Clinical Markers of Dysgraphia According to Intellectual Quotient in Children with Developmental Coordination Disorder
Article Information
Soukaina Hamdioui 1,2, Laurence Vaivre-Douret 2,3,4,5,6*
1Faculty of Society and Humanity, Division of Psychology, Université de Paris, France
2National Institute of health and Medical Research (INSERM UMR 1018-CESP), Faculty of Medicine, University of Paris-Saclay, UVSQ, Villejuif, France
3 Faculty of Health, Division of Medicine Paris Descartes, Université de Paris, France
4 University Institute of France (Institut Universitaire de France, IUF), Paris, France
5Department of Child Psychiatry, Assistante Publique-Hôpitaux de Paris (AP-HP. Centre), Necker-Enfants Malades University hospital, Paris, France
6Department of Endocrinology, IMAGINE Institute, Necker-Enfants Malades Université de Paris, France
*Corresponding Author: Prof. Laurence Vaivre-Douret, PhD, Hôpital Universitaire Necker-Enfants Malades, INSERM UMR 1018-CESP, Carré Necker Porte N4,149, rue de Sèvres, 75015 Paris, France
Received: 18 September 2020; Accepted: 25 September 2020; Published: 09 November 2020
Citation:
Soukaina Hamdioui, Laurence Vaivre-Douret, Clinical Markers of Dysgraphia According to Intellectual Quotient in Children with Developmental Coordination Disorder. Journal of Psychiatry and Psychiatric Disorders 4 (2020): 366-382.
View / Download Pdf Share at FacebookAbstract
Objective: Handwriting difficulties are commonly observed in children with high intellectual quotient (HIQ) and generally associated to developmental coordination disorder (DCD) but remain poorly understood. The aim of our study is to refine the specific clinical features of handwriting disorders in children with DCD regarding their IQ level.
Method: Neurovisual, neuroimaging, neuropsychological, neuropsychomotor functions, and handwriting performances of 38 children (6-to-12 years-old: mean 9y, SD 2.7), diagnosed with DCD (DSM-5 criteria), were analyzed. Among them, two matched groups were studied according to the Full IQ (FIQ) level: 19 typical-children (FIQ=90-110) and 19 HIQ children (FIQ> 120).
Results: Handwriting difficulties were present in both groups without statistical difference. Dysgraphia was significantly related to pyramidal-tract-dysfunction (p=0.01) and electroretinogram abnormalities (p=0.03) in both groups. In HIQ children, a specific marker was identified: visual gnosis impairment associated to deficit of visual-spatial memory.
Conclusions: Dysgraphia is a severe diagnosis involving the central nervous system, affecting the motor and neurovisual pathways and it rather appears as a comorbidity to DCD. Neurological-soft-signs and electroretinogram abnormalities can be considered as interesting clinical markers of dysgraphia in children with DCD. Children having FIQ>120 display visual gnosis disorder as a specific clinical marker of dysgraphia.
Keywords
Handwriting disorders; Dysgraphia; Developmental coordination disorder; High intellectual quotient; Neurological soft signs; Developmental assessment
Handwriting disorders articles; Dysgraphia articles; Developmental coordination disorder articles; High intellectual quotient articles; Neurological soft signs articles; Developmental assessment articles
Handwriting disorders articles Handwriting disorders Research articles Handwriting disorders review articles Handwriting disorders PubMed articles Handwriting disorders PubMed Central articles Handwriting disorders 2023 articles Handwriting disorders 2024 articles Handwriting disorders Scopus articles Handwriting disorders impact factor journals Handwriting disorders Scopus journals Handwriting disorders PubMed journals Handwriting disorders medical journals Handwriting disorders free journals Handwriting disorders best journals Handwriting disorders top journals Handwriting disorders free medical journals Handwriting disorders famous journals Handwriting disorders Google Scholar indexed journals Dysgraphia articles Dysgraphia Research articles Dysgraphia review articles Dysgraphia PubMed articles Dysgraphia PubMed Central articles Dysgraphia 2023 articles Dysgraphia 2024 articles Dysgraphia Scopus articles Dysgraphia impact factor journals Dysgraphia Scopus journals Dysgraphia PubMed journals Dysgraphia medical journals Dysgraphia free journals Dysgraphia best journals Dysgraphia top journals Dysgraphia free medical journals Dysgraphia famous journals Dysgraphia Google Scholar indexed journals Developmental coordination disorder articles Developmental coordination disorder Research articles Developmental coordination disorder review articles Developmental coordination disorder PubMed articles Developmental coordination disorder PubMed Central articles Developmental coordination disorder 2023 articles Developmental coordination disorder 2024 articles Developmental coordination disorder Scopus articles Developmental coordination disorder impact factor journals Developmental coordination disorder Scopus journals Developmental coordination disorder PubMed journals Developmental coordination disorder medical journals Developmental coordination disorder free journals Developmental coordination disorder best journals Developmental coordination disorder top journals Developmental coordination disorder free medical journals Developmental coordination disorder famous journals Developmental coordination disorder Google Scholar indexed journals High intellectual quotient articles High intellectual quotient Research articles High intellectual quotient review articles High intellectual quotient PubMed articles High intellectual quotient PubMed Central articles High intellectual quotient 2023 articles High intellectual quotient 2024 articles High intellectual quotient Scopus articles High intellectual quotient impact factor journals High intellectual quotient Scopus journals High intellectual quotient PubMed journals High intellectual quotient medical journals High intellectual quotient free journals High intellectual quotient best journals High intellectual quotient top journals High intellectual quotient free medical journals High intellectual quotient famous journals High intellectual quotient Google Scholar indexed journals Neurological soft signs articles Neurological soft signs Research articles Neurological soft signs review articles Neurological soft signs PubMed articles Neurological soft signs PubMed Central articles Neurological soft signs 2023 articles Neurological soft signs 2024 articles Neurological soft signs Scopus articles Neurological soft signs impact factor journals Neurological soft signs Scopus journals Neurological soft signs PubMed journals Neurological soft