Clinicopathological Evaluation of Colorectal Growth in a Tertiary Level Hospital in Bangladesh: A Series of Hundred Cases
Article Information
Syeda Rumman Aktar Siddiqui1, Md. Ariful Islam2, Ratim Mir3, Mohammad Zillur Rahman4
1Associate Professor, Department of Biochemistry, Chittagong Medical College, Chattogram, Bangladesh, Email: siddiquirumman@gmail.com, Orcid Id: 0009-0002-2553-8491
2Assistant Professor, Department of Pathology, International Medical College, Gazipur, Dhaka, Bangladesh, Email: maislam870@gmail.com, Orcid Id: 0009-0008-0504-0104
3Assistant professor, Department of Pathology, Shaheed M Monsur Ali Medical College, Sirajganj, Bangladesh, Email: ratim.uni.48@gmail.com, Orcid Id: 0009-0006-6393-1891
4Professor and Chairman, Department of Pathology, Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, Bangladesh, Email: drzillur@gmail.com, Orcid Id: 0000-0003-3222-1118
*Corresponding Author: Dr. Syeda Rumman Aktar Siddiqui, Associate Professor, Department of Biochemistry, Chittagong Medical College, Chattogram, Bangladesh
Received: 20 August 2024; Accepted: 09 September 2024; Published: 05 September 2025.
Citation:
Syeda Rumman Aktar Siddiqui, Md. Ariful Islam, Ratim Mir, Mohammad Zillur Rahman. Clinicopathological Evaluation of Colorectal Growth in a Tertiary Level Hospital in Bangladesh: A Series of Hundred Cases. Journal of Cancer Science and Clinical Therapeutics 9 (2025): 132-139.
View / Download Pdf Share at FacebookAbstract
Introduction: Colorectal cancer (CRC) ranks as the third most commonly diagnosed cancer worldwide, with incidence and mortality rates of 6.1% and 9.2%, respectively. In 2020, CRC accounted for an estimated 10 million deaths globally, with 6 million occurring in low-income countries. Despite being less prevalent in Bangladesh compared to other underdeveloped nations, it poses a significant health burden. Methods: This retrospective cross-sectional study, conducted at the Department of Histopathology in Ahsania Mission Cancer and General Hospital, Dhaka, Bangladesh, from July to December 2023, aimed to assess the clinicopathological characteristics of colorectal growth among patients undergoing colonoscopic biopsy and excision procedures. A purposive sampling technique was employed, enrolling 100 cases irrespective of age and sex. Results: Of the study participants, 50% underwent colonoscopic biopsy, with 52% being malignant and 48% non-malignant. All patients (50%) undergoing excision procedures had malignant cases. Female patients comprised the majority (60.5%) of malignant cases, with the ascending colon being the most frequent site (31.0%) of colorectal carcinoma. Adenocarcinoma, NOS, was the predominant histopathological type (63.2%). Most cases were diagnosed at an advanced stage, with Grade II being the most frequent (54, 71.1%). PT2 was the most common T stage (18, 51.4%), while PN0 dominated among N stages (19, 52.8%), with the highest lymph node involvement observed in N1b (18.0%). Conclusion: Colorectal carcinoma predominantly affects middle-aged individuals, with a notable female predominance. Adenocarcinoma, NOS, represents the most frequent histopathological subtype, primarily affecting the ascending colon. The advanced stage at diagnosis underscores the necessity for screening programs to facilitate early detection in Bangladesh.
