Clinical Observation of Applying Intrabeam® Intraoperative Radiotherapy for the Treatment of Invasive Thymoma
Article Information
Tian-xiang Cui1#, Xie-wan Chen2#, Jing-meng Li3, Jin-dong Qian1, Guang-hui Li1*
#Both the authors contributed equally
1Cancer Institute of People's Liberation Army, Xinqiao Hospital, Army Medical University, Chongqing, 400037, China
2Medical English Department, College of Basic Medical Sciences, Army Medical University, Chongqing, 400037, China
3Department of Thoracic Surgery, Xinqiao Hospital, Army Medical University, Chongqing, 400037, China
*Corresponding Author: Dr. Guang-hui Li, Cancer Institute of People's Liberation Army, Xinqiao Hospital, Army Medical University, Chongqing, 400037, China
Received: 16 August 2019; Accepted: 12 September 2019; Published: 01 October 2019
Citation: Tian-xiang Cui, Xie-wan Chen, Jing-meng Li, Jin-dong Qian, Guang-hui Li. Clinical Observation of Applying Intrabeam® Intraoperative Radiotherapy for the Treatment of Invasive Thymoma. Journal of Cancer Science and Clinical Therapeutics 3 (2019): 152-165.
View / Download Pdf Share at FacebookAbstract
Purpose: Intraoperative radiotherapy (IORT) has been used to treat various solid tumors, and demonstrated advantages. However, no IORT has been reported to treat invasive thymoma. The current study tried to determine the safety profile of applying INTRABEAM IORT to the treatment of invasive thymoma.
Materials and methods: Among the patients admitted to our hospital from September to December, 2016 who was diagnosed with invasive thymoma, 14 were selected as subjects. They were inquired for medical history and divided into 8 stage IIA cases and 6 stage IIB cases according to Masaoka staging system. Of the 14 patients, 5 had myasthenia gravis (MG). INTRABEAM radiation (8–10 Gy, low energy) was delivered to the postoperative tumor bed of each patient during operation. The intra- and post-operative complications were detected and evaluated, and the improvement of symptoms assessed.
Results: One month after operation, only one patient presented cough and increased hemogram levels, chest CT (computerized tomography) showed pulmonary inflammation. Postoperative chest CT at 3 and 12 months showed that all the patients returned to normal, without cough or pulmonary fibrosis surrounding the radiation field. In addition, ultrasonic cardiography and electrocardiogram (ECG) demonstrated no significant difference before and after surgery. At the end of the follow-up, all the patients were alive, no relapse or remote metastasis had been found.
Conclusion: It is safe to administer INTRABEAM IORT at a dose of about 10 Gy with low energy to patients with stage II invasive thymoma. The INTRABEAM IORT does not significantly increase operation- or radiation-related complications, has no significant effect on the vital organs around the radiation field, such as lung and hear
Keywords
<p>Intraoperative radiotherapy; INTRABEAM; Thymoma; Surgery</p>
Intraoperative radiotherapy articles, INTRABEAM articles, Thymoma articles, Surgery articles
Intraoperative radiotherapy articles Intraoperative radiotherapy Research articles Intraoperative radiotherapy review articles Intraoperative radiotherapy PubMed articles Intraoperative radiotherapy PubMed Central articles Intraoperative radiotherapy 2023 articles Intraoperative radiotherapy 2024 articles Intraoperative radiotherapy Scopus articles Intraoperative radiotherapy impact factor journals Intraoperative radiotherapy Scopus journals Intraoperative radiotherapy PubMed journals Intraoperative radiotherapy medical journals Intraoperative radiotherapy free journals Intraoperative radiotherapy best journals Intraoperative radiotherapy top journals Intraoperative radiotherapy free medical journals Intraoperative radiotherapy famous journals Intraoperative radiotherapy Google Scholar indexed journals INTRABEAM articles INTRABEAM Research articles INTRABEAM review articles INTRABEAM PubMed articles INTRABEAM PubMed Central articles INTRABEAM 2023 articles INTRABEAM 2024 articles INTRABEAM Scopus articles INTRABEAM impact factor journals INTRABEAM Scopus journals INTRABEAM PubMed journals INTRABEAM medical journals INTRABEAM free journals INTRABEAM best journals INTRABEAM top journals INTRABEAM free medical journals INTRABEAM famous journals INTRABEAM Google Scholar indexed journals Thymoma articles Thymoma Research articles Thymoma review articles Thymoma PubMed articles Thymoma PubMed Central articles Thymoma 2023 articles Thymoma 2024 articles Thymoma Scopus articles Thymoma impact factor journals Thymoma Scopus journals Thymoma PubMed journals Thymoma medical journals Thymoma free journals Thymoma best journals Thymoma top journals Thymoma free medical journals Thymoma famous journals Thymoma Google Scholar indexed journals Surgery articles Surgery Research articles Surgery review articles Surgery PubMed