signs medical journals Neurological soft signs free journals Neurological soft signs best journals Neurological soft signs top journals Neurological soft signs free medical journals Neurological soft signs famous journals Neurological soft signs Google Scholar indexed journals Developmental assessment articles Developmental assessment Research articles Developmental assessment review articles Developmental assessment PubMed articles Developmental assessment PubMed Central articles Developmental assessment 2023 articles Developmental assessment 2024 articles Developmental assessment Scopus articles Developmental assessment impact factor journals Developmental assessment Scopus journals Developmental assessment PubMed journals Developmental assessment medical journals Developmental assessment free journals Developmental assessment best journals Developmental assessment top journals Developmental assessment free medical journals Developmental assessment famous journals Developmental assessment Google Scholar indexed journals Attention Deficit-Hyperactivity Disorder articles Attention Deficit-Hyperactivity Disorder Research articles Attention Deficit-Hyperactivity Disorder review articles Attention Deficit-Hyperactivity Disorder PubMed articles Attention Deficit-Hyperactivity Disorder PubMed Central articles Attention Deficit-Hyperactivity Disorder 2023 articles Attention Deficit-Hyperactivity Disorder 2024 articles Attention Deficit-Hyperactivity Disorder Scopus articles Attention Deficit-Hyperactivity Disorder impact factor journals Attention Deficit-Hyperactivity Disorder Scopus journals Attention Deficit-Hyperactivity Disorder PubMed journals Attention Deficit-Hyperactivity Disorder medical journals Attention Deficit-Hyperactivity Disorder free journals Attention Deficit-Hyperactivity Disorder best journals Attention Deficit-Hyperactivity Disorder top journals Attention Deficit-Hyperactivity Disorder free medical journals Attention Deficit-Hyperactivity Disorder famous journals Attention Deficit-Hyperactivity Disorder Google Scholar indexed journals Full Intellectual Quotient articles Full Intellectual Quotient Research articles Full Intellectual Quotient review articles Full Intellectual Quotient PubMed articles Full Intellectual Quotient PubMed Central articles Full Intellectual Quotient 2023 articles Full Intellectual Quotient 2024 articles Full Intellectual Quotient Scopus articles Full Intellectual Quotient impact factor journals Full Intellectual Quotient Scopus journals Full Intellectual Quotient PubMed journals Full Intellectual Quotient medical journals Full Intellectual Quotient free journals Full Intellectual Quotient best journals Full Intellectual Quotient top journals Full Intellectual Quotient free medical journals Full Intellectual Quotient famous journals Full Intellectual Quotient Google Scholar indexed journals Visual Spatial Index articles Visual Spatial Index Research articles Visual Spatial Index review articles Visual Spatial Index PubMed articles Visual Spatial Index PubMed Central articles Visual Spatial Index 2023 articles Visual Spatial Index 2024 articles Visual Spatial Index Scopus articles Visual Spatial Index impact factor journals Visual Spatial Index Scopus journals Visual Spatial Index PubMed journals Visual Spatial Index medical journals Visual Spatial Index free journals Visual Spatial Index best journals Visual Spatial Index top journals Visual Spatial Index free medical journals Visual Spatial Index famous journals Visual Spatial Index Google Scholar indexed journals Verbal Comprehension Index articles Verbal Comprehension Index Research articles Verbal Comprehension Index review articles Verbal Comprehension Index PubMed articles Verbal Comprehension Index PubMed Central articles Verbal Comprehension Index 2023 articles Verbal Comprehension Index 2024 articles Verbal Comprehension Index Scopus articles Verbal Comprehension Index impact factor journals Verbal Comprehension Index Scopus journals Verbal Comprehension Index PubMed journals Verbal Comprehension Index medical journals Verbal Comprehension Index free journals Verbal Comprehension Index best journals Verbal Comprehension Index top journals Verbal Comprehension Index free medical journals Verbal Comprehension Index famous journals Verbal Comprehension Index Google Scholar indexed journals
Article Details
Abbreviations
ADHD = Attention Deficit-Hyperactivity Disorder
DCD = Developmental Coordination Disorder
ERG = Electroretinogram
FIQ = Full Intellectual Quotient
HIQ = High Intellectual Quotient
IM = Ideomotor
MANOVA = Multivariate Analysis of Variance
MX = Mixed subtype
NP-MOT = Developmental neuropsychomotor battery
NSS = Neurological Soft Signs
PSR = Phasic Stretch Reflex
VCI = Verbal Comprehension Index
VEP = Patterns of Visual Evoked Potential
VSC = Visuo-Spatial/ or Constructional
VSI = Visual Spatial Index
1. Introduction
Handwriting is a complex developmental process requiring perceptual, motor cognitive, and emotional capacities [1, 2]. A literature review highlights the different terms used to describe handwriting disorder: poor writing [3], handwriting dysfunction [4], dysgraphia [5] or learning disability handwriting (including poor handwriting and dysgraphia) [6]. Other authors defined “motor dysgraphia” as a disorder in fine motor coordination, proprioception, and visual perception, characterized by slowing only in writhing activity [7]. However, there is still no consensus in the literature regarding the definition of handwriting disorder and their etiology remaining poorly understood. Lopez et al. [8, 9] differentiated dysgraphia from poor handwriting identifying six specific handwriting criteria (Table 1). In the current study, we dissociated between dysgraphia and poor handwriting, considering dysgraphia to be a marked degree of handwriting impairment.
|
Criteria |
Details |
|
Criterion 1: Irregular handwriting |
Irregular spaces between letters, words and lines, irregularity in letter size. |
|
Criterion 2: Immaturity of handwriting gesture |
Gestural developmental organization of the upper limb (according to a joint repository of gestural organization shoulder-elbow-wrist-fingers) allowing the child to perform the fine motor gestures required to form letters. |
|
Criterion 3: Excessive pressure of the open on the paper |
Pen pressure on the paper. |
|
Criterion 4: Neuro-vegetative responses |
Sweating, pain, respiratory breaks. |
|
Criterion 5: Trembling |
Handshake when writing. |
|
Criterion 6: Slow handwriting velocity |
Scores based on interquartile range (≤Q3). |
Table 1: Handwriting qualitative criteria identified in the study of Lopez et al. (2016).
