Clinical Significance of Psoriasiform Sarcoidosis
Article Information
Halil Yanardag1, Cuneyt Tetikkurt2*, Muammer Bilir1, Seza Tetikkurt3, Ozge Askin4
1Department of Internal Medicine Medicine, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
2Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
3Department of Pathology, Demiroglu Bilim University Medical Faculty, Turkey
4Department of Dermatology, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
*Corresponding Author: Dr. Cuneyt Tetikkurt, Department of Pulmonary Medicine, Cerrahpasa Medical Faculty, Istanbul Cerrahpasa University, Turkey
Received: 02 April 2020; Accepted: 20 April 2020; Published: 18 May 2020
Citation: Halil Yanardag, Cuneyt Tetikkurt, Muammer Bilir, Seza Tetikkurt, Ozge Askin. Clinical Significance of Psoriasiform Sarcoidosis. Archives of Clinical and Medical Case Reports 4 (2020): 471-482.
View / Download Pdf Share at FacebookAbstract
Sarcoidosis is a chronic granulomatous inflammatory disease characterized by the presence of noncaseating granulomas in various organs. Psoriasis is a persistent and recurrent autoimmune disorder mainly affecting the skin. Cutaneous involvement is observed in one fourth of the sarcoidosis patients and psoriatic plaques may be a manifestation of cutaneous sarcoidosis. The pathogenic mechanism of psoriasis is relevant to the overstimulation of CD4 TH1 and TH17 lymphocytes that are also involved in the granuloma formation of sarcoidosis. We present seven cases of sarcoidosis patients presenting with psoriasiform sarcoidosis. All the patients had cutaneous plaques indistinguishable from psoriasis. Diagnosis of sarcoidosis was confirmed by biopsy in at least two organs. Psoriasiform sarcoidosis was identified by skin biopsy of the involved cutaneous lesions. All patients had stage III sarcoidosis and presented with one or more clinical or laboratory manifestations of sarcoidosis. BAL was negative for infectious agents. Histopathologic examination of the bronchial and transbronchial biopsy samples revealed noncaseiting granulomatous inflammation. Differentiation of sarcoidosis and psoriasis may constitute a diagnostic dilemma for the clinician in terms of the identical skin lesions. The presence of psoriatic plaques may suggest an overlap between the underlying pathogenetic mechanisms of both disorders. Despite the great clinical similarity, the only way to differentiate the two syndromes is pathological examination. The common pathogenetic mechanism involving the TH1 and TH17 pathways for both sarcoidosis and psoriasis may be the fundamental linkage for this occurrence. We present seven cases of sarcoidosis patients presenting with psoriasiform lesions to define the clinical profile of sarcoidosis in these patients including presentation, laboratory findings and prognosis. The coexistence of sarcoidosis and psoriasis, on the other hand may s
Keywords
Sarcoidosis; Psoriasis; Cutaneous sarcoidosis; Psoriasiform sarcoidosis
Sarcoidosis articles, Psoriasis articles, Cutaneous sarcoidosis articles, Psoriasiform sarcoidosis articles
Sarcoidosis articles Sarcoidosis Research articles Sarcoidosis review articles Sarcoidosis PubMed articles Sarcoidosis PubMed Central articles Sarcoidosis 2023 articles Sarcoidosis 2024 articles Sarcoidosis Scopus articles Sarcoidosis impact factor journals Sarcoidosis Scopus journals Sarcoidosis PubMed journals Sarcoidosis medical journals Sarcoidosis free journals Sarcoidosis best journals Sarcoidosis top journals Sarcoidosis free medical journals Sarcoidosis famous journals Sarcoidosis Google Scholar indexed journals Psoriasis articles Psoriasis Research articles Psoriasis review articles Psoriasis PubMed articles Psoriasis PubMed Central articles Psoriasis 2023 articles Psoriasis 2024 articles Psoriasis Scopus articles Psoriasis impact factor journals Psoriasis Scopus journals Psoriasis PubMed journals Psoriasis medical journals Psoriasis free journals Psoriasis best journals Psoriasis top journals Psoriasis free medical journals Psoriasis famous journals Psoriasis Google Scholar indexed journals surgery articles surgery Research articles surgery review articles surgery PubMed articles surgery PubMed Central articles surgery 2023 articles surgery 2024 articles surgery Scopus articles surgery impact factor journals surgery Scopus journals surgery PubMed journals surgery medical journals surgery free journals surgery best journals surgery top journals surgery free medical journals surgery famous journals surgery Google Scholar indexed journals Cutaneous sarcoidosis articles Cutaneous sarcoidosis Research articles Cutaneous sarcoidosis review articles Cutaneous sarcoidosis PubMed articles Cutaneous sarcoidosis PubMed Central articles Cutaneous sarcoidosis 2023 articles Cutaneous sarcoidosis 2024 articles Cutaneous