articles Surgery PubMed Central articles Surgery 2023 articles Surgery 2024 articles Surgery Scopus articles Surgery impact factor journals Surgery Scopus journals Surgery PubMed journals Surgery medical journals Surgery free journals Surgery best journals Surgery top journals Surgery free medical journals Surgery famous journals Surgery Google Scholar indexed journals radiation therapy articles radiation therapy Research articles radiation therapy review articles radiation therapy PubMed articles radiation therapy PubMed Central articles radiation therapy 2023 articles radiation therapy 2024 articles radiation therapy Scopus articles radiation therapy impact factor journals radiation therapy Scopus journals radiation therapy PubMed journals radiation therapy medical journals radiation therapy free journals radiation therapy best journals radiation therapy top journals radiation therapy free medical journals radiation therapy famous journals radiation therapy Google Scholar indexed journals myasthenia gravis articles myasthenia gravis Research articles myasthenia gravis review articles myasthenia gravis PubMed articles myasthenia gravis PubMed Central articles myasthenia gravis 2023 articles myasthenia gravis 2024 articles myasthenia gravis Scopus articles myasthenia gravis impact factor journals myasthenia gravis Scopus journals myasthenia gravis PubMed journals myasthenia gravis medical journals myasthenia gravis free journals myasthenia gravis best journals myasthenia gravis top journals myasthenia gravis free medical journals myasthenia gravis famous journals myasthenia gravis Google Scholar indexed journals electrocardiography articles electrocardiography Research articles electrocardiography review articles electrocardiography PubMed articles electrocardiography PubMed Central articles electrocardiography 2023 articles electrocardiography 2024 articles electrocardiography Scopus articles electrocardiography impact factor journals electrocardiography Scopus journals electrocardiography PubMed journals electrocardiography medical journals electrocardiography free journals electrocardiography best journals electrocardiography top journals electrocardiography free medical journals electrocardiography famous journals electrocardiography Google Scholar indexed journals International Thymic Malignancy articles International Thymic Malignancy Research articles International Thymic Malignancy review articles International Thymic Malignancy PubMed articles International Thymic Malignancy PubMed Central articles International Thymic Malignancy 2023 articles International Thymic Malignancy 2024 articles International Thymic Malignancy Scopus articles International Thymic Malignancy impact factor journals International Thymic Malignancy Scopus journals International Thymic Malignancy PubMed journals International Thymic Malignancy medical journals International Thymic Malignancy free journals International Thymic Malignancy best journals International Thymic Malignancy top journals International Thymic Malignancy free medical journals International Thymic Malignancy famous journals International Thymic Malignancy Google Scholar indexed journals post-operative radiotherapy articles post-operative radiotherapy Research articles post-operative radiotherapy review articles post-operative radiotherapy PubMed articles post-operative radiotherapy PubMed Central articles post-operative radiotherapy 2023 articles post-operative radiotherapy 2024 articles post-operative radiotherapy Scopus articles post-operative radiotherapy impact factor journals post-operative radiotherapy Scopus journals post-operative radiotherapy PubMed journals post-operative radiotherapy medical journals post-operative radiotherapy free journals post-operative radiotherapy best journals post-operative radiotherapy top journals post-operative radiotherapy free medical journals post-operative radiotherapy famous journals post-operative radiotherapy Google Scholar indexed journals overall survival articles overall survival Research articles overall survival review articles overall survival PubMed articles overall survival PubMed Central articles overall survival 2023 articles overall survival 2024 articles overall survival Scopus articles overall survival impact factor journals overall survival Scopus journals overall survival PubMed journals overall survival medical journals overall survival free journals overall survival best journals overall survival top journals overall survival free medical journals overall survival famous journals overall survival Google Scholar indexed journals
Article Details
Abbreviation:
IORT: Intraoperative radiotherapy; EBRT: external beam radiation therapy; MG:myasthenia gravis; ECG: electrocardiography; ITMIG: International Thymic Malignancy Interest Group; PORT: post-operative radiotherapy; OS: overall survival; PFS: progression-free survival.