The international DSM-5 classifications “Impairment in written expression” [10] and DSM-4 “disorder of writing expression” [11] does not include “handwriting difficulties”. However, DSM-4-TR page 56 states a disorder in spelling or handwriting alone, in the absence of other difficulties of written expression, generally does not qualify for a diagnosis of disorder of written expression. If poor handwriting is due to impairment in motor coordination, a diagnosis of Developmental Coordination Disorder (DCD) should be considered [12]. Handwriting difficulties are often linked to learning disabilities (e.g. dyslexia, dysorthographia, attention deficit-hyperactivity disorder (ADHD), DCD). It is a public health issue often alerting clinicians whatever the level of intelligence quotient (IQ), but it is often noted in children with high IQ (HIQ). Certain authors putatively explained this by a dysynchrony between intellectual and motor performances as a developmental fact in these children, because they think faster than they write, causing a poor handwriting [13-15]. According to the normal distribution and mainly to the Gaussian curve of the Wechsler intelligence scale [16], a Full IQ (FIQ) from 120 to 129 (6.7% of the general population) is considered as superior. Very superior FIQ beginning at 130 (2.5%), two standard deviations from the mean, qualified as high intellectual potential. Thus, are children with a high FIQ really characterized systematically and significantly by handwriting difficulties compared to children with an average FIQ? To answer this question, it is interesting to study the clinical features of handwriting disorders in a group of children with high FIQ compared to a matched group with average FIQ. Handwriting disorders are often assessed with a single test based on writing product only, without deep analyze of other functions involved in handwriting: primarily the Concise Children's Handwriting Rating Scale (BHK) [17] or Scale for rapid assessment of children's writing [18], the Children's Handwriting Assessment Tool (ETCH) [19], the Minnesota Handwriting Assessment (MHA) [20], the Detailed Assessment of Writing Speed (DASH) [21].
Children with DCD are particularly affected by handwriting disorders (87-88%) [22, 23] but no study has analyzed these difficulties in children with DCD regarding their IQ in inclusion criteria. The study of handwriting disorders in DCD is all the more complex because, despite the consensus of terminology and diagnosis reached by international researchers [24], DCD remains poorly understood and studies do not use the same tools to identify their groups of children with DCD. Certain studies shed light on the features of DCD, using clustering method and normative age-related data with a deep standardized assessment including cognitive, neuromotor and neurovisual tests, highlighting DCD subtypes with specific diagnostic markers [23, 25-27]. They identified two mainly pure DCD subtypes: ideomotor-DCD (MI) (8%), visuo-spatial/or visuo-constructional-DCD (VSC) (52%) and a mixed subtype (MX) (40%). Children with IM-DCD had as diagnostic markers significant alterations in manual practice, digital perception, and imitation of gestures. The VSC subtype was characterized by impairments in visual spatial motor structuring and/or construction skills visual motor integration, while the MX subgroup shared impairments common to IM and VSC, with in addition poor manual dexterity, specific impairments in motor coordination of the lower and upper limbs, and neurological soft signs (NSS) suggesting synkinesis, adiadochokinesis, and other comorbidities (e.g. impaired executive function, impaired auditory memory). All children in VSC and MX subtype had handwriting disorders. It thus appears very interesting to specify the clinical features of handwriting disorders according to DCD subtypes which could be useful for clinical decision-making processes (diagnosis and remediations).
The purpose of the current study was to analyze neuropsychological, neuropsychomotor, neuroimaging and neurovisual characteristics, to refine the specific features of handwriting disorders (dysgraphia/poor handwriting) in children with DCD, regarding their IQ. The first hypothesis explores if dysgraphia appears such as a developmental fact in children with DCD comparing a group with high FIQ and another with an average FIQ. The second explores if there are specific clinical features of dysgraphia in both groups, using neuroimaging, standardized developmental assessment of neuropsychological, neurovisual and neuropsychomotor functions, including neurological soft signs evaluation.
2. Methods
2.1 Ethical approval
The Intuitional ethic committee of Paris Descartes University approved our study (CER-PD 2019-49; CER-PD 2019-93). Participants provided written informed consent before the start of the study, signed by a parent or legal representative and children before enrolment in the study.
2.2 Participants
Data from 38 DCD children aged 6 to 12 years-old (mean 9y, SD 2.7) were recruited in the outpatient consultation of child psychiatry departments at Necker Hospital University in Paris, France. All of them met the DCD criteria DSM-5 [10] to be included. Inclusion criteria were strict: Children with sensory and severe visual abnormalities (e.g. strabismus, amblyopia, nystagmus), a diagnosis of severe language disorders, genetic disorder, traumatic brain injury, general medical abnormalities, or ADHD, autism spectrum disorder, psychiatric abnormalities (according to DSM-5 criteria) were not included. Nor was any child born premature (<37 weeks) and no physical therapy neither medication. Children who met inclusion criteria were assigned to the three DCD subtypes validated in a previous study and described above [23, 25, 28]: IM, VSC, and MX-DCDs. Among the 38 children, we dissociated two matched groups in accordance with Gaussian curve of Weschler intelligence scale [16]: 19 typical children (FIQ=90-110) with DCD and 19 HIQ children (>120) with DCD. Within this last group, we distinguished superior FIQ starting at +1.33 SD (FIQ=120-129), and very superior FIQ at +2 SD (FIQ≥130) who are identified as gifted children by the Wechsler intelligence scale [16]. The two groups were matched according to sociodemographic data, age, language disorder, and ophthalmological abnormalities.
All children underwent extensive neuropsychological, brain Magnetic Resonance Imaging (MRI) scans, handwriting assessment, neurovisual, anamnestic form, and developmental neuropsychomotor assessment.