sarcoidosis Scopus articles Cutaneous sarcoidosis impact factor journals Cutaneous sarcoidosis Scopus journals Cutaneous sarcoidosis PubMed journals Cutaneous sarcoidosis medical journals Cutaneous sarcoidosis free journals Cutaneous sarcoidosis best journals Cutaneous sarcoidosis top journals Cutaneous sarcoidosis free medical journals Cutaneous sarcoidosis famous journals Cutaneous sarcoidosis Google Scholar indexed journals Psoriasiform sarcoidosis articles Psoriasiform sarcoidosis Research articles Psoriasiform sarcoidosis review articles Psoriasiform sarcoidosis PubMed articles Psoriasiform sarcoidosis PubMed Central articles Psoriasiform sarcoidosis 2023 articles Psoriasiform sarcoidosis 2024 articles Psoriasiform sarcoidosis Scopus articles Psoriasiform sarcoidosis impact factor journals Psoriasiform sarcoidosis Scopus journals Psoriasiform sarcoidosis PubMed journals Psoriasiform sarcoidosis medical journals Psoriasiform sarcoidosis free journals Psoriasiform sarcoidosis best journals Psoriasiform sarcoidosis top journals Psoriasiform sarcoidosis free medical journals Psoriasiform sarcoidosis famous journals Psoriasiform sarcoidosis Google Scholar indexed journals treatment articles treatment Research articles treatment review articles treatment PubMed articles treatment PubMed Central articles treatment 2023 articles treatment 2024 articles treatment Scopus articles treatment impact factor journals treatment Scopus journals treatment PubMed journals treatment medical journals treatment free journals treatment best journals treatment top journals treatment free medical journals treatment famous journals treatment Google Scholar indexed journals pericoronitis articles pericoronitis Research articles pericoronitis review articles pericoronitis PubMed articles pericoronitis PubMed Central articles pericoronitis 2023 articles pericoronitis 2024 articles pericoronitis Scopus articles pericoronitis impact factor journals pericoronitis Scopus journals pericoronitis PubMed journals pericoronitis medical journals pericoronitis free journals pericoronitis best journals pericoronitis top journals pericoronitis free medical journals pericoronitis famous journals pericoronitis Google Scholar indexed journals CT scan articles CT scan Research articles CT scan review articles CT scan PubMed articles CT scan PubMed Central articles CT scan 2023 articles CT scan 2024 articles CT scan Scopus articles CT scan impact factor journals CT scan Scopus journals CT scan PubMed journals CT scan medical journals CT scan free journals CT scan best journals CT scan top journals CT scan free medical journals CT scan famous journals CT scan Google Scholar indexed journals patients articles patients Research articles patients review articles patients PubMed articles patients PubMed Central articles patients 2023 articles patients 2024 articles patients Scopus articles patients impact factor journals patients Scopus journals patients PubMed journals patients medical journals patients free journals patients best journals patients top journals patients free medical journals patients famous journals patients Google Scholar indexed journals MRI articles MRI Research articles MRI review articles MRI PubMed articles MRI PubMed Central articles MRI 2023 articles MRI 2024 articles MRI Scopus articles MRI impact factor journals MRI Scopus journals MRI PubMed journals MRI medical journals MRI free journals MRI best journals MRI top journals MRI free medical journals MRI famous journals MRI Google Scholar indexed journals
Article Details
1. Introduction
Sarcoidosis is a chronic multisystemic disorder of unknown cause characterized by the presence of granulomatous inflammation and non-caseified epitheloid granulomas in various organs [1-3]. Cutaneous involvement occurs in 20 to 35 percent of the patients and skin is the second most common organ affected after the lungs [4-8]. Almost any type of skin lesion may be a manifestation of cutaneous sarcoidosis. Psoriasiform cutaneous sarcoidosis is rare and occurs only in 0.9% of the sarcoidosis patients [5, 9-11]. The role of the TH1 cell in sarcoidal granuloma formation has been well documented while the TH17 pathway in sarcoidosis is currently under investigation. TH17 cells are known to be involved in the pathogenesis of psoriasis. The coexistence of sarcoidosis and psoriasis is related to the shared underlying immunologic and pathogenic mechanisms. Recent data indicates that both sarcoidosis and psoriasis share the common TH1/TH17 pathway while psoriasis may occur in patients with sarcoidosis as a comorbid condition as both disorders are TH1/TH17 mediated diseases [12-14]. TH17 cells are recently described cells that may play a role in the pathogenesis of autoimmune diseases, including predominantly psoriasis and sarcoidosis [13, 15, 16].