Keywords
<p>Clinicopathological, Colorectal Carcinoma, CRC, Growth in the colon, Colonoscopy, Biopsy, Excision.</p>
Article Details
1. Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer globally with an incidence and mortality rate of 6.1% and 9.2% respectively [1]. Nowadays, it is a major health burden across the world. An estimated 10 million deaths occurred in 2020 due to cancers with CRC accounting for 9.4% of these deaths. In low- and middle-income countries (LMICs), it accounts for over 600,000 deaths yearly [2]. In the male population, CRC ranks third in incidence, following lung and prostate cancers, and in females, it ranks second, following breast cancer [3]. There is an over 10-fold geographical variation of CRC incidence throughout the world. The highest incidence rates are observed in Australia and New Zealand, with the estimated age-standardized rates being 44.8 per 100000 populations in men and 32.2 in women. CRC incidence increases with age, and cases are fairly uncommon before the 4th decade of life [4]. This is the reason why most screening programs are targeted at people over 50 years old. Moreover, recent studies have revealed an alarming increase in incidence between the ages of 40 to 44 years prompting consideration of lowering the recommended screening age [5]. Mortality rates have progressively declined in most economically developed countries, in contrast with poorer regions of the world, where mortality is either stable or increasing. However, the prevalence of colorectal cancer is lower in Asia than in Western countries. However, the incidence has been increasing rapidly in countries of the Asia-Pacific region during the last two decades due to the Westernization of lifestyles [6]. In Bangladesh, for 5 years, the prevalence of colon and rectal cancer has been observed at 3.28 and 3.1 per 100,000 populations, respectively [7]. The probability of developing CRC can be increased by both genetic and acquired/environmental factors [8]. Although the impact of genetic susceptibility in an individual is much greater than the impact of acquired factors, the vast majority of CRC cases could be prevented through modifications in environmental factors [8]. Hereditary syndromes, family history and inflammatory bowel diseases are the main risk factors that are recommended for screening [9]. In practice, risk factors that do not affect screening are the target of primary prevention strategies. Hereditary colorectal syndromes comprise numerous specific genetic disorders that are associated with the development of CRC, altogether accounting for about 10% of CRC cases. The most common form is hereditary non-polyposis CRC, which reportedly accounts for only 2%-5% of CRC cases. Family history, in addition to the genetic syndromes thus far known, constitutes a very significant risk factor for developing CRC, which appears to account for up to 25% of cases [10]. Although the mechanisms underlying this observation are not completely understood, studies have shown that individuals having first-degree family members diagnosed with CRC are at 2-3 times greater risk of developing CRC than the general population [11-13]. There is insufficient data on the growth of colorectal carcinoma at the national level of Bangladesh. Hence, the determination of clinicopathological characteristics of colorectal carcinoma is the demand of time which might be of great use to guide the proper management and prognosis of colorectal cancer patients in Bangladesh. This paper aimed to determine the clinicopathological evaluation of the growth of colorectal cancer in Bangladesh.
2. Methods
This retrospective cross-sectional study was conducted at the Department of Histopathology in Ahsania Mission Cancer and General(AMCGH), Uttara, Dhaka, Bangladesh from July 2023 to December 2023. A consecutive sampling technique was used. Clinically suspected 100 colorectal carcinoma cases irrespective of age and sex were retrospectively enrolled in this study. Among the study cases, 50 underwent Colonoscopic Biopsy and 50 underwent Excision procedure. The tumours were classified based on World Health Organization (WHO) classification of tumour guideline, 2019[14] and the grading of tumours was classified by using the Canadian Cancer Society (CCS) specified three-tiered grading system [15]. Staging was performed following the TNM Classification of Colorectal Carcinoma of the American Cancer Society, 2020[16]. Data were collected using an Excel data sheet from the registry of the Histopathology Department of Ahsania Mission Cancer and General(AMCGH), Uttara, Dhaka, Bangladesh
Data Analysis:
The study coordinators performed random checks to verify data collection processes. Completed data forms were reviewed, edited and processed for computer data entry. Descriptive statistical analysis was performed and the results were presented in tables and charts. Chi-square tests were performed to determine the association between the study variables, and the ANOVA test was applied to indicate the difference in mean between the study groups, where p<0.05 was considered as the level of significance with 95% CI. Statistical analyses were carried out by using the Statistical Package for Social Sciences version 26.0 for Windows (SPSS Inc., Chicago, Illinois, USA). The quantitative observations were indicated by frequencies and percentages.