2.3 Measures
2.3.1. Neuropsychological and Handwriting assessment: All children completed a standard measure of intelligence, the Wechsler Intelligence Scale for children (WISC-4) according to the age [16]. Verbal Comprehension Index (VCI), Visual Spatial Index (VSI), and FIQ scores were expressed as standardized scores (mean 100, SD=15). Auditory memory and working memory were also analyzed in addition to visual spatial structuring with Kho’s cubes, and mental executive functions of planning with the Porteus Labyrinth test and Tower of London test [29]. Visual spatial organization and memory were evaluated with copying and memorization of a complex geometric figure [30], and the visual motor integration by Beery-VMI test [31]. Visual perception was assessed with tangled lines and naming of animals seen in outline from the rear and outlines of mudded fruits (standardized and normed tests) [23]. Kinesthetic perception was assessed with a ‘‘status test’’, in which the child’s arm and finger are positioned in a posture that he must remember and repeat with eyes closed. Language was assessed by the ODEDYS test and the N-EEL battery [32-33].
Regarding handwriting evaluation, we used the French handwriting scale of the Ajuriaguerra test correlated at 0.78 with the BHK French adaptation scale [17], including an assessment of quality of gesture, of the regularity and form of letters, and a specific dysgraphia scale with criteria allowing to detect dysgraphia. Then, we analyzed poor handwriting and dysgraphia (scoring higher than 19, following the scoring guidelines of author). We also extracted the six qualitative handwriting criteria described in the study of Lopez et al. [8].
2.3.2. Neuropsychomotor developmental assessment: To evaluate neuropsychomotor physical functions, all children have been assessed with a standardized developmental neuropsychomotor battery (NP-MOT) [34]. It is a validated battery, applicable to children from 4 years old and has adequate test-retest reliability and internal consistency. Correlation coefficients of the NP-MOT with the Bruininks-Oseretsky Test Motor Proficiency (BOTMP) [35] range from 0.72 to 0.84, for motor coordination and balance. It allows physical standardized assessment of passive/active muscular tone of limbs and axial tone, NSS such as synkinesis, hypotonia, hypertonia with the presence of mild lower limb pyramidal tract abnormality such as the pyramidal stretch reflex (PSR), control and regulation in gross motor tasks (gait, balance, coordination), bodily spatial integration, laterality, and manual praxis, completed by imitation of gestures.
2.3.3. Brain MRI scans: T1, T2, FLAIR, neurovisual assessment and other measures: Anatomical MRI was performed with a 1.5 Tesla (Signa General Electric) scanner using the following sequences: 3D T1-weighted sequence, axial and coronal FSE T2-weighted imaging and coronal FLAIR sequences.
The electro-physiological examination of neurovisual functions consisted of Electroretinogram test (ERG), Patterns of Visual Evoked Potential (VEP) were also recorded to detect brain abnormalities and analyze the visual motor pathways. The stimuli consisted of a high contrast black and white check of 60, 30, and 15 patterns element size in minutes, in a field of 30° as recommended by the International Society for Clinical Electrophysiology in Vision (ISCEV) standards. Amplitudes and peak latencies of ERG and VEP were compared to normative values. This assessment was preceded by ophthalmologic and orthoptic examinations including refraction.
An anamnesis form was also used to collect data about pregnancy and delivery, psychomotor development (e.g., sitting alone, walking), and any difficulties with constructional play, such as puzzles and Lego blocks following a model relative to developmental markers found in previous study [23].
2.4. Statistical analysis
We used R-software [36] for statistical treatment. Data were analyzed according to the intention-to-treat principle. We used p-values at 0.05 to indicate statistical significance and p-values at 0.01 if reached. We also applied the statistical error correction especially Bonferroni method to adjust for multiple comparisons. To analyze dependence between IQS and all DCD variables (continuous outcomes according to one or more classification factors), parametric Multivariate Analysis of Variance (MANOVA) was used. Comparisons of scores for the two groups were performed by Student's T-test (t). For the dichotomous variables the Pearson chi-square test (χ2) was used (Two-way cross-classification between qualitative variables). Variables were coded as 0 for success to a test (meaning no disorder) or 1 for failure (indicating a probable disorder) based on regular scoring (standard deviation: 1 < SD or < 20th percentile according to the test). A Spearman's ρ test (ρ) for non-parametric correlations were used.
3. Results
3.1 Sample descriptive analyzes and parametric Multivariate Analysis of Variance (MANOVA)
Sociodemographic and clinical characteristics of all children are presented in Table 2. Groups (HIQ children with DCD versus typical children with DCD) were matched according to sociodemographic data; age (t=- 0.069, df=18, p=0.945 [95% CI, 95.16 to 124.24]), language disorder (χ2 (1)=0.2, p=0.655 [95% CI, 0.72 to 0.74]), and ophthalmological abnormalities (χ2 (1)=0, p=1 [95% CI, 1 to 1). Only FIQ scores are identified as differentiation criterion of groups (t=12.87, df=18, p<0.0001 [95% CI, 127.36 to 138.01]). However, we found 11% of language disorders, previously undiagnosed, especially dyslexia in HIQ children vs 16% in typical children with DCD, and 32% of ophthalmological abnormalities in both groups.
We identified 100% of handwriting disorders in both groups with high rate of poor handwriting compared to dysgraphia. We distinguished 95% of poor handwriting in both groups and 21% in typical children versus 26% in HIQ children with DCD of dysgraphia. In all our analyzes, no significant difference was shown within the HIQ group.
|
Typical children with DCD (n=19) |
HIQ children with DCD (n=19) |
|
|
Age (months): mean (SD) |
109.84 (33.72) |
109.79 (33.34) |
|
Gender (%) Female Male |
5 95 |
10 90 |
|
IQ mean (SD) FIQ VSI VCI |
100.79 (6.83) 89.21 (11.21) 109.84 (13.78) |
133.68 (11.95) 116 (21.10) 134.89 (13.82) |
Legend: IQ: Intellectual Quotient; FIQ: Full Intellectual Quotient; VSI: Visual Spatial Index); VCI: Verbal Comprehension Index; HIQ: High intellectual quotient; DCD: Developmental coordination disorder; SD: Standard deviation.