We present a series of seven patients with psoriasiform sarcoidosis. Psoriasiform sarcoidosis clinically resembles psoriasis that is indistinguishable with erythematous and overlying silvery plaques [11, 15-17]. The potential overlap in the immunologic and pathogenetic pathway of sarcoidosis with psoriasis suggests a common mechanism underlying the occurrence of these diseases. On the other hand, this common pathway may play a crucial and a dominant role in the development of psoriasiform sarcoidosis that is suggested by the presence of identical cutaneous lesions in both disorders. The primary objective of this case series is to define the clinical manifestations of sarcoidosis with psoriasiform cutaneous sarcoidosis. The second aim is to define the pathogenesis of sarcoidosis due to the existence of clinically identical skin lesions in psoriasiform sarcoidosis and psoriasis. The close similarity between these two diseases may shed light on the pathogenesis of sarcoidosis through the mutual pathologic mechanism of psoriasis. Presence of psoriatic cutaneous lesions may also be a crucial triangulation point in determining the prognosis and the treatment options for sarcoidosis patients.
2. Case Reports
2.1 Case 1
A 39 year old female presented with scaly plaques on the soles. Past history revealed sarcoidosis of ten years diagnosed by the histopathologic examination of the mediastinal lymph nodes. Family history was excellent. Physical examination demonstrated silvery, scaly and lichenified plaques on the soles of both feet (Figure 1). Basic laboratory tests were normal except for an elevated serum ACE (angiotensin-converting enzyme) at 94 U/L and a high urinary calcium (450 mg/day) level. Pulmonary function tests revealed a mild restrictive abnormality with a mild decrease in DLCO/VA. Chest x-ray showed bilateral infiltrations. Current lung CT revealed bilateral parenchymal infiltrations in the upper lobes of both lungs. Biopsy of the cutaneous lesions showed psoriasiform hyperplasia with underlying compact granulomas in the dermis that were composed of epithelioid histiocytes, multinucleated giant cells and lymphocytes compatible with psoriasiform sarcoidosis. Transbronchial biopsy demonstrated non-caseified granulomas compatible with sarcoidosis. BAL analysis revealed lymphocytosis with an increased CD4/CD8 ratio of 4.0 while culture was negative for bacteria, fungus and mycobacteria. Ocular examination demonstrated anterior uveitis due to sarcoidosis.
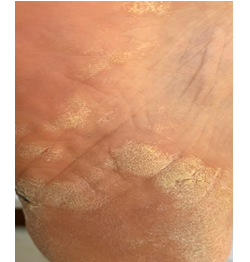
Figure 1: Scaly plaques on the sole of the left foot.
2.2 Case 2
A 30 year old female was admitted for bilateral hilar lymphadenopathy on chest x-ray with scaly and a red scaly rash below the knee. She had no relevant personal or family history. Physical examination was normal other than the cutaneous scaly lesions (Figure 2) below the right knee. Laboratory evaluation showed a high ACE level of 118 U/L and a high urinary calcium (560 mg/day) level. Pulmonary function tests revealed a mild restrictive defect with a moderate decrease in DLCO/VA. Chest x-ray demonstrated bilateral parenchymal infiltrations. Thorax CT revealed stage III sarcoidosis showing parenchymal infiltrations in the upper and middle zones of both lungs. Pathology of the mediastinal lymph nodes deduced non-caseified granulomatous inflammation. BAL culture was negative for bacteria, fungus and mycobacteria. Biopsy of the skin lesions demonstrated hyperkeratosis, psoriasiform hyperplasia and non-caseified granulomas in the dermis that were compatible with psoriasiform sarcoidosis. Ocular examination demonstrated anterior uveitis due to sarcoidosis.