3. Results
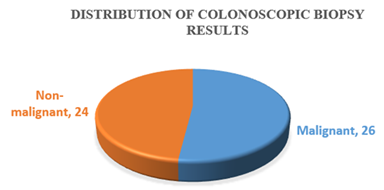
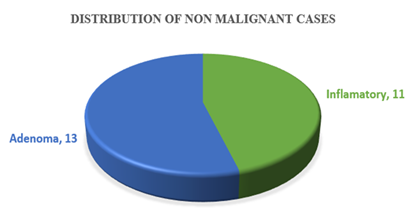
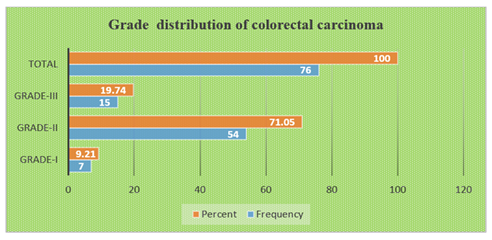
Table-1 shows the age distribution of patients with colorectal growth. Among the study patients, the most frequent age group was 31-45 years (38%). The mean age of the patients was (48.89±14.54) years. Table-2 shows the sex distribution of patients with colorectal growth. Among the study patients. The majority of the patients (55, 55%) were female and rest of the patients (45, 45%) were male. Table-3 shows the site distribution of colorectal growth with the study patients. According to site distribution of colorectal growth, the most frequent site of colorectal growth of carcinoma was observed in the ascending colon, sigmoid colon and colon not specified were 31%, 26% and 23% respectively. Figure 1 shows the distribution of colonoscopic biopsy results. Of 50 patients who underwent Colonoscopic Biopsy procedure, 26 patients had malignant tumours and 24 patients had non-malignant growth. Figure 1 shows the distribution of colonoscopic biopsy results. Of 50 patients who underwent Colonoscopic Biopsy procedure, 26 patients had malignant tumours and 24 patients had non-malignant growth. Figure 2 shows among the non-malignant cases, 13(54.16%) were Adenoma and 11(45.83%) were inflamatory bowel diseases. Table-4 shows that 50 cases underwent biopsy procedure, of them, inflammatory disease, adenoma and malignant cases were observed in 22%, 26% and 52% cases respectively. At the same time, 50(50%) cases underwent excision procedure whereas all the cases 50(100%) had malignant growth. Table-5 shows the distribution of histological types of colorectal carcinoma of the study patients. According to the distribution of histological types of colorectal carcinoma, the most frequent type of colorectal carcinoma was observed Adenocarcinoma, NOS 48(63.15%) followed by Mucinous adenocarcinoma 15(19.73%), Adenocarcinoma with Mucinous features 7(9.21%), Poorly differentiated carcinoma 3(3.94%), Signet ring cell carcinoma 2(2.63%) and Papillary Adenocarcinoma 1(1.31%). Table-6 shows the distribution of histological types of colorectal carcinoma by age groups of the study patients. According to the distribution of histological types of colorectal carcinoma by age groups, the most frequent Adenocarcinoma, NOS (20,26.31%) were observed in the age group 46-60 years and followed by (15, 19.93%) in 31-45 years, 10(13.16%) in 61-75 years. The most frequent Adenocarcinoma with mucinous features were observed (4, 5.26%) in the age group (46-60) years and followed by 2(2.63%) in 31-45 years and 1(1.32%) in ≤30 years. The mucinous type of adenocarcinoma was observed 8(10.53%) in the age group 31-45 years and followed by 3(3.95%) in 46-60 years, 2(2.63%) in 61-75 years and followed the same 2(2.63%) in ≤30 years. Papillary adenocarcinoma was observed only 1(1.32%) in the age group 61-75 years. Signet ring cell carcinoma was observed 1(1.32%) in the age group ≤30 years and followed the same 1(1.32%) in 46-60 years. Poorly differentiated carcinoma was observed 1(1.32%) in the age group 31-45 years and followed the same 1(1.32%) in 46-60 years and 61-75 years. Table-7 shows the distribution of histological types of colorectal carcinoma by sex of the study patients. According to the distribution of histological types of colorectal carcinoma by sex, the most frequent colorectal carcinoma in male 20(26.32%) was observed Adenocarcinoma, NOS and followed by Mucinous Adenocarcinoma 8(10.53%), Adenocarcinoma with Mucinous Features 1(1.32%) and papillary Adenocarcinoma followed the same (1.32%). The most frequent colorectal carcinoma in female was also observed Adenocarcinoma, NOS (28, 36.84%). Figure 4 shows the grade distribution of observed colorectal carcinoma with the study patients. According to grade distribution, Grade-II were observed the most frequent (54, 71.05%) and followed by Grade-III (15, 19.74%) and Grade-I (7,9.21%). Table-8 shows the grade distribution of colorectal carcinoma by age groups of the study patients. According to grade distribution by age groups, the maximum Grade-II (22, 28.95%) were observed in the age group (46-60) years. The maximum Grade-III (6, 7.89%) were identified in the age group (46-60) years (Table 9). Table-10 shows Grade-II were observed 44.73% in female and 26.31% in male. Table-10 shows the most extent of invasion of colorectal carcinoma was observed muscularis propria 25(50%). According to the distribution of stages of colorectal carcinoma of both treated and untreated cases, PT2 ((36%) and PN0 (36%) were observed the most frequent stages (Table 11). Table-12 shows the distribution of stages of colorectal carcinoma of non-treated cases. According to the primary tumour, most frequent stage was PT2 (18,50%) and based on regional lymph node involvement, the most frequent stages were observed PN0 (19,52.77%). According to the stages distribution of treated cases, among, T stages, the most frequent was found Y2 (6, 42.85%) (Table 13). According to the distribution of lymph node involvement, the most lymph node involvement was found N1b (9,18%) (Table-14). Table 15 shows the comparison of stages of colorectal carcinoma between treated and non-treated cases and the group mean of the stages of both treated and nontreated cases was not statistically significant (P>0.05).