1. Background
Intraoperative radiotherapy (IORT) is the application of a single dose of radiation to the tumor bed, possibly affected site, and lymphatic drainage area while the areas are exposed during surgery in a single session [1]. The single high dose in a single session can generate a biological effect 2–3 times that of fractionated radiations by external beam radiation therapy (EBRT) [2]. For example, 20 Gy of IORT generates biological effects comparable to that generated by 70 Gy of EBRT [3]. Moreover, by collaboration of surgeons and radiation therapists, IORT can be delivered immediately after the resection of tumor tissue and directly to the target region, thus shortening the interval between operation and radiation and decreasing the proliferation of tumor cells. Further, the radiation field can be precisely determined by moving or covering the normal tissues out of the field of vision, thus greatly reducing the harm to surrounding tissues and the incidence of radiation-related complications [4-6].
Given the above advantages, IORT has been widely applied to the treatment of breast cancer, pancreatic cancer, gastric cancer, rectal cancer, and solitary metastatic brain tumor in recent years, and demonstrated its superiority [7-11]. However, to our best knowledge, no application of IORT to invasive thymoma has been reported. Therefore, we
recruited 14 patients from all the cases of stage II thymoma proved by pathological examination admitted to our hospital from September to December, 2016. The patients were administered radiation (8-12 Gy, median dose 10 Gy, 50KVe) for the tumor bed after radical surgery for thymoma, and the safety and short-term effectiveness of IORT were investigated.
2. Materials and Methods
2.1 Patients collection
Patients were collected from September to December, 2016. For preliminary selection, the patients who consulted the Department of Thoracic Surgery in our hospital due to mediastinal mass and myasthenia gravis (MG) were considered candidate subjects of the current study. These candidates were confirmed with mediastinal mass by thoracic CT scanning and ruled out with remote metastasis, and planned to receive surgical treatment. For secondary selection, the patients meeting the following criteria were included. Intra-operative frozen section was confirmed as thymoma, tumor invasion to the capsule not to adjacent tissues or organs was observed with naked eyes, and the tumor was in stage II according to Masaoka staging system. The pathological subtype and Masaoka stage of the tumor were determined by post-operative pathological examination. Till the end of December, 2016, a total of 15 patients were collected. Of them, one case was confirmed with thymic carcinoma by post-operative pathological
examination. Finally, a total of 14 patients became the subjects of the current study. Each patient has signed an informed consent, and all the procedures were conducted after the approval of the Ethics Committee of Xinqiao Hospital, Army Medical University. This work was in accordance with the Helsinki Declaration as revised in 2013.
2.2 Surgical procedures
The surgery was performed under intravenous anesthesia with double-lumen endotracheal tube intubation and left-lung ventilation. All the patients were treated with right thoracic approach and placed in a left lateral recumbent position of 30–45 degrees in angle. The operation was completed through two ports in the chest wall. A 1.5 to 2-cm port for observation and auxiliary operation was created in the 5th or 6th intercostal space along the right middle axillary line, and a 2 to 4 cm port for major operation created in the 3rd or 4th intercostal space along the right anterior axillary line. An incision protection retractor was attached to the major operation port. After insertion of instruments, extended thymectomy was performed as follows. The mediastinal pleura was longitudinally incised from top to bottom anterior to the phrenic nerve which was protected, providing adequate mobilization to the structures and full exposure to the superior and inferior poles of tumor and the normal part of thymus. The tumor was pulled up, and then treated together with the pericardial reentry, and subsequently underwent a blunt separation to the mediastinal pleura. After a full mobilization of the prevascular space and retrosternal space, the thymic veins were treated using an ultrasonic knife and titanium clips and the tumor was excised. Finally, the fat tissue in anterior mediastinum and surrounding the cardiophrenic angle was removed. The excised tumor was transferred into the specimen bag and extracted through the major operation port. At end of the operation, a closed thoracic drainage tube was placed through the observation port. All the surgical procedures were completed using the video-assisted thoracoscope.