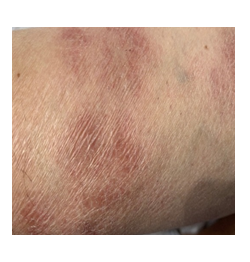
Figure 2: Scaly and erythematous psoriasiform cutaneous lesions below the right knee.
2.3 Case 3
A 52 year female presented with erythematous silvery scales on the arms. She had a past medical history of pneumonia. On physical examination erythematous plaques with silvery scales (Figure 3) were noted on both forearms. Laboratory evaluation was notable for an elevated serum ACE level at 112 U/L with a normal complete blood count and a normal serum calcium level. Urine calcium was high (620 mg/day) above normal. Bilateral parenchymal infiltrations were noted in the chest x-ray and thorax CT in the upper and middle lung zones. Pulmonary function tests revealed a mild restrictive abnormality. Pathologic examination of the transbronchial lung revealed non-caseified granulomatous infiltration while skin biopsy demonstrated non-caseified granulomas with hyperkeratosis, psoriasiform hyperplasia and non-caseified granulomas with lymphocytes throughout the dermis compatible with sarcoidosis. BAL culture was negative for bacteria, fungus and mycobacteria. Ocular examination showed anterior uveitis.
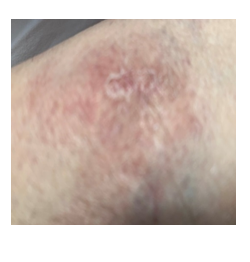
Figure 3: Scaly and erythematous psoriasiform cutaneous lesions in the left arm.
2.4 Case 4
A 31 year old male presented with scaly erythematous plaques. He had allergic rhinitis for five years. On physical examination sharply demarcated erythematous, silvery and scaly white scales were observed on both elbows. Serum laboratory results were normal other than an elevated serum ACE (98 IU/L) and a high serum calcium (10.2 mg/dL) level. Chest x-ray showed infiltrative lesion in the middle zones. Parenchymal infiltrations were observed on chest CT in both lungs. Pulmonary function tests revealed a mild restrictive defect and a mild decrease in DLCO/VA. Histopathology of the transbronchial lung biopsy revealed non-caseified granulomatous inflammation compatible with sarcoidosis. BAL culture was negative for bacteria, fungus and mycobacteria. Pathologic examination of the skin lesions showed hyperkeratosis and psoriasiform acanthosis with non-caseified granulomas in the dermis. Ophtalmologic examination demonstrated anterior uveitis with conjunctival granulomas.
2.5 Case 5
A 59 year old female was admitted for scaly erythematous plaques and blurred vision. She had a sarcoidosis history of ten years. Physical examination was unremarkable other than the scaly, white, silvery and erythematous plaques just below the right knee. Laboratory results were within normal limits except for a high serum calcium (10.5 mg/dL) level. Pulmonary function tests revealed a moderate restrictive abnormality with a mild decrease in DLCO/VA. Chest x-ray showed infiltrations and bronchiectasis in the upper and mid zones. Thorax CT revealed parenchymal infiltrations, bronchiectatic and fibrotic changes in both upper and middle lung (Figure 4). BAL culture was negative for infectious agents. Slit lamp examination revealed anterior uveitis while pathologic examination of the punch biopsy of the skin lesions showed non-caseified granulomatous inflammation with aggregates of epithelioid macrophages, CD4+ T cells, hyperkeratosis and irregular psoriasiform hyperplasia in the dermis.
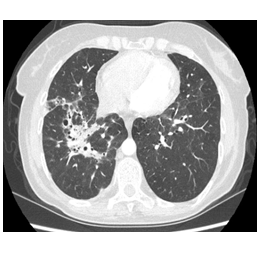
Figure 4: Thorax CT stage revealing stage III sarcoidosis with bronchiectatic and fibrotic lesions.
