Clinical Evaluation of General Anaesthesia using Ketamine Hydrochloride with and without Diazepam in Sheep
Article Information
Mst. Antora Akter1§, Kazi Afsana Homayra Orchy1§, Md. Moshiur Rahman Khan1, Mahdi Hasan2, Moinul Hasan1, Md. Mahmudul Alam1,*
1Department of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural, University, Mymensingh-2202, Bangladesh
2Department of Microbiology and Hygiene, Faculty of Veterinary Science, Bangladesh Agricultural, University, Mymensingh-2202, Bangladesh
§Contributed equally.
?Corresponding Author: Md. Mahmudul Alam, Professor, Department of Surgery and Obstetrics, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, E-mail: mahmud.dso@bau.edu.bd
Received: 14 July 2020; Accepted: 13 September 2020; Published: 22 September 2020
Citation: Mst. Antora Akter, Kazi Afsana Homayra Orchy, Md. Moshiur Rahman Khan, Mahdi Hasan, Moinul Hasan, Md. Mahmudul Alam. Clinical Evaluation of General Anaesthesia using Ketamine Hydrochloride with and without Diazepam in Sheep. Archives of Veterinary Science and Medicine 3 (2020): 63-75.
View / Download Pdf Share at FacebookAbstract
The study has been carried out to evaluate the clinical efficacy of ketamine with or without diazepam in sheep. Six healthy sheep were used in this study and randomly divided into two groups; Group A (atropine-diazepam-ketamine) and Group B (atropine-ketamine). Anaesthesia was induced with administration of diazepam (0.4 mg/kg b.wt) and ketamine (15 mg/kg b.wt) intramuscularly (IM) after five minutes of IM administration of atropine sulphate (0.05 mg/kg b.wt) in group A and ketamine (22 mg/kg b.wt) IM after five minutes of IM injection of atropine sulphate (0.05 mg/kg b.wt) in group B. Clinical parameters were recorded before and after (5, 10, 15, 20, 25, 30 mins, 1h and 24h post- recovery) administration of peripheral blood was collected at the same interval for haemato-biochemical observations. The clinical, hematological and serum biochemical profile differed from the values obtained at pre-experimental control level in both groups A and B. However, in group A, the changes were smooth and steady whereas these were irregular, fluctuated and inconsistent in certain clinical and biochemical parameters in sheep of group B. The present study indicates that the use of atropine-diazepam-ketamine anaesthesia is safe and useful in sheep than atropine-ketamine anaesthesia for major invasive procedures.
Keywords
Anaesthesia; Diazepam; Ketamine; Sheep
Anaesthesia articles, Diazepam articles, Ketamine articles, Sheep articles
Article Details
1. Introduction
General anaesthesia reversibly modifies consciousness, without global brain shutdown. It can produce different states of consciousness depending on the anaesthetic agent and dosage, including a complete absence of subjective experience, a conscious experience without the perception of the environment or episodes of focused consciousness with the awareness of the environment [1]. Although ruminant usually undergoes a variety of surgical procedures under a combination of physical restraint, sedation, and local or regional anaesthesia, general anaesthesia may be preferred to other techniques, especially in the case of complex and prolonged surgical procedures, where the technical and anatomical aspects of the surgical procedure warrant absolute control over pain and movement during operation. Various forms of anaesthetic medications are used in the practice of anaesthesia by small ruminants while certain hazards are involved in anaesthetic practices. To minimize anaesthetic complications, balanced anaesthesia is typically followed [2, 3]. The balanced anaesthetic approach involves combining two or more anaesthetic drugs to achieve the desired components of general anaesthesia while mitigating the adverse effects on cardiopulmonary function of individual drugs [4]. Ketamine is a versatile agent with such a unique profile that allows it to be used safely in a range of circumstances around the world including animals for pets and zoos [4, 5]. It raises heart rate and mean arterial pressure, enhances cardiovascular functions and can cause undesirable effects including muscle hypertonicity, myoclonus and seizures when used as a single agent [6]. Use of ketamine was reported as a single agent and in combination with other hypnotics such as benzodiazepines [7]. In conjunction with ketamine, diazepam helps to relieve harmful cardiovascular effects of ketamine and displays anticonvulsant, amnestic, and muscle relaxing effects by central pathways [8]. Used alone, because of its association with poor muscle relaxation, tachycardia, and catalepsy or muscle rigidity, ketamine does not provide a true anaesthetic state in dogs even at high dosages. Therefore, it is commonly used in combination with other premedicants to minimize the adverse effects in pet [8] and large ruminants [9]. However, the ketamine effect has rarely been studied in sheep that are very frequently subjected to various surgical interventions. Therefore, determining the ketamine effects in combination with other sedative agents can help to achieve the safest combination for surgical procedures in small ruminants particularly in sheep. Thus, the goal of the present study is to test the anaesthetic effect of ketamine with or without premedicants in sheep.