2.3 INTRABEAM IORT technique
IORT in the current study was realized by using INTRABEAM. INTRABEAM® treatment system (Carl Zeiss, Oberkochen, Germany, Figure 1) is a mobile miniature electron beam-driven device to deliver radiation directly to the tumor bed during surgery, with a flexible arm and a balanced stand [12]. It can provide a point source of low energy X-rays (50 kV max) at the tip of an electron beam drift tube (3.2 mm in diameter) fixed at the end of the manipulator. The tube is sheathed by a spherical applicator (ranged 1.5 to 5.0 cm in diameter) with a cone-shaped shank at the bottom, which is placed upside down during IORT [13, 14]. The low-energy X-rays are emitted after the electron beam hits the gold target at the tip of the tube and are modulated by the applicator to give a uniform dose in a spherical field [13].
Based on the specific size of the tumor and tumor bed, an applicator in appropriate size was deployed above and against the tumor bed. A saline-soaked gauze was then folded into a shape of 2 cm in thickness, and the folded gauze was set around the applicator to block X-rays from entering the normal tissues and thus ensure the localization of radiation to the tumor bed area (Figure 2). Totally, a single dose of 8–10 Gy at the applicator surface was administered in the current study. The specific radiation time was determined by comprehensively considering the radiation dose, the depth of invasion and the size of the applicator. Different IORT parameters are shown in Table 1.

Figure 1: INTRABEAM device (A) and spherical applicators with cone-shaped shanks (B).
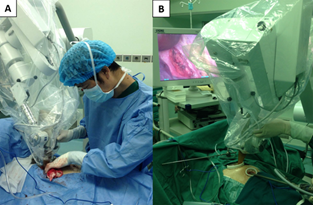
Figure 2: The operator placed an applicator in a proper size above and against the tumor bed through the major operation port (A and B).
Table 1: Surgical Conditions of Patients and IORT Parameters.
2.4 Follow-up and assessments
All the patients were required to return for examination at 1 month, 3 months and 1 year after discharge from hospital, respectively. The examination included blood routine test, liver and kidney function tests, CT scanning of the lungs, electrocardiography (ECG), ultrasonic cardiography, etc. Later in the first year, the patients were followed up every 3 months by phone call or out-patient consultation. The content included the improvement of myasthenia gravis, survival, quality of life, etc. The last follow-up date was May 31st, 2019, and the whole process lasted 29–32 months, with a median duration of 30 months.
3. Results
3.1 General characteristics of patients
A total of 15 patients were collected from September to December, 2016. One of the patients was confirmed with thymic carcinoma by post-operative pathological examination and delivered radiochemotherapy after surgery, and thus excluded. Therefore, 14 patients were observed in the current study, consisting of 9 males and 5 females, aged between 31–72y with a median age of 53y. According to Masaoka stage, 8 patients had stage IIA disease and 6 had stage IIB disease. According to pathological type, 2 cases had B1 type, 6 cases had B2 type, 6 had AB type. For comorbidity, 5 patients had MG. The detailed data for general characteristics are listed in Table 2.
3.2 Complications related to IORT
During the surgery, the blood loss and rehydration are shown in Table 1. The time for installation and operation the radiation equipment are listed in Table 1. The longest time is 72 min, the minimum is 48min, and the mean is 57.6 min. No serious complications of surgery or IORT were observed in the 14 patients, and the 5 patients with MG did not develop myasthenic crisis after surgery.
3.3 Conditions of patients during the follow-up
During the follow-up, CT scanning of the lungs at 1 month after discharge revealed that 5 patients developed moderate pulmonary inflammation, of whom 1 had symptoms of cough and increased hemogram levels, and 2 had a small amount of pleural effusion. However, CT scanning of the lungs at 3 and 12 months after discharge showed that all the patients returned to normal, without cough, short of breath after activity, or pulmonary fibrosis surrounding the radiation field (Figure 3). In addition, ultrasonic cardiography and ECG demonstrated no significant difference before and after surgery (Table 3).
3.4 Effectiveness of intrabeam IORT
At the end of the follow-up, no relapse or remote metastasis had been found. Of the 5 patients with MG, 4 had symptoms significantly improved after treatment, while 1 did not show any obvious improvement in symptoms, presented with blepharoptosis and inability to comb hair, administered Neostigmine orally.
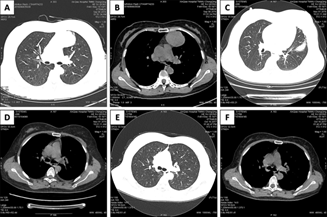
Figure 3: Pre-operative chest CT showed a mass in the left anterior mediastinum (A and B); chest CT at post-operative 1 month showed pulmonary infection in left upper lobe (C) and a small amount of pleural effusion on the left side (D); and chest CT at post-operative 6 months showed normal lungs and no pulmonary fibrosis in the irradiated region (E and F).
aECG, Electrocardiogram; UCG, Ultrasonic Cardiogram.