Table 1: Age distribution of patients with colorectal growth (N=100).
|
Age groups(years) |
Frequency (N) |
Percentage (%) |
|
≤30 |
8 |
8 |
|
31-45 |
38 |
38 |
|
46-60 |
34 |
34 |
|
61-75 |
18 |
18 |
|
>75 |
2 |
2 |
|
Total |
100 |
100 |
|
Mean Age(years): |
48.89±14.54 |
|
|
Median |
48.5 |
|
|
Mode |
36 |
|
|
Range: |
16-94 |
Table 2: Sex distribution of patients with Colorectal Carcinoma (n=100).
|
Sex |
Frequency (n) |
Percentage (%) |
|
Male |
45 |
45 |
|
Female |
55 |
55 |
|
Total |
100 |
100 |
Table 3: Site distribution of colorectal growth with the study patients (n=100).
|
Site of Colorectal Carcinoma |
Frequency(n) |
Percentage (%) |
|
Colon not specified |
23 |
23 |
|
Ileocaecal |
1 |
1 |
|
Ascending colon |
31 |
31 |
|
Transverse colon |
2 |
2 |
|
Descending colon |
2 |
2 |
|
Sigmoid |
26 |
26 |
|
Recto sigmoid |
6 |
6 |
|
Rectum |
9 |
9 |
|
Total |
100 |
100 |
Table 4: Distribution of study cases according to procedure modality(n=100).
|
Cases |
Biopsy (n=50) |
Percentage (%) |
Excision (n=50) |
Percentage (%) |
|
Inflammatory Disease |
11 |
22 |
0 |
0 |
|
Adenoma |
13 |
26 |
0 |
0 |
|
Malignant |
26 |
52 |
50 |
100 |
Table 5: Distribution of histological types of colorectal carcinoma of the study patients (n=76).
|
Histological type of colorectal carcinoma |
Frequency (n) |
Percentage (%) |
|
Adenocarcinoma, NOS |
48 |
63.15 |
|
Adenocarcinoma with Mucinous features |
7 |
9.21 |
|
Mucinous adenocarcinoma |
15 |
19.73 |
|
Papillary Adenocarcinoma |
1 |
1.31 |
|
Signet ring cell carcinoma |
2 |
2.63 |
|
Poorly differentiated carcinoma |
3 |
3.94 |
|
Total |
76 |
100 |
Table 6: Distribution of histological types of colorectal carcinoma by age groups of the study patients (n=76).
|
Age group of the patients with the colorectal carcinoma |
Total |
P-value |
|||||
|
Histological types of colorectal carcinoma |
≤30 |
31-45 |
46-60 |
61-75 |
>75 |
||
|
N(%) |
N(%) |
N(%) |
N(%) |
N(%) |
N(%) |
||
|
Adenocarcinoma, NOS |
1(1.32) |
15(19.93) |
20(26.31) |
10(13.16) |
2(2.63) |
48(63.15) |
|
|
Adenocarcinoma with mucinous features |
1(1.32) |
2(2.63) |
4(5.26) |
0(0) |
0(0) |
7(9.21) |
0.543 |
|
Mucinous adenocarcinoma |
2(2.63) |
8(10.53) |
3(3.95) |
2(2.63) |
0(0) |
15(19.74) |
|
|
Papillary adenocarcinoma |
0(0) |
0(0) |
0(0) |
1(1.32) |
0(0) |
1(1.32) |
|
|
Signet ring cell carcinoma |
1(1.32) |
0(0) |
1(1.32) |
0(0) |
0(0) |
2(2.63) |
|
|
Poorly differentiated carcinoma |
0(0) |
1(1.32) |
1(1.32) |
1(1.32) |
0(0) |
3(3.95) |
|
|
Total |
5(6.58) |
26(34.21) |
29(38.16) |
14(18.42) |
2(2.63) |
76(100) |
|
Table 7: Distribution of histological types of colorectal carcinoma by sex of the study patients (n=76).