2.Materials and Methods
2.1 Experimental animals
With the permission and approval from the Animal Experiment and Ethics Committee (AEEC) of the Department of Surgery and Obstetrics, Bangladesh Agricultural University, Mymensingh, (permission number: AEEC/DSO-BAU/02/2017, the experiment was carried out on six healthy female nonpregnant indigenous sheep with 6-8 months old weighing between 12- 14 kg. The animals were randomly divided into two groups comprising three animals. The animals were kept under the same management and nutritional conditions during the anaesthetic trials. Animals were kept off feed one night before the time of anaesthesia but had access to water ad libitum. Each animal was allowed to acclimatize to a room with temperature for at least 30 min before each experiment was commenced. Baseline cardiovascular, pulmonary, and body temperature measurements were obtained in conscious animals before the injection of any drugs. The skin wool overlying the left jugular vein was clipped, shaved, and washed with 70% alcohol and povidone-iodine before the experiment for aseptic blood sampling. After primary preparation and monitoring of the cardiovascular function, 2×3 ml blood samples (one for determination of normal levels of biochemical parameters and the other for normal hematological parameters) were collected from each animal via the left jugular vein.
Table 1: Anaesthetic agents with induction protocol.
|
Groups |
Anaesthetic agents |
Dose |
Time of administration |
Manufacturer |
|
Group A |
Atrovet (Atropine Sulphate 1 mg/ml) |
0.05 mg/kg body weight (IM) |
5 minutes prior to induction of anaesthesia |
Techno Drugs Limited, Narsingdi, Bangladesh |
|
Sedil (Diazepam 5 mg/ml) |
0.4 mg/kg body weight (IM) |
0 min (baseline) |
Square Pharmaceuticals, Dhaka,Bangladesh |
|
|
Ketalar (Ketamine hydrochloride 50 mg/ml) |
15 mg/kg body weight (IM) |
5 minutes following diazepam administration |
Popular Pharmaceuticals, Tongi, Bangladesh |
|
|
Group B |
Atrovet (Atropine Sulphate 1 mg/ml) |
0.05 mg/kg body weight (IM) |
5 minutes prior to induction of anaesthesia |
Techno Drugs Limited, Narsingdi, Bangladesh |
|
Ketalar (Ketamine hydrochloride 50 mg/ml) |
22 mg/kg body weight (IM) |
0 min (baseline) |
Popular Pharmaceuticals, Tongi, Bangladesh |
2.2 Anaesthesia protocol
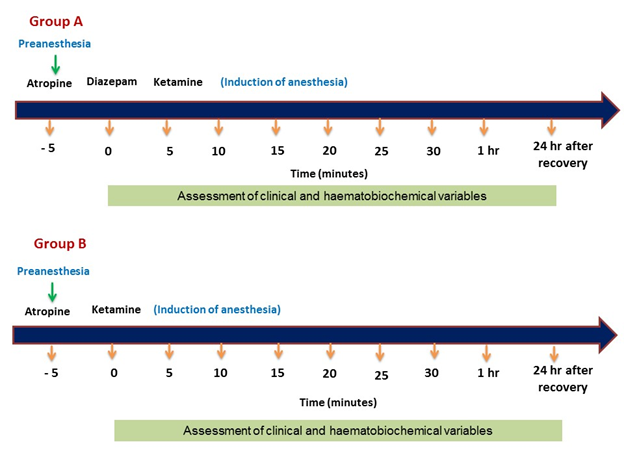
The anaesthesia protocol and the agent administered in prepared animals during the study are illustrated in Table 1 and Figure 1. The prepared animals were randomly divided into two groups; group A: Atropine-Diazepam –Ketamine and group B: Atropine-Ketamine.
2.3 Assessment of physiological parameters
The physiological parameters; (body temperature, heart rate, respiratory rate, and peripheral capillary oxygen saturation) were recorded before administration of the anaesthetics (baseline, 0), and then at 5, 10, 15, 20, 25, 30, 1h post-induction and 24h post-recovery. Heart rate, body temperature, and peripheral capillary oxygen saturation (SpO2) were monitored and recorded from Patient Monitor (Oxysmart- M, Oxycon Co. Ltd, China).
For SpO2, pulse, and body temperature, the machine sensor was attached with the tongue of the animal. Simultaneously, rectal temperature and heart rate were recorded with the help of a thermometer and stethoscope respectively to avoid error in patient monitor. Respiratory rate was recoded with a stethoscope and visual observation of chest movement.
2.4 Monitoring of anaesthesia
After the administration of premedicants and anaesthetics, the animals were kept under close observation. The onset of anaesthesia was judged by the presence of muscular relaxation and absence of swallowing reflexes. The depth of anaesthesia was checked by pricking the skin and underlying tissues with a sterile needle.
2.5 Assessment of blood pictures and serum biochemical parameters
For measurement of blood pictures, blood samples were collected in tubes pre-coated with heparin to avoid blood coagulation at 0, 5, 10, 15, 20, 25, 30mins, 1h post-induction, and 24h post-recovery of anaesthesia in both the groups. A fraction of blood was transferred in the clot activator tube for serum biochemical tests including Alanine Aminotransferase (ALT), Aspartate Aminotransferase (AST), Blood Urea Nitrogen (BUN), Total Protein (TP) and Creatinine.
2.6 Statistical analysis
Data were expressed as mean ± standard error of mean in each group. Data were analyzed by SPSS Statistics version 22 using t-test and one-way ANOVA test. A probability value less than or equal to 0.05 (p ≤ 0.05) was considered statistical where applicable.
3.Results
3.1 Physiological effects of single and combined anaesthetic regimens