2.6 Case 6
A 65 year old female presented with macular rash, scaly plaques, redness in the eye and an abnormal sensitivity to light. Personal history revealed a previous sarcoidosis diagnosis of 20 years. Physical examination was normal other than the macular rash on the anterior chest, scaly plaques on the right sole and redness in both eyes. Laboratory results were within normal limits. Urine calcium level was high (260 mg/day) above normal. Chest x-ray showed cystic and bronchiectatic lesions in the middle lung. Thorax CT revealed cystic and bronchiectatic lesions in the lung parenchyma revealing stage III sarcoidosis (Figure 5). BAL culture was negative for bacteria, fungus and mycobacteria. Pulmonary function tests revealed a mild restrictive defect. Evaluation of the skin lesions was compatible with cutaneous psoriasiform involvement. Cutaneous biopsy revealed non-caseified granulomatous inflammation and psoriasiform hyperplasia with granulomas in the dermis that were composed of epithelioid histiocytes, multinucleated giant cells and CD4+ T lymphocytes. Opthalmologic examination demonstrated bilateral anterior uveitis.
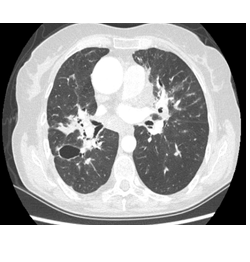
Figure 5: Thorax CT showing stage III sarcoidosis with bronchiectatic and cystic lesions.
2.7 Case 7
A 56 year old male presented with erythematous plaques with silvery scales. Personal history revealed a sarcoidosis history of 15 years diagnosed by the histopathologic examination of the transbronchial biopsy samples. Physical examination was normal other than scaly plaques on the right elbow. Laboratory findings were within normal limits except for a high serum calcium (10.8 mg/dL) level and a high serum ACE (92 IU/L) level. Pulmonary function tests revealed a mild restrictive abnormality with a moderate decrease in DLCO/VA. Chest x-ray showed bilateral infiltrative lesions. Thorax CT revealed bilateral alveolar sarcoidosis pattern. BAL demonstrated lymphocytic alveolitis with a 4.2 CD4 /CD8 ratio while culture was negative for infectious organisms including bacteria, fungus or mycobacteria. Cutaneous biopsy showed non-caseified granulomas in the dermis with psoriasiform changes in the epidermis. Opthalmologic consultation revealed normal ocular findings.
3. Results
All patients had a final diagnosis of stage III sarcoidosis with a psoriasiform cutaneous involvement and were commenced on methylprednisolone 0.5mg/kg/day with a total daily dose ranging from 24 to 32 mg per day. Azathioprine was added to treatment for persistent uveitis and hypercalcemia in one patient. The patients are under follow-up and showed a stabile course following treatment with regression of psoriasiform skin lesions, uveitis and hypercalcemia. The common conspicuous clinical points in our patients were the coexistence of stage III sarcoidosis, a mild restrictive pulmonary function test abnormality, mild or moderate DLCO/VA decrease, psoriasiform skin lesions, uveitis and hypercalcemia and/or hypercalcuria. The hallmark of the case series was the presence of psoriasiform cutaneous involvement associated with persistent sarcoidosis. Consequently, the clinical profile of our patients was consistent with chronic sarcoidosis that required treatment. The presence of psoriasiform cutaneous lesions in sarcoidosis patients may reveal that the disease may have a chronic and a persistant character indicating the necessity of treatment.
4. Discussion
Sarcoidosis is a granulomatous disease characterized by the presence of non-caseified granulomas in various organs such as the lung, skin, lymph nodes, eyes, joints, brain, kidneys, and heart. The immunological hallmark is the presence of CD4 T cells at the sites of inflammation leading to a TH1 cytokine profile that results in granulomatous inflammation at the involved organs [18]. Skin manifestations of sarcoidosis occur in approximately 25 percent of the patients [19-24] that may appear as specific and non-specific lesions based upon the histopathologic features [25]. Cutaneous lesions present with a variety of morphologies, including papules, nodules, plaques and infiltrated scars. Skin manifestations other than erythema nodosum and lupus pernio are designated as non-specific lesions. Specific skin lesions contain non-caseified granulomas that is the classical hallmark of the histopathologic findings. Specific and non-specific cutaneous lesions may coexist simultaneously in sarcoidosis patients [26] while any type of skin lesion may develop during the course of the disease. On the other hand, psoriasiform cutaneous lesions of sarcoidosis are rare and may occur only in 0.9% of the patients [5, 9-11]. We analyzed seven sarcoidosis patients with a psoriasiform cutaneous involvement to define the clinical significance of this manifestation in regard to the clinical profile of sarcoidosis.