|
Sex of the patients |
Total |
P-value |
||
|
Histological types of carcinoma |
Male |
Female |
||
|
N(%) |
N(%) |
N(%) |
||
|
Adenocarcinoma, NOS |
20(26.32) |
28(36.84) |
48(63.15) |
|
|
Adenocarcinoma with Mucinous Features |
1(1.32) |
6(7.89) |
7(9.21) |
|
|
Mucinous Adenocarcinoma |
8(10.53) |
7(9.21) |
15(19.74) |
|
|
papillary Adenocarcinoma |
1(1.32) |
0(0) |
1(1.32) |
0.149 |
|
Signet ring cell carcinoma |
0(0) |
2(2.63) |
2(2.63) |
|
|
Poorly differentiated Carcinoma |
0(0) |
3(3.95) |
3(3.95) |
|
|
Total |
30(39.47) |
46(60.53) |
76(100) |
|
Table 8: Grade distribution of colorectal carcinoma by age groups of the study patients (n=76).
|
Age Groups |
Grade of colorectal carcinoma |
Chi-square |
|||||||||
|
Grade-I |
% |
Grade-II |
% |
Grade-III |
% |
Total |
% |
df |
P-value |
||
|
≤30 |
0 |
0 |
3 |
3.95 |
2 |
2.63 |
5 |
6.58 |
|||
|
31-45 |
5 |
6.58 |
16 |
21.05 |
5 |
6.58 |
26 |
34.21 |
7.709 |
8 |
0.462 |
|
46-60 |
1 |
1.32 |
22 |
28.95 |
6 |
7.89 |
29 |
38.16 |
|||
|
61-75 |
1 |
1.32 |
11 |
14.47 |
2 |
2.63 |
14 |
18.42 |
|||
|
>75 |
0 |
0 |
2 |
2.63 |
0 |
0 |
2 |
2.63 |
|||
|
Total |
7 |
9.21 |
54 |
71.05 |
15 |
19.74 |
76 |
100 |
|||
Table 9: Grade distribution of colorectal carcinoma by sex of the study patients (n=76).
|
Sex |
Grade-I |
% |
Grade-II |
% |
Grade-III |
% |
Total |
% |
Chi-square |
df |
P-value |
|
Male |
4 |
5.26 |
20 |
26.31 |
6 |
7.89 |
30 |
39.47 |
|||
|
Female |
3 |
3.95 |
34 |
44.73 |
9 |
11.84 |
46 |
60.52 |
0.005 |
2 |
0.998 |
|
Total |
7 |
9.21 |
54 |
71.05 |
15 |
19.74 |
76 |
100 |
Table 10: Distribution of extent of invasion of colorectal carcinoma of excised cases (n=50).
|
Extent of invasion of the colon carcinoma |
Frequency (n) |
Percentage (%) |
|
Muscularis propria |
25 |
50 |
|
Pericolorectal tissue |
2 |
4 |
|
Serosa/ mesenteric |
1 |
2 |
|
Perforated part |
1 |
2 |
|
Mesenteric fat |
2 |
4 |
|
Lamina propria |
2 |
4 |
|
Perirectal fatty tissue |
3 |
6 |
|
Mesentery |
1 |
2 |
|
Pericolic fatty tissue |
11 |
22 |
|
Submucosa |
1 |
2 |
|
Vesceral |
1 |
2 |
|
Total |
50 |
100 |
Table 11: Distribution of stages of colorectal carcinoma of both treated and non-treated cases (n=50).