Table 2: Sociodemographic and clinical characteristics in HIQ FIQ>120) and typical children (FIQ=90-110) with DCD (n=38).
Regarding DCD, the outcomes show a significant difference between groups regarding frequency of IM-DCD [F (1, 36)=4.67, p=0.035] and VSC-DCD [F (1, 36)=5.28, p=0.03] in favor of HIQ children but not for MX-DCD [F (1, 36)=0.66, p=0.41]. In HIQ children, we found 58% of MX, 5% of IM, and 37% of VSC DCD. About typical children with DCD, there were 21% of MX, 11% of IM, and 68% of VSC. Handwriting disorders were represented in both groups without significant difference whether poor handwriting [χ2 (1)=0.12, p=0.73)] neither dysgraphia [χ2 (1)=0.11, p=0.74)]. Regarding the DCD subtypes, dysgraphia was present only in MX-DCD in typical children with DCD and was present in MX and VSC-DCD in HIQ children with DCD. Otherwise, all subgroups displayed poor handwriting in all DCD subtypes. Regarding qualitative handwriting criteria, we identified the same in both groups: immaturity of gesture (p<0.0001), excessive pressure of the pen on the paper (p<0.0001), and neuro-vegetative responses (p<0.0001). They are identified in all DCD subtypes in typical children while there only found in VSC and MX-DCD in HIQ children (Figures 1and 2):
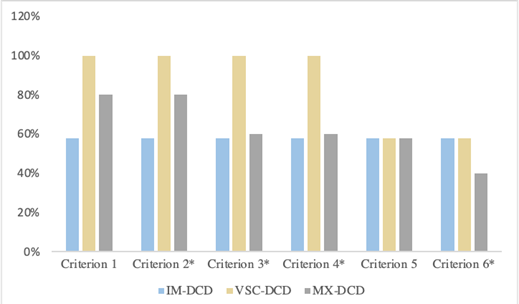
Figure 1: Distribution of qualitative handwriting criteria in HIQ children (FIQ>120) with DCD.
Legend: DCD: Developmental Coordination Disorder; HIQ: High intellectual quotient; IM-DCD: Ideomotor-DCD; VSC-DCD: Visuo-spatial/ or constructional-DCD; MX-DCD: Mixed-DCD; Criterion 1: Irregular handwriting; Criterion 2: Immaturity of handwriting gesture; Criterion 3: Excessive pressure of the open on the paper; Criterion 4: Neuro-vegetative responses; Criterion 5: Trembling; Criterion 6: Slow handwriting velocity; *: significant difference p<0.05.
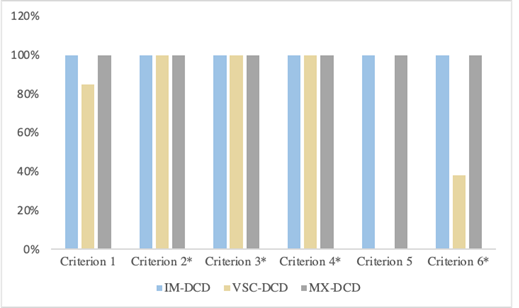
Figure 2: Distribution of qualitative handwriting criteria in typical children (FIQ=90-110) with DCD.
Legend: HIQ: High intellectual quotient.
3.2 Relationship between neuropsychological functions and the level of handwriting disorder (poor handwriting, dysgraphia)
VCI, VSI, and FIQ scores were not significantly associated with either poor handwriting (respectively p=0.58, p=0.52, and p=0.96) or with dysgraphia (respectively p=0.28, p=0.38, and p=0.15) in all children. Only deficit of visual spatial memory is significantly related to dysgraphia in HIQ children (p=0.01). As well, IM-DCD has a significant, strong, and positive correlation with executive functions disorder (p=0.001) in HIQ children with DCD, and negative correlation between Kho’s’ cubes test failure (p=0.006), visual spatial memory (p=0.05) and VSC-DCD. We identified 16% of auditory-memory deficits and 5% of auditory-attention difficulties in typical children with DCD vs respectively 26% and 37% in HIQ children with DCD.
3.3 Relationship between brain MRI investigations and neurovisual functions regarding the level of handwriting disorder (poor handwriting, dysgraphia)
We displayed more abnormal MRI in typical children with DCD (42%) than in HIQ children with DCD (32%) but not significantly. The abnormalities detected were: posterior peri ventricular white matter anomalies, unilateral ventricular dilatation with small left hippocampus, ventricular dilatation and white matter posterior hyper-intensities with anomalies of hippocampus; hyper-intensity on T2 and FLAIR in the left pallidum; multiple punctate white matter hyper-intensities on T2 and FLAIR sequences and dilated Virchow-Robin spaces, small hippocampus (Interuncal Index), dysgenesis of the corpus callosum (Vermis Cerebellar Atrophy), and non-specific cysts. These abnormalities are heterogeneous and non-specific to dysgraphia neither to PSR but were significantly (p=0.014) related to criterion-1 (irregular handwriting) in typical children with DCD.
Regarding electrophysiological examination of neurovisual functions, no significant difference was shown between both groups either dysgraphia or poor handwriting, but we identified significant correlations between dysgraphia and ERG in both groups (typical children with DCD: p=0.04; HIQ children with DCD: p=0.03). In Typical children with DCD, we identified 75% of abnormal horizontal pursuit, 50% of abnormal vertical pursuit, 100% of abnormal VEP, and 100% of abnormal ERG with dysgraphia. Depending poor handwriting in the same group, we have respectively found 61%, 33%, 94%, and 100%. In HIQ children with DCD children, there were 20% of abnormal horizontal pursuit, 60% of abnormal vertical pursuit, 60% of abnormal VEP, and 100% of abnormal ERG with dysgraphia. Depending poor handwriting in this group, we respectively found 65%, 65%, 88%, and 100%. No significant difference was shown between both groups.