The patients had several common points that may be attributed to psoriasiform cutaneous sarcoidosis. All the patients had a progressively worsening stage III sarcoidosis. Serum ACE, serum and urinary calcium were above normal almost in all of the subjects. In addition, the patients had at least three extrapulmonary organ involvements while anterior uveitis was the cardinal manifestation of sarcoidosis in our patients. The hallmark of this case series was that the psoriasiform cutaneous involvement is a reliable clinical sign for a severe and a persistent sarcoidosis outcome that necessisates treatment. None of the previous or current studies have comprehensively surveyed or evaluated all the potential risk factors for progressive sarcoidosis. Presence of psoriasiform cutaneous involvement should therefore be included among the presumptive hazards for persistent and chronic sarcoidosis outcome that necessitates immunosuppressive treatment. As a second endpoint, this manifestation can be regarded as a crucial sign for a worse prognosis in sarcoidosis patients.
Black race has been shown as a risk factor for chronic and persistent disease while the pattern of sarcoidosis may indicate the likelihood of resolution [27-30]. Three or more organ involvement increases the risk of persistent or progressive disease [29-31]. Specific organ disease such as cardiac, osseous, renal, spleen, upper airways, lupus pernio or nephrocalcinosis may be associated with a chronic disease or a worse prognosis [32]. Some other features that may correlate with persistent sarcoidosis include radiologic pattern, genetic polymorphisms, and nature of organ involvement [33]. Psoriasiform cutaneous involvement in sarcoidosis is a rare manifestation that may develop in only 0.9% of the patients [5, 9-11]. Although an unusual feature of sarcoidosis, psoriasiform cutaneous involvement may be a crucial sign for persistent and chronic sarcoidosis disease probability. The presence of high serum or urinary calcium, stage III sarcoidosis and anterior uveitis were also identified in our patients. The results of our case series indicate that sarcoidosis patients presenting with psoriasiform cutaneous lesions clearly carry a significant risk for a severe and a persistent disease. This is the first study to suggest that the presence of psoriasiform skin lesions emerges as an important indicator of the need for treatment in sarcoidosis and as a marker for a chronic disease course.
There are several limitations of this study. Our case series is limited by the small sample size. This drawback is associated with the rare existence of psoriasiform involvement among sarcoidosis patients. The second shortcoming is that our population consisted of only the Caucasian people. Consequently, studies with larger sample sizes including patients with different genetic and race profiles are needed to reveal the real incidence of psoriasiform cutaneous lesions for determining the prognostic outcome of this finding. Follow-up time may be considered to be insufficient and brief but it is well known that two years is usually accepted as sufficient for a stable sarcoidosis course. The follow-up period was minimum four years while two patients were followed for six and seven years, respectively.
The prognostic significance of psoriasiform cutaneous lesions may be associated with the TH1 and the TH17 pathway. TH17 cells are involved in the pathogenesis of psoriasis. Psoriasis may occur in patients with sarcoidosis as a comorbid condition as both disorders are TH1/TH17 mediated diseases. The coexistence of sarcoidosis and psoriasiform cutaneous involvement is related to the shared underlying immunologic and pathogenic mechanisms. The similar pathogenesis of TH1 and TH17 in both sarcoidosis and psoriasis suggests a common pathway for the association of these two disorders [10-12]. The same underlying pathogenetic mechanism may be involved in sarcoidosis patients with a psoriasiform disease. The TH17 pathway is associated with a worse and a severe prognosis in psoriasis patients [14]. There are now increasing number of studies relevant to the TH17 cells or IL-17 release in sarcoidosis [34-36]. The TH17 pathway may be the dominant pathogenetic mechanism in psoriasiform sarcoidosis for the development of a worse or a severe outcome as it is the case in psoriasis patients.
Sarcoidosis is rarely associated with other diseases that are mostly characterized by a TH1/TH2 imbalance [37]. Moreover, the skin lesions of sarcoidosis are not always granulomatous and may arise from a systemic immunological reaction. Both sarcoidosis and psoriasis share a common pathway that result from a variable response to a common antigenic response. Enhancement of the TH1 response appears to be a feature of both disorders. Treatment response to anti-TNF agents further justifies this hypothesis [38, 39]. Furthermore, it has also been reported that the pso p27, a psoriatic scale antigen linked to the pathogenesis of psoriasis, is markedly increased in the lungs of sarcoidosis patients [40]. The presence of psoriasiform cutaneous lesions among our patients may elucidate the existence of such a systemic immunological response. The literature data on psoriasis closely supports the worse clinical profile and the severe prognostic outcome among our sarcoidosis patients [40-42]. As clearly seen in our patients, psoriasiform skin involvement appears to be a crucial landmark for defining the clinical and the prognostic severity of sarcoidosis. The presence of psoriasiform cutaneous disease justifies a severe disease and a worse prognostic outcome in sarcoidosis as the results of our study suggests.
