency virus infection, both apoptotic epithelial cells and IELs are increased [20]. In our analysis, cleaved caspase-3-immunoreactive fragmented apoptotic bodies were more frequently observed in IBD-like CN than in MC-like CN, but CB8-positive IELs and CD68-positive phagocytizing lamina proprial macrophages were not significantly altered [6]. It is supposed that the accelerated apoptosis of debris type may be related to clinical association of occult/gross haematochezia. A recent study [21] showed in the UC rat model that microRNA-21-5p inhibited interleukin-6 receptor/signal transducer and activator of transcription signal-mediated pathway to decrease the level of inflammatory cytokines and apoptosis. Therefore, the authors implicated microRNA-21-5p as a candidate of therapeutic molecular target of UC in the human. The microRNA-21-5p-targeted therapy may be hopefully applicable to IBD-like CN.
The clinical and epidemiological features of MC have been established during the past 30 years [2,3]. We proposed a new variant of MC, “CN”. It is of note that more than half (60%) of colorectal biopsy specimens sampled from patients with UC in a remission state showed microscopic appearance resembling CN [6]. The chained nuclear migration to the middle part of the surface-lining columnar cells may be correlated with the abnormality in apoptotic processes of the colorectal surface epithelial cells. Further clinicopathological studies are needed to evaluate whether CN can really be regarded as a distinct disease entity in the category of MC, and to settle unanswered questions in this perplexing condition, including the cause, pathophysiology, and optimal treatment.
Contributors
MT and YT contributed equally to the present analysis. YT proposed a basic idea of CN. Both authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content, cooperatively designed the study, and analyzed both light and electron microscopic features.
Funding
The authors received no financial support for the research, authorship, and/or publication of the present article.
Competing Interests
None declared.
Ethics Approval
All the procedures were in accordance with the ethical standards of the responsible institutional committee on human experimentation and with the Helsinki Declaration of 1964 and later versions. The study was approved in December 2019 by the Ethics Committee for Clinical Research of Shimada Municipal Hospital, Shimada, Shizuoka, Japan (approval number R01-10).
Provenance and Peer Review
Not commissioned; externally peer reviewed.
Patient Consent for Publication
Written informed consent was obtained from the respective patients after selection of cases, including negative control cases.
Acknowledgments
Tomohiko Hanaoka, M.D., Shinya Watanabe, M.D., Masahiro Matsushita, M.D., and Tadahiro Isono, M.D. at the Departments of Gastroenterology (TH, SW, MM) and Surgery (TI), Shimada Municipal Hospital, Shimada, Shizuoka, contributed to providing valuable clinical information. We cordially thank Naoki Ooishi, M.T., Kuniaki Muramatsu, M.T., and Kazuhiro Nakajima, M.T., Department of Diagnostic Pathology, Shimada Municipal Hospital, Shimada, Shizuoka, Japan. Kohei Watanabe, M.T., Special Reference Laboratories, Hamura, Tokyo, Japan, are also acknowledged for his support in the electron microscopic analysis.
References
- Choi EK, Appelman HD. Chronic colitis in biopsy samples. Surg Pathol Clin 10 (2017): 841-861.
- Tysk C, Bohr J, Nyhlm N, et al. Diagnosis and management of microscopic colitis. World J Gastroenterol 14(2008): 7280-7288.
- Park T, Cave D, Marshall C. Microscopic colitis: a review of etiology, treatment and refractory disease. World J Gastroenterol 21 (2015): 8804-8810.
- Baert F, Wouters K, D’Haens G, et al. Lymphocytic colitis: a distinct clinical entity? A clinicopathological confrontation of lymphocytic and collagenous colitis. Gut 45(1999): 375-381.
- Williams JJ, Beck PL, Andrews CN, et al. Microscopic colitis: a common cause of diarrhoea in older adults. Age Ageing 39 (2010): 162-168.
- Tachibana M, Hanaoka T, Watanabe S, et al. Colitis nucleomigrans: the third type of microscopic colitis (part 1). Pathol Int 70 (2020): 752-760.
- Tachibana M, Tsutsumi Y. Colitis nucleomigrans: the third type of microscopic colitis (part 2). An ultrastructural study. Pathol Int 70 (2020): 761-766.
- Olesen M, Eriksson S, Bohr J, et al. Microscopic colitis: a common diarrhoeal disease. An epidemiological study in Orebro, Sweden, 1993-1998. Gut 53 (2004): 346-350.
- Nakahashi-Oda C, Udayanga KGS, Nakamura Y, et al. Apoptotic epithelial cells control the abundance of Treg cells at barrier surfaces. Nat Immunol 17 (2016): 441-450.
- Bach JF. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat Rev Immunol 18 (2018): 105-120.
- Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature 535 (2016): 75-84.
- Sträter J, Koretz K, Günthert AR, et al. In situ detection of enterocytic apoptosis in normal colonic mucosa and in familial adenomatous polyposis. Gut 37 (1995): 819-825.
- Iwanaga T, Han H, Adachi K, et al. A novel mechanism for disposing of effete epithelial cells in the small intestine of guinea pigs. Gastroenterology 105 (1993): 1089-1097.
- Ramachandran A, Madesh M, Balasubramanian KA. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J Gastroenterol Hepatol 15 (2000): 109-120.
- Negroni A, Cucchiara S, Stronati L. Apoptosis, necrosis, and necroptosis in the gut and intestinal homeostasis. Mediators Inflamm 2015 (2015): 250762.
- Henkart PA. Lymphocyte-mediated cytotoxicity: two pathways and multiple effector molecules. Immunity 1 (1994): 343-346.
- Mitomi H, Ohkura Y, Yokoyama K, et al. Contribution of TIA-1+ and granzyme B+ cytotoxic T lymphocytes to cryptal apoptosis and ulceration in active inflammatory bowel disease. Pathol Res Pract 203 (2007): 717-723.
- Ciccocioppo R, Di Sabatino A, Parroni R, et al. Cytolytic mechanisms of intraepithelial lymphocytes in coeliac disease (CoD). Clin Exp Immunol 120 (2000): 235-240.
- Ciccocioppo R, Di Sabatino A, Parroni R, et al. Increased enterocyte apoptosis and Fas-Fas ligand system in celiac disease. Am J Clin Pathol 115 (2001): 494-503.
- Epple HJ, Allers K, Tröger H, et al. Acute HIV infection induces mucosal infiltration with CD4+ and CD8+ T cells, epithelial apoptosis, and a mucosal barrier defect. Gastroenterology 139 (2010): 1289-1300.
- Lu X, Yu Y, Tan S. The role of the miR-21-5p-mediated inflammatory pathway in ulcerative colitis. Exp Ther Med 19 (2020): 981-989.