Regarding visual perception, we have significantly shown [χ2 (1)=3.6, p=0.05)], more visual gnosis disorder in HIQ children with DCD (89%) than in typical children with DCD (5%), and it was significantly related to dysgraphia in HIQ children (p=0.03).
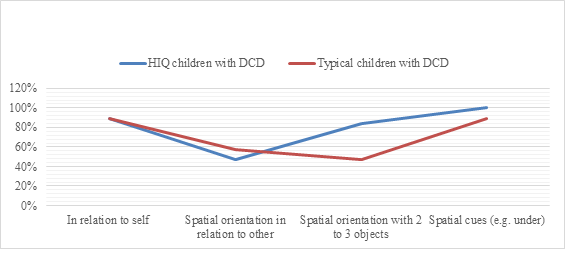
Figure 3: The rate of success in bodily spatial orientation test in HIQ group (FIQ>120) compared to typical children (FIQ=90-110) with DCD.
Legend: HIQ: High intellectual quotient.
3.4 Relationship between neuropsychomotor functions and the level of handwriting disorder (poor handwriting, dysgraphia)
Regarding neuropsychomotor features evaluated by NP-MOT battery, when comparing poor handwriting in typical versus HIQ children with DCD, we have respectively found 56% vs 17% who achieved object spatial orientation skill (χ2 (1)=3.77, p=0.05 [95% CI, 86 to 97]), 39% vs 6% of buccofacial praxis success (χ2 (1)=4.5, p=0.03 [95% CI, 65 to 75]). There was no significant difference between both groups regarding dysgraphia. In contrast, when comparing HIQ and typical children with DCD without poor handwriting, we respectively noted 84% vs 47% of success in object spatial orientation skill (Figure 3).
Regarding functional laterality, we found a statistical difference beginning at QI=130 (χ2 (1)=4.57, p=0.03) [95% CI, 100 to 100]), with 33% of right-handed and 67% of left-handed vs 65% of right-handed and 35% of left-handed respectively.
Regarding dysgraphia, it was significantly related to distal pyramidal tract dysfunction (p=0.01) in whole sample without significant difference between groups. All dysgraphic children showed PSR and significantly adiadochokinesia (p=0.002), dynamic balance disorder (p=0.012), coordination impairments between upper and lower limbs (p=0.01). Frequency of coordination impairments between upper and lower limbs was higher in HIQ children but not significantly (respectively 50% vs 100%, χ2(1)=1.29, p=0.26 [95% CI, 45 to 47) higher dynamic balance disorder (respectively 25% vs 100%, χ2(1)=2.67, p=0.1 [95% CI, 20 to 22]), and higher heterogenous laterality (gestual+psychosocial+spontaneous laterality) but not significantly (respectively 25% vs 100%).
4. Discussion
High IQ as in gifted children is characterized by an early maturation of the central nervous system manifested by early development, especially motricity and language [37]. However, this early maturation can be disturbed by a neurodevelopmental impairment as DCD. Nevertheless, we displayed a lower frequency of IM and VSC-DCD in HIQ children with DCD children while the frequency of MX-DCD is higher compared to typical children with DCD. Neuropsychological assessments results showed a significant and positive correlation between better executive functions and lower frequency of IM pure DCD in HIQ children group compared to typical children with DCD, also a significant negative correlation between Kho’s’ cubes test failure and lower frequency of VSC-DCD. Neuropsychomotor assessment showed a high rate of success of “spatial orientation with 2 or 3 objects” items in favor of HIQ group, highlighting a better mental representation. Thus, children with DCD and having FIQ>120 have better skills of mental imagery and evocation through internal language to plan and to control constructive abilities. No significant difference is shown between children having FIQ>120 and those having FIQ>130. It highlights in HIQ group better activation and connectivity of the fronto-parietal lobe and cerebral cortex improving fluid reasoning [38]. Higher frequency of MX-DCD can be explained, in consistence with literature [23, 25, 27], by specific impairments such as neurological soft signs suggesting adiadochokinesia, and difficulties in buccofacial praxis involving impairment of orofacial motricity in relation with the motor pathway and other comorbidities (high rate of auditory-memory and auditory-attention difficulties in HIQ children with DCD). It is essential not to neglect comorbidities influence on IQ scores for HIQ children as it might lower the FIQ (120-129).
Although it is well known that children with DCD have handwriting difficulties, this is the first study to our knowledge that analyze in-depth characteristics of poor handwriting and dysgraphia. There is no significant difference between the HIQ and typical children with DCD. These findings differed from literature studies [13, 14, 15] attesting a high rate in HIQ children and using non-in-depth evaluation and non-standardized assessments with developmental normative data for age. The high prevalence of poor handwriting in both groups (95%) and the low prevalence of dysgraphia (26% in HIQ vs 21% in typical children with DCD) is in accordance with the literature [9, 22] but not consistent with Graham’s et al. study [3]. This could be explained by the non-differentiation between dysgraphia and poor handwriting. Moreover, we identified a higher rate of dyslexia in typical children with DCD compared to HIQ group but with a lower rate of dysgraphia and any significant correlation. Thus, in the present study, dysgraphia cannot be explained by ophthalmological abnormalities nor by language disorders. Therefore, dysgraphia appeared as a graphomotricity disorder independent from the language disorders (given the rigorous inclusion criteria excluding severe language disorders), and as a comorbidity to DCD. While poor handwriting in both groups seems due to an immaturity coordination of handwriting gesture, characterized by excessive pressure of the pen on paper and neurovegetative responses consecutive to disorders of coordination programming in DCD, affecting the synergic coordination of the upper limb.