5. Conclusions
Prediction of the prognosis and the clinical outcome is one of the most crucial aspects in sarcoidosis patients. Many clinical or laboratory criteria that may indicate a severe and persistent disease like lupus pernio, nephrocalcinosis, three or more extrapulmonary organ involvement, chest radiology, pulmonary function tests, DLCO/VA or thorax CT have been proposed up to now while none of these criteria have provided sufficient conclusions for determining the prognosis of sarcoidosis patients. Considering the psoriasiform cutaneous lesions of sarcoidosis as a prognostic factor, a significant correlation was observed between the clinical manifestations, laboratory and radiological findings along with the disease profile in all our patients. Although a rare finding, the presence of psoriasiform cutaneous lesions is a noteworthy clinical sign to designate the clinical and the prognostic outcome of sarcoidosis patients. Psoriasiform cutaneous sarcoidosis appears to be a significant marker for chronic and persistent disease that challenges treatment.
Authors Contribution
Halil Yanardag is responsible for patient data, follow-up and wrote the clinical course of the patients.
Cuneyt Tetikkurt is the pulmonary consultant for the cases and wrote the pulmonary findings of the cases.
Muammer Bilir prepared and wrote the references.
Seza Tetikkurt wrote the pathologic aspects of cutaneous and psoriasiform involvement in sarcoidosis.
Ozge Askin is the dermatology consultant for cutaneous involvement of sarcoidosis and wrote the cutaneous aspects of the cases.
References
- Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med 160 (1999): 736-755.
- Costabel U, Hunninghake GW. ATS/ERS/WASOG statement on sarcoidosis. Sarcoidosis Statement Committee. American Thoracic Society. European Respiratory Society. World Association for Sarcoidosis and Other Granulomatous Disorders. Eur Respir J 14 (1999): 735-737.
- Hunninghake GW, Costabel U, Ando M, et al. ATS/ERS/WASOG statement on sarcoidosis. American Thoracic Society/European Respiratory Society/World Association of Sarcoidosis and other Granulomatous Disorders. Sarcoidosis Vasc Diffuse Lung Dis 16 (1999): 149-173.
- Kenneth E, Kataria YP. Cutaneosus manifestations of sarcoidosis. Arch Intern Med 145 (1985): 1811-1814.
- Lodha S, Sanchez M, Prystowsky S. Sarcoidosis of the skin. Chest 136 (2009): 583-586.
- Sharma OP. Sarcoidosis: a historical perspective. Clin Dermatol 25 (2007): 232-241.
- Olive K, Kataria YP. Cutaneous manifestations of sarcoidosis: relationship to other organ system involvement, abnormal measurements, and disease course. Arch Intern Med 145 (1985): 1811-1815.
- Veien NK, Stahl D, Brodthagen H. Cutaneous. sarcoidosis in caucausians. J Acad Dermatol 16 (1987): 534-540.
- Elgart ML. Cutaneous sarcoidosis: definition and types of lesions. Clin Dermatol 4 (1986): 35-35.
- Vega ML, Abrahams J, Keller M. Psoriasiform Sarcoidosis: Collision of Two Entities or Expression of One Common Pathogenesis? J Clin Aesthet Dermatol 9 (2016): 55-57.
- Burgoyne JS, Wood MG. Psoriasiform sarcoidosis. Arch Dermatol 106 (1972): 896-898.
- Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin Chest Med 29 (2008): 379-390.
- Wanat KA, Rosenbach M. Cutaneous sarcoidosis. Baughman RP, Culver DA (eds). Clinics in Chest Medicine 36 (2015): 685-702.
- Mitsuishi T, Nogita T, Kawashima M. Psoriasiform sarcoidosis with ulceration. Int J Dermatol 31 (1992): 339-340.
- M Facco, A Cabrelle, A Teramo, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 66 (2011): 144-150.
- Di Cesare A, Di Meglio P, Nestle FO. The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J Invest Dermatol 129 (2009): 1339-1350.