Table 2: General Characteristics of the Patients.
Table 3: Conditions of Each Patient during the Follow-Up.
4. Discussion
Thymoma is derived from thymic epithelium and accounts for approximately 50% of all types of anterior mediastinal tumor. Despite the fact that most thymoma is benign, thymoma has a potential of transforming into malignancy that will invade local tissue or metastasize to remote sites. According to the statistics from the National Cancer Institute, the incidence of thymoma is 0.13 per 100,000 person-years in the United States [15]. In addition, over 30% of patients with thymoma has a comorbidity of MG [16]. Consistent with the previous report, MG was present in 5 of the 14 patients in the current study. Therefore, it is vitally important to treat thymoma properly.
Surgical removal of thymoma is the standard treatment for thymomas that can be completely excised. However, whether the tumor can be completely removed remains a problem and determines the prognosis of thymoma patients. It has been reported that the completion of surgical resection is a factor that substantially affects local recurrence and survival [17]. According to the International Thymic Malignancy Interest Group (ITMIG), the complete resection can only be achieved by excising the whole thymus and surrounding tissue [18].
To realize complete resection and enhance the effects of surgery, other treatments are also adjunctly used, such as post-operative radiotherapy (PORT). However, controversies still exist over the effectiveness of PORT for thymoma [19]. Some studies reported that radiotherapy could significantly improve the prognosis of patients with stage II or III thymoma [20], and PORT is needed to decrease the local recurrence and increase the survival of patients with stage II or even more invasive thymoma [21]. Other studies revealed that radiotherapy could only improve the prognosis of stage III patients rather than stage II patients. In a study based on the National Cancer Database of US, Matthew et al. recruited 4056 thymoma patients between 2004 and 2012, of whom 2001 (49%) received PORT. The results demonstrated that PORT improved the overall survival (OS) of the patients with stage IIB (HR=0.61, p=0.035), stage III (HR=0.69, p=0.020) and positive margins (HR=0.53, p<0.001) disease, whereas did not improve the prognosis of patients with stages I-IIA disease (HR=0.76, p=0.156) [22].
Increasing evidence supports the ineffectiveness of PORT in recent years. The rate of complete removal reached 88% in stage II patients [23], and the patients with thymic tumor completely resected did not benefit from PORT [24]. A meta-analysis by Korst and coworkers showed that PORT could not decrease the recurrence in stages II and III patients who underwent complete tumor resection [25]. Consistently, after analyzing 1320 patients with stage II or III thymoma, Kondo et al. found that PORT neither significantly inhibited local recurrence nor improved patient outcome [26]. In another study, no significant difference was observed in the 10-year survival rate between PORT group and surgery alone group (92.8% vs. 94.4%, P=0.22) [27]. Additionally, no improvement of OS or progression-free survival (PFS) was found in 71 stage II patients who underwent complete resection and PORT [28]. Omasa and colleagues revealed that PORT only improved the PFS of patients with stage II or III thymic carcinoma, but ineffective for stage II or III thymoma [29].
Despite the ineffectiveness of PORT, surgery alone cannot prevent relapse and metastasis. Once the tumor breaks through its envelope or invades the mediastinal adipose tissue in stage II thymoma, the rate of local recurrence and metastasis will arrive to 11% [23]. Therefore, radiation therapy is still needed in combination with surgical removal, but the time of delivery can be adjusted. Since IORT is more precise and earlier than PORT, IORT can be considered to optimize the treatment. Simultaneous with surgery, radiation can be precisely and timely delivered to the tumor bed and surrounding tissue, thus maximally protecting the normal tissue and decreasing the time for tumor proliferation [30]. Furthermore, IOPT can inhibit local recurrence by changing the tumor microenvironment. The exudates collected at the surgical wound in 24 h after breast-conserving surgery could stimulate the proliferation and migration of breast cancer cells in vitro, while the exudates from the wound that underwent IORT could not [30]. Although the single dose of IORT is much higher than that of EBRT, low-energy X rays have weak penetrating power; when the radiation dose for tumor bed was 20 Gy, the power would decrease to 5-7 Gy at 1 cm from the surface [12]. Deployment of the spherical radiation applicator at a certain distance from the skin and the thoracic wall can effectively reduce radiation injury, thus making radiation protection easier than EBRT. In the current study, we protected the normal tissues by covering the radiation applicator with a wet gauze of 2 cm in thickness.