Our findings highlight common clinical markers of dysgraphia for the two groups (HIQ and typical children with DCD), affecting the motor and neurovisual pathways, thus underlining a severe diagnosis involving the central nervous system. Dysgraphia was characterized by the association of nearly all handwriting qualitative criteria (1, 2, 3, 4, 6) in both groups. It was significantly related to a distal pyramidal tract dysfunction (PSR) in both groups without significant difference, linked significantly to dynamic balance disorder and to coordination impairments between upper-lower limbs. These results are consistent with the literature [39, 9] suggesting association between motor coordination impairments and dysgraphia, underlining motor programing impairment related to DCD. This may suggest the implication of a dysfunction of the pyramidal pathway, and of the occipito-temporo-parietal cortex [40]. In addition, the significant presence of neurological soft signs as adiadochokinesia, PSR, and higher heterogenous laterality confirms the alteration of the voluntary motor movement underpowered by the premotor cortex (motor pathway) with an alteration in the control of the motoneurons as was observed in the studies of Vaivre-Douret et al. and Chang et al. [39, 41]. At the anatomical level, the current outcomes displayed more abnormal MRI in typical children DCD (42%) than in HIQ children with DCD (32%) but not significantly. Theses abnormalities including the posterior peri-ventricular white matter anomalies, unilateral ventricular dilatation with small left hippocampus, small hippocampus, dysgenesis of the corpus callosum, and non-specific cysts, are heterogeneous and non-specific to dysgraphia neither to PSR but were significantly related to criterion 1 (irregular handwriting) only in typical children with DCD. Although, the criterion-D of DSM-5 requires that the deficit in motor skills in DCD cannot be explained by a neurological impairment (often tested by Babinski’s reflex), our results highlight the relevant contribution of a fine standardized clinical neuromotor examination of neurological soft signs (39%-80%) as in the developmental NP-MOT battery [34]. It should systematically be undertaken by pediatricians, physiotherapists, and occupational therapists to look for motor impairments such as dysdiadochokinesia of the hand and discreet spastic hypertonia (PSR) tested by the dorsiflexion of the foot.
Moreover, children with DCD identified with dysgraphia showed ERG abnormalities in both groups suggesting a disturbance of visual perception. There is also a high rate of abnormal horizontal, vertical pursuits, and abnormal VEP regarding the norms [42]. These findings support the conclusions of previous research using the same outcome measures [28]. Furthermore, we identified a specific significant clinical marker in HIQ children with DCD: visual gnosis impairment rarely examined but associated to cognitive disorders, especially in visual spatial memory, suggesting more involvement of the right cortex, that may explain the high rate of left-handed in very superior IQ (> 130) found in the recent study of Vaivre-Douret et al. [43].
The distribution of dysgraphia regarding DCD subtypes in the both groups suggests that children with DCD and having FIQ>120 consult when they are severely affected about handwriting and cannot used their compensation strategies described above.
5. Conclusions
In conclusion, these findings contribute on one hand to a better understanding and screening with specific markers of poor handwriting vs dysgraphia in DCD, linked to their neurocognitive and neuropsychomotor profiles, and on the other hand, to clarify the impact of the IQ level. The results of this study could support both the hypothesis of a deficit of motor programing in handwriting disorder and the hypothesis of neuromotor noise and neurovisual impairment in dysgraphia. This argue for new approach to implement the remediations. Although we highlighted specific markers basing on a depth multidimensional assessment, this has been a limitation to the sample of subgroups collection in the current study. Furthermore, a group of typically developing children with poor handwriting/dysgraphia without DCD could be interesting to compare in further research.
Acknowledgements
We are grateful to the children and their families who took part in the study. This research was supported by the Institut Universitaire de France (IUF) [grant numbers L17P99, IUF004], and the Foundation of France [Fondation pour la Recherche en Psychomotricité et Maladies de Civilisation (FRPMC) sous l’égide de Fondation de France]. The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the article.
Declaration of Interest Statement
The authors declare that they have no known competing financial interests or personal relationships that might be perceived as posing a conflict or bias could influence the work reported in this paper.
References
- Cornhill H, Case-Smith J. Factors that relate to good and poor handwriting. American Journal of Occupational Therapy 50 (1996): 732-739.
- Costa LJC, Edwards CN, Hooper SR. Writing disabilities and reading disabilities in elementary school students: Rates of co-occurrence and cognitive burden. Learning Disability Quarterly 39 (2016): 17-30.
- Graham S, Struck M, Santoro J, et al. Dimensions of good and poor handwriting legibility in first and second graders: motor programs, visual-spatial arrangement, and letter formation parameter setting. Developmental Neuropsychology 29 (2006): 43-60.
- Goyen TA, Duff S. Discriminant validity of the Developmental Test of Visual-Motor Integration in relation to children with handwriting dysfunction. Australian Occupational Therapy Journal 52 (2005): 109-115.
- Berninger VW, Richards TL, Abbott RD. Differential diagnosis of dysgraphia, dyslexia, and OWL LD: behavioral and neuroimaging evidence. Reading and Writing 28 (2015): 1119-1153.
- Berninger VW, May MO. Evidence-based diagnosis and treatment for specific learning disabilities involving impairments in written and/or oral language. Journal of Learning Disabilities 44 (2011):167-183.
- Fournier del Castillo MC, Maldonado Belmonte MJ, Ruiz-Falcó Rojas ML, et al. Cerebellum atrophy and development of a peripheral dysgraphia: a paediatric case. Cerebellum 9 (2010): 530-536.
- Lopez C, Hemimou C, Vaivre-Douret L. Nature des troubles de l’écriture chez des enfants porteurs d’un Trouble de l’Acquisition de la Coordination (TAC) [Nature of handwriting disorders in children with Developmental Coordination Disorder (DCD)]. In: Entretiens de Bichat « Les Entretiens de Psychomotricité ». Paris: Expansion scientifique française (2016).
- Lopez C, Hemimou C, Golse B, et al. Developmental dysgraphia is often associated with minor neurological dysfunction in children with developmental coordination disorder (DCD). Neurophysiologie Clinique/Clinical Neurophysiology 48 (2018): 207-217.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders, 5th (DSM V). Washington, DC, USA: American Psychiatric Publishing (2013).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders, 4th (DSM IV). Washington, DC, USA: American Psychiatric Publishing (1994).