- Wanat KA, Schaffer A, Richardson V, et al. Sarcoidosis and psoriasis: a case series and review of the literature exploring co-incidence vs coincidence. JAMA Dermatol 149 (2013): 848-852.
- Kim HS, Choi D, Lim LL, et al. Association of interleukin 23 receptor gene with sarcoidosis. Dis Markers 31 (2011): 17-24.
- Hanno R, Needelman A, Eiferman RA, et al. Cutaneous sarcoidal granulomas and the development of systemic sarcoidosis. Arch Dermatol 117 (1981): 203-207
- English JC 3rd, Patel PJ, Greer KE. Sarcoidosis. J Am Acad Dermatol 44 (2001): 725-743.
- Samtsov AV. Cutaneous sarcoidosis. Int J Dermatol 31 (1992): 385-391.
- Mangas C, Fernández-Figueras MT, Fité E, et al. Clinical spectrum and histological analysis of 32 cases of specific cutaneous sarcoidosis. J Cutan Pathol 33 (2006): 772-777.
- Elgart MD. Cutaneous sarcoidosis: Definitions and types of lesions. Clinics in Dermatology 4 (1986): 35-45.
- Marcoval J, Mañá J, Rubio M. Specific cutaneous lesions in patients with systemic sarcoidosis: relationship to severity and chronicity of disease. Clin Exp Dermatol 36 (2011): 739-744.
- Lodha S, Sanchez M, Prystowsky S. Sarcoidosis of the skin: a review for the pulmonologist. Chest 136 (2009): 583-596.
- Sanchez M, Haimovic A, Prystowsky S. Sarcoidosis. Dermatol Clin 33 (2015): 389-416.
- Judson MA, Baughman RP, Thompson BW, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lug Dis 20 (2003): 204-211.
- Chappel AG, Cheung WY, Hutchins HA. Sarcoidosis: a long-term follow up study. Sarcoidosis Vasc Diffuse Lug Dis 17 (2000): 219-225.
- Mana J, SalaazarA, Manresa F. Clinical factors predicting persistence of activity in sarcoidosis: a multivariate analysis of 193 cases. Respiration 61 (1994): 219-225.
- Prasse A, Katic C, Germann M, et al. Phenotyping sarcoidosis, from a pulmonary perspective. Am J Respir Crit Care Med 177 (2008): 330-336.
- Neville E, Walker AN, James DG. Prognostic factors predicting the outcome of sarcoidosis:an analysis of 818 patients. Q J Med 52 (1983): 525-533.
- Patel CD, Budev M and Culver DA. Advanced (“End-Stage”) Pulmonary Sarcoidosis. Judsan MA (ed). Pulmonary Sarcoidosis. Humana Press (2014): 79-110.
- Lazar CA, Culver DA. Treatment of sarcoidosis. Semin Respir Crit Care Med 31 (2010): 501-508.
- Facco M, Cabrelle A, Teramo A, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 66 (2011): 144-150.
- Ten Berge B, Paats MS, Bergen IM, et al. Incresed IL-17A expression in granuloma and in circulating memory T cells in sarcoidosis. Rheumatology 51 (2012): 37-46.
- Huang H, Lu Z, Jinag C, et al. Imbalance between Th17 and regulatory T-cells in sarcoidosis. Int J Mol Sci 14 (2013): 21463-72143.
- Moller DR. Rare manifestations of sarcoidosis. Sarcoidosis, Eur Respir Monograph. Edited by: Drent M, Costabel U 10 (2005): 233-250.
- Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 174 (2006): 795-802.
- Saleh S, Ghodsian S, Yakimova V, et al. Effectiveness of infliximab in treating selected patients with sarcoidosis. Respir Med 100 (2006): 2053-2059.
- Jacobsen T, Lie BA, Lysvand H, et al. Detection of psoriasis-associated antigen pso p27 in sarcoidosis bronchoalveolar lavage fluid using monoclonal antibodies. Clin Immunol Immunopathol 81 (1996): 82-87.
- Schön MP, Boehncke WH: Psoriasis. N Eng J Med 352 (2005): 1899-1912.
- Griffiths CEM, Iaccarino L, Naldi L, et al. Psoriasis and psoriatic arthritis: Immunological aspects and therapeutic guidelines. Clin Exp Rheumatol 24 (2006): s72-s78.