|
Stages |
N(%) |
Stages |
N(%) |
|
T |
N |
||
|
PTx |
0(0%) |
PNx |
0(0%) |
|
PT0 |
0(0%) |
PN0 |
18(36%) |
|
PTis |
0(0%) |
PN1 |
2(4%) |
|
PT1 |
1(2%) |
PN1a |
1(2%) |
|
PT2 |
18(36%) |
P N1b |
5(10%) |
|
PT3 |
12(24%) |
PN1c |
1(2%) |
|
P T4 |
4(8%) |
PN2 |
2(4%) |
|
PT4a |
1(2%) |
PN2a |
3(6%) |
|
PT4b |
0(0%) |
P N2b |
4(8%) |
|
Total |
36(100%) |
36(100%) |
Table 12: Distribution of stages of colorectal carcinoma of non-treated cases (n=36).
|
Stages |
N(%) |
Stages |
N(%) |
|
T |
N |
||
|
PTx |
0 |
PNx |
1(2.77) |
|
PT0 |
0 |
PN0 |
19(52.77) |
|
PTis |
0 |
PN1 |
2(5.55) |
|
P T1 |
1(2.77) |
PN1a |
1(2.77) |
|
PT2 |
18(50) |
PN1b |
5(13.88) |
|
PT3 |
12(33.33) |
PN1c |
1(2.77) |
|
P T4 |
4(11.11) |
PN2 |
0(0) |
|
PT4a |
1(2.77) |
PN2a |
3(8.33) |
|
PT4b |
0(0) |
P N2b |
4(11.11) |
|
Total |
36(100) |
Total |
36(100) |
Table 13: Distribution of stages of colorectal carcinoma of treated cases (n=14).
|
Stages |
N(%) |
Stages |
N(%) |
|
T |
N |
||
|
Y0 |
2(14.28%) |
Y0 |
6(42.85) |
|
Y2 |
6(42.85%) |
Y1a |
1(7.14) |
|
Y3 |
4(28.57%) |
Y1b |
2(14.28 |
|
Y4a |
2(14.28%) |
Y2a |
2(14.28) |
|
Y2b |
3(21.42) |
||
|
Total |
14(100) |
Total |
14(100) |
Table 14: Distribution of lymph node involvement of excised cases (n=50).
|
Lymph node involvement |
Frequency |
Percent |
|
N1a |
2 |
4 |
|
N1b |
9 |
18 |
|
N2a |
7 |
14 |
|
N2b |
7 |
14 |
Table 15: Comparison of stages of colorectal carcinoma between treated and non-treated cases (n=50)
|
Treated Cases(n=14) |
Non-treated Cases(n=36) |
P-value |
||||||
|
Stages |
N(%) |
Stages |
N(%) |
Stages |
N(%) |
Stages |
N(%) |
|
|
T |
N |
T |
N |
|||||
|
Y0 |
2(14.28) |
Y0 |
6(42.86) |
PTx |
0(0) |
PNx |
1(2.77) |
|
|
Y2 |
6(42,85) |
Y1a |
1(7.14) |
PT0 |
0(0) |
PN0 |
19(52.77) |
|
|
Y3 |
4(28.57) |
Y1b |
2(14.28) |
PTis |
0(0) |
PN1 |
2(5.55) |
|
|
Y4a |
2(14.28) |
Y2a |
2(14.28) |
PT1 |
1(2.77) |
PN1a |
1(2.77) |
|
|
Y2b |
3(21.42) |
PT2 |
18(50) |
PN1b |
5(13.88) |
|||
|
PT3 |
12(33.33) |
PN1c |
1(2.77) |
0.627 |
||||
|
PT4 |
4(11.11) |
PN2 |
0(0) |
|||||
|
PT4a |
1(2.77) |
PN2a |
3(8.33) |
|||||
|
PT4b |
0(0) |
PN2b |
4(11.11) |
|||||
- • One one-way ANOVA test was performed to compare the mean between the groups with a p-value of 0.05 as the level of significance.