After the surgery, we closely followed up the patients and revealed that it is safe to deliver INTRABEAM IORT to the tumor bed at a dose of about 10 Gy with low-energy X rays. The extension of operation time did not significantly increase radiation-related complications. Although 4 patients were presented with pulmonary infections after surgery, only 1 of them had cough and increased hemogram levels, which met the diagnosis criteria of pulmonary infection, and the other 3 were most probably due to the reactional inflammation after surgery. Till the end of the follow-up on May 31st, 2019, no radiation pneumonitis or pulmonary fibrosis around the radiation field and no tumor recurrence had been found in any patient.
Moreover, because thymoma is located in special places, generally near the heart and great vessels, intra-operative radiation may exert effects on the conduction system and the systolic and diastolic functions of the heart. We therefore compared the ultrasonic cardiograms before IORT with those after IORT at 6 months and 1 year, respectively. No significant change was observed in the systolic and diastolic functions and chamber size of the heart, suggesting that IORT does not affect the cardiac functions. Similarly, no obvious change was detected in electrocardiogram before and after surgery, indicating that IORT does not affect the cardiac conduction system.
In summary, the current study has demonstrated the safety of delivering INTRABEAM IORT to patients with invasive thymoma. However, due to the limited follow-up time and sample size and the design of not being a random controlled trial, the current study cannot fully confirm the effectiveness of INTRABEAM IORT in treating invasive thymoma, which deserves further investigation in the near future.
5. Conclusions
It is safe to administer INTRABEAM IORT at a dose of about 10 Gy with low energy to patients with stage II invasive thymoma. The INTRABEAM IORT does not significantly increase operation- or radiation-related complications, has no significant effect on the vital organs around the radiation field, such as lung and heart. The long-term efficacy is worth expecting.
Acknowledgments
Not applicable
Funding
No funding was received.
Availability of Data and Materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
(I) Conception and design: Tian-xiang Cui and Xie-wan Chen.
(II) Administrative support: Guang-hui Li.
(III) Provision of study materials or patients: Jing-meng Li.
(IV) Collection and assembly of data: Jin-dong Qian.
(V) Data analysis and interpretation: Tian-xiang Cui and Xie-wan Chen.
(VI) Manuscript writing: All authors.
(VII) Final approval of manuscript: All authors.
Ethics Approval and Consent to Participate
This study was approved by the institutional review board and ethics committee of Xinqiao Hospital, Army Medical University, Chongqing, China.
Consent for publication
Not applicable.
Competing Interests
We declare that we do not have any commercial or associative interests that represent a conflict of interest in connection with the work submitted.
References
- Vaidya JS, Baum M, Tobias JS, et al. Targeted intraoperative radiotherapy (TARGIT) yields very low recurrence rates when given as a boost. Int J Radiat Oncol Biol Phys 66 (2006): 1335-1338.
- Avinash Pilar, Meetakshi Gupta, Sarbani Ghosh Laskar, et al. Intraoperative radiotherapy: review of techniques and results. Ecancermedicalscience 11 (2017): 750.
- Herskind C, Griebel J, Kraus-Tiefenbacher U, et al. Sphere of equivalence-a novel target volume concept for intraoperative radiotherapy using low-energy X rays. Int J Radiat Oncol Biol Phys 72 (2008): 1575-1581.
- Bentzen SM, Agrawal RK, Aird EG, et al. The UK standardisation of breast radiotherapy (START) trial A of radiotherapy of ractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol 9 (2008): 331-341.
- Vaidya JS, Wenz F, Bulsara M, et al. An international randomised controlled trial to compare TARGeted Intraoperative radiotherapy (TARGIT) with conventional postoperative radiotherapy after breast-conserving surgery for women with early-stage breast cancer (the TARGIT-A trial). Health Technol Assess 20 (2016): 1-188.
- Coombs NJ, Coombs JM, Vaidya UJ, et al. Environmental and social benefits of the targeted intraoperative radiotherapy for breast cancer: data from UK TARGIT-A trial centres and two UK NHS hospitals offering TARGIT IORT. BMJ Open 6 (2016): e010703.