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental disorders, 4th Edition text revised. (DSM IV-TR). Washington, DC, USA: American Psychiatric Publishing (2000).
- Winner E. The origins and ends of giftedness. American Psychologist 55 (2000): 159-169.
- Terrassier JC. Les enfants intellectuellement précoces [Early intellectually children]. Arch Pédiatrie 16 (2009): 1603-1606.
- Terrassier JC. Les enfants surdoués: ou la précocité embarrassante [Gifted children: or embarrassent precocity]. 12th Paris: ESF Sciences Humaines (2018).
- Wechsler D. WISC-IV: Wechsler Intelligence Scale for Children: Technical and Interpretive Manual. Paris: ECPA-Pearson (2003).
- Hamstra-Bletz L, DeBie J, Den Brinker BPLM. Beknopte Beoordelingsmethode voor Kinderhandschriften [The concise assessment method for children’s handwriting]. Dutch: Lisse: Swets and Zeitlinger (1987).
- Charles M, Soppelsa R, Albaret JM. BHK-Echelle d’évaluation rapide de l’écriture de l’enfant. [BHK-Scale for rapid assessment of children's writing. Paris: Editions of the Center for Applied Psychology]. Paris: ECPA-Pearson (2003).
- Amundson SJ. Evaluation tool of children’s handwriting. Homer, AL: OT Kids (1995).
- Reisman JE. Development and reliability of the research version of the Minnesota Handwriting Test. Phys Occup Ther Pediatr 13 (1999): 41-55.
- Barnett A, Henderson S, Scheib B, et al. The detailed assessment of speed of handwriting. London: H. Assessment (2007).
- Overvelde A, Hulstijn W. Handwriting development in grade 2 and grade 3 primary school children with normal, at risk, or dysgraphic characteristics. Research in Developmental Disabilities 32 (2011): 540-548.
- Vaivre-Douret L, Lalanne C, Cabrol D, et al. Identification de critères diagnostiques des sous-types de troubles de l’acquisition de la coordination (TAC) ou dyspraxie développementale [Identification of diagnostic criteria for subtypes of coordination acquisition disorders (TAC) or developmental dyspraxia]. Neuropsychiatrie de l’Enfance et de l’Adolescence 59 (2011a): 443-453.
- Blank R, Smits-Engelsman B, Polatajko H, et al. European Academy for Childhood Disability (EACD): Recommendations on the definition, diagnosis and intervention of developmental coordination disorder. Developmental Medicine and Child Neurology 54 (2012): 54-93.
- Lalanne C, Falissard B, Golse B, et al. Refining developmental coordination disorder subtyping with multivariate statistical methods. BMC Medical Research Methodology 1 (2012): 107.
- Vaivre-Douret L. Developmental Coordination Disorder: state of art, Neurophysiologie Clinique/Clinical Neurophysiology 44 (2014): 13-23.
- Vaivre-Douret L, Lalanne C. Specific impairments, and predictive markers for Developmental Coordination Disorder subtypes in children: the importance of multidimensional developmental assessments in cluster analysis. Journal of Translational Science 5 (2019): 1-13.
- Robert MP, Ingster-Moati I, Albuisson E, et al. Vertical and horizontal smooth pursuit eye movements in children with developmental coordination disorder. Developmental Medicine and Child Neurology 56 (2014): 595-600.
- Korkman M, Kirk U, Kemp S. Developmental neuropsychological assessment manual. Paris: ECPA-Pearson (2003).
- Rey A. Manuel test de copie d’une figure complexe [Manual of complex Rey’s figure]. Paris: ECPA-Pearson (1960).
- Beery KE, Beery NA. The Beery-Buktenica Developmental Test of Visual-Motor Integration. Beery VMI: With Supplemental Developmental Tests of Visual Perception and Motor Coordination and Steppingstone Age Norms From Birth to Age Six. Administration, Scoring, and Teaching Manual. Minneapolis: Pearson (2010).
- Odédys. Outil de dépistage des dyslexies version 2 [Dyslexia screening tool, 2nd version]. Grenoble: Cogni-Sciences IUFM (2005).
- Chevrie-Muller C, Plaza M. N-EEL: nouvelles épreuves pour l'examen du langage [New tests for language examination]. Paris: ECPA-Pearson (2001).
- Vaivre-Douret L. Batterie d’évaluation des fonctions neuro-psychomotrices (NP-MOT) [Tests battery of developmental neuro-psychomotor functions in children (NP-MOT)]. Paris: ECPA-Pearson (2006).
- Bruininks O. Buininks-Oseretsky Test of Motor Profiency. Circle Pines, MN: American Guidance Service (1978).
- R Core Development Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2004).
- Vaivre-Douret L. Developmental and cognitive characteristics of high-level potentialities (highly gifted) children. International Journal of Pediatrics (2011b): 2011.
- Nusbaum F, Hannoun S, Kocevar G, et al. Hemispheric Differences in White Matter Microstructure between Two Profiles of Children with High Intelligence Quotient vs. Controls: A Tract-Based Spatial Statistics Study. Frontiers in Neuroscience 11 (2017): 173.
- Vaivre-Douret L, Lalanne C, Golse B. Developmental coordination disorder, an umbrella term for motor impairments in children: nature and co-morbid disorders. Frontiers in psychology 7 (2016): 502.
- Hamdioui S, Vaivre-Douret L. Sémiologie de la dysgraphie chez l’enfant porteur d’un trouble du développement de la coordination en fonction du quotient intellectual [Semiology of dysgraphia in children with a developmental coordination disorder related to intelligence quotient]. Neurophysiologie Clinique/Clinical Neurophysiology (2020): Abstract in press.
- Chang SH, YU NY. Characterization of motor control in handwriting difficulties in children with or without developmental coordination disorder. Developmental Medicine and Child Neurology 52 (2010): 244-250.