4. Discussion
CRCs are commonly to occur in the late age of life, but nowadays, younger people are increasingly affected by different types of CRCs [14].This study was conducted in a tertiary level hospital and a total of 100 cases colorectal carcinoma patients who underwent colonoscopic biopsy and excision procedures were retrospectively enrolled in this study.In this current study, the majority of the cases (38%) belonged to the age group >31-45 years with a mean age was 48.89±14.54 which is similar to another study conducted in the pathology department of Bangabandhu sheikh mujib medical university from November 2009 to July 2010 where mean age of the study population was 47±14.8 [15] and is lower than the Western World [16-23]. However, Saha et al., 2016 and Raza et al., 2016 recorded the mean age as 50.77 years and 47 ± 14.8 years, respectively in Bangladeshi colorectal cancer patients [15,22], which are also almost similar to this present study. The occurrence of CRCs especially in relatively young age group may be attributed to the recent change in food habits and life style. Median age of the study population of current study was 48.50. Another study was carried out at the surgical outdoor of ABUTH hospital in Zaria, Nigeria in the year 2020 found median age of study population was 41 [24]. In the current study, 60.53% patients were female among all malignant cases (n=76). These findings of our study indicates the higher incidence of CRCs in female than the male. This female dominance may be occurred due to sedentary life style of the female more than their counterpart. But some studies conducted in Europe and Asia observed male dominance in CRCs cases [15, 23, 24]. This present study investigated that the most frequent site of colorectal growth of carcinoma was observed Ascending colon (31, 31%). A retrospective study was conducted on 1443 colorectal cancer cases in the Pathology department of Farhet Hached University Hospital, Tunisia and explored most frequent tumour site was ascending colon (47%) [25].
In the current study, adenocarcinoma (63.15%) was noticed mostly frequent histopathological type of malignant tumour. Morever, another study was carried out at the surgical outdoor of ABUTH hospital in Zaria, Nigeria in the year 2020 had similar outcome of our study where adenocarcinoma was observed 73% amid total malignant patients [24]. In the present study, according to the distribution of stages of colorectal carcinoma of both treated and untreated cases, PT2 (36%) and PN0 (36%) were observed the most frequent stages. The retrospective study that was carried out at the surgical outdoor of ABUTH hospital in Zaria, Nigeria in the year 2020 had similar outcome of our study where adenocarcinoma was observed 86% patients was presented with advance stage disease [24]. Late stage disease is always associated wih worst prognosis. Many study revealed that ignorance and high cost of disease are the cardinal reason of bad prognosis of colorectal cancer [26, 27]. Advance stage disease also reflects the absence and the necessity of screening programme [28]. Advanced age and late disease hault the cancer prognosis. Thus screening is the main intervention of CRC control [29]. Many studies proved that patients who are diagnosed at their early stage has better prognosis [30]. To detect the early stage disease, screening test has great role. However, significant progress of CRC treatment is seen now-a-days [31]. Another study explored that if CRC patients were diagnosed at early stage disease, there would be 90% probability of 4 years of survival [31]. Promoting guidelines and adaptation of screening programme enhanced the survival rate of CRC patients with early stage disease [31]. Targeted health programmes and engagement of medical professional in screening programmes would assist to spread the role of screening in CRC prognosis. It is validated that awareness would raise the participation in screening test.
Conclusion
This study investigated that the most of the colorectal carcinoma was observed in the middle-aged people. Majority of the cases were diagnosed at an advanced grade and stage. the widespread utilization of screening programme could save greater percentage of live annually. Thus, maximum efforts should be given to expand the screening programme
Limitations of the study
This was study was conducted in a single center with a limited consecutive sampling technique. Therefore, the results of this study may not reflect the whole scenario of the entire nation.
Recommendations
Necessary policies should be adopted to promote awareness about the growth of colorectal cancer especially among the adults’ population to promote colorectal health care at an early stage. screening program should be implemented at the national level and people should be encouraged to attain CRC screening programme based on the guidelines. To get robust data, multicenter studies are in great need of policymakers to interpret the demonstrable scenario and to take necessary steps towards mitigating this problem. Further research is also needed to detect the burden in an attempt to reduce disease burden and facilitate the prognosis of such condition.
Conflict of Interest
None declaredFunding
Self-funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68 (2018): 394-424.
- Globocan 2020: New Global Cancer Data (2020) UICC (2020).
- Torre L A, Bray F, Siegel R L, et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians 65 (2015):87-108.
- Jemal A, Bray F, Center M M, et al. Global cancer statistics. CA: a cancer journal for clinicians 61 (2011): 69-90.
- Eddy D M. Screening for colorectal cancer. Annals of Internal Medicine 113 (1990): 373-384.
- Wong M C, Ding H, Wang J, et al. Prevalence and risk factors of colorectal cancer in Asia. Intestinal research 17 (2019): 317-329.
- The International Agency for Research on Cancer (IARC) (no date) Global cancer observatory, Global Cancer Observatory (2024).