- Vaidya JS, Wenz F, Bulsara M, et al. Risk-adapted targeted intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet 383 (2014): 603-613.
- Ogawa K, Karasawa K, Ito Y, et al. Intraoperative radiotherapy for resected pancreatic cancer: a multi-institutional retrospective analysis of 210 patients. Int J Radiat Oncol Biol Phys 77 (2010): 734-742.
- Miller RC, Haddock MG, Gunderson LL, et al. Intraoperative radiotherapy for treatment of locally advanced and recurrent esophageal and gastric adenocarcinomas. Dis Esophagus 19 (2006): 487-495.
- Guo S, Reddy CA, Kolar M, et al. Intraoperative radiation therapy with the photon radiosurgery system in locally advanced and recurrent rectal cancer: retrospective review of the Cleveland clinic experience. Radiat Oncol 7 (2012): 110.
- Weil RJ, Mavinkurve GG, Chao ST, et al. Intraoperative radiotherapy to treat newly diagnosed solitary brain metastasis: initial experience and long-term outcomes. J Neurosurg 122 (2015): 825-832.
- Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): an international, prospective, randomised, non-inferiority phase 3 trial. Lancet 376 (2010): 91-102.
- Pan L, Zheng W, Ye X, et al. A novel approach of INTRABEAM intraoperative radiotherapy for nipple-sparing mastectomy with breast reconstruction. Clin Breast Cancer 14 (2014): 435-441.
- Njeh CF, Saunders MW, Langton CM. Accelerated Partial Breast Irradiation (APBI): A review of available techniques. Radiat Oncol 5 (2010): 90.
- Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 5 (2010): S260-S265.
- Qu YJ, Liu GB, Shi HS, et al. Preoperative CT findings of thymoma are correlated with postoperative Masaoka clinical stage. Acad Radiol 20 (2013): 66-72.
- Filosso PL, Guerrera F, Rendina AE, et al. Outcome of surgically resected thymic carcinoma: a multicenter experience. Lung Cancer 83 (2014): 205-210.
- Kirti Bushan, Sanjay Sharma, Harish Verma. A Review of Thymic Tumors. Indian J Surg Oncol 4 (2013): 112-116.
- Lim YJ, Kim E, Kim HJ, et al. Survival impact of adjuvant radiotherapy in Masaoka stage II-IV thymomas: A systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 94 (2016): 1129-1136.
- Lombe DC, Jeremic B. A review of the place and role of radiotherapy in thymoma. Clin Lung Cancer 16 (2015): 406-412.
- Chang JH, Kim HJ, Wu HG, et al. Postoperative radiotherapy for completely resected stage II or III thymoma. J Thorac Oncol 6 (2011): 1282-1286.
- Jackson MW, Palma DA, Camidge DR, et al. The impact of postoperative radiotherapy for thymoma and thymic carcinoma. J Thorac Oncol 12 (2017): 734-744.
- Detterbeck FC. Evaluation and treatment of stage I and II thymoma. J Thorac Oncol 5 (2010): S318-S322.
- Mangi AA, Wain JC, Donahue DM, et al. Adjuvant radiation of stage III thymoma: is it necessary? Ann Thorac Surg 79 (2005): 1834-1839.
- Korst RJ, Kansler A L, Christos PJ, et al. Adjuvant radiotherapy for thymic epithelial tumors: a systematic review and meta-analysis. Ann Thorac Surg 87 (2009): 1641-1647.
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1320 patients from Japan. Ann Thorac Surg 76 (2003): 878-884.
- Utsumi T, Shiono H, Kadota Y, et al. Postoperative radiation therapy after complete resection of thymoma has little impact on survival. Cancer 115 (2009): 5413-5420.
- Aydiner A1, Toker A, Sen F, et al. Association of clinical and pathological variables with survival in thymoma. Med Oncol 29 (2012): 2221-2228.
- Omasa M, Date H, Sozu T, et al. Postoperative radiotherapy is effective for thymic carcinoma but not for thymoma in stage II and III thymic epithelial tumors: the Japanese Association for Research on the Thymus Database Study. Cancer 121 (2015): 1008-1016.
- Belletti B, Vaidya JS, D’Andrea S, et al. Targeted intraoperative radiotherapy impairs the stimulation of breast cancer cell proliferation and invasion caused by surgical wounding. Clin Cancer Res 14 (2008): 1325-1332.