- Chan A T, and Giovannucci E L. Primary prevention of colorectal cancer. Gastroenterology 138 (2010): 2029-2043.
- Wilschut J A, Steyerberg E W, van Leerdam M E, et al. How much colonoscopy screening should be recommended to individuals with various degrees of family history of colorectal cancer?. Cancer 117 (2011): 4166-4174.
- Jasperson K W, Tuohy T M, Neklason D W, et al. Hereditary and familial colon cancer. Gastroenterology 138 (2010): 2044-2058.
- Gala M, and Chung D C. August. Hereditary colon cancer syndromes. In Seminars in oncology 38 (2011): 490-499.
- Zhang K, Civan J, Mukherjee S, et al. Genetic variations in colorectal cancer risk and clinical outcome. World journal of gastroenterology: WJG 20 (2014): 4167.
- Burt MD, R W, DiSario MD, J A, and Cannon-Albright, Ph. D, L. Genetics of colon cancer: impact of inheritance on colon cancer risk. Annual review of medicine 46 (1995): 371-379.
- Ahadi M, Sokolova A, Brown I, et al. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: an update and critical assessment. Pathology 53 (2021): 454-461.
- Raza A M, Kamal M, Begum F, et al. Clinico-demographic characteristics of colorectal carcinoma in Bangladeshi patients. Journal of Current and Advance Medical Research 3 (2016): 22-25.
- Nelson R L, Dollear T, Freels S, et al. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society 80 (1997): 193-197.
- Nelson R L, Dollear T, Freels S, et al. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer: Interdisciplinary International Journal of the American Cancer Society 80 (1997): 193-197.
- Turner J. (no date) ‘The Gastrointestinal Tract’, in Robbins and Cotran Pathologic Basis of Disease, Professional Edition E-Book. 9th edn. Elsevier Health Sciences (2014).
- Saha M, Shil B C, Saha S K, et al. Study of Clinicopathological Profile of Sporadic Cases of Colorectal Cancer. Euroasian Journal of Hepato-Gastroenterology 6 (2016): 134.
- Hayne D, Brown R S, McCormack M, et al. Current trends in colorectal cancer: site, incidence, mortality and survival in England and Wales. Clinical oncology 13 (2001): 448-452.
- Goh K L, Quek K F, Yeo G T S, et al. Colorectal cancer in Asians: a demographic and anatomic survey in Malaysian patients undergoing colonoscopy. Alimentary pharmacology & therapeutics 22 (2005): 859-864.
- Malik K A. Colorectal carcinoma: a six years experience at a tertiary care hospital of Sindh. JLUMHS 76 (2007).
- Hashmi AA, Ali R, Hussain ZF, et al. Mismatch repair deficiency screening in colorectal carcinoma by a four-antibody immunohistochemical panel in Pakistani population and its correlation with histopathological parameters. World Journal of Surgical Oncology 15 (2017): 116.
- Theyra-Enias H, Adewuyi S A, Alabi A, et al. Socio-demographic and clinico-pathologic pattern of patients with colorectal cancers seen in Ahmadu Bello University Teaching Hospital, Zaria. West African Journal of Radiology 27 (2020): 136-142.
- Missaoui N, Jaidaine L, Ben Abdelkader A, et al. Clinicopathological patterns of colorectal cancer in Tunisia. Asian Pacific J Cancer Prev 11 (2010):1719-1722.
- Ohayi SA, Nzegwu MA, Silas AA, et al. Histopathological pattern of colorectal tumours in Jos university Teaching Hospital (JUTH), Jos. A 5 year retrospective review from (January 1999- December 2003) Adv Biores 2 (2011): 127-131
- Rutter CM, Johnson EA, Feuer EJ, et al. Secular trends in colon and rectal cancer relative survival. J Natl Cancer Inst 105 (2013):1806-1813.
- DeSantis CE, Lin CC, Mariotto AB, et al. Cancer treatment and survivorship statistics. CA Cancer J Clin 64 (2014): 252-271.
- Issa IA, Noureddine M. Colorectal cancer screening: An updated review of the available options. World J Gastroenterol 23 (2017): 5086-5096.
- National Cancer Institute. Cancer Stat Facts: Colorectal Cancer (2018).
- Doubeni CA. The impact of colorectal cancer screening on the US population: is it time to celebrate? Cancer 120 (2014): 2810-2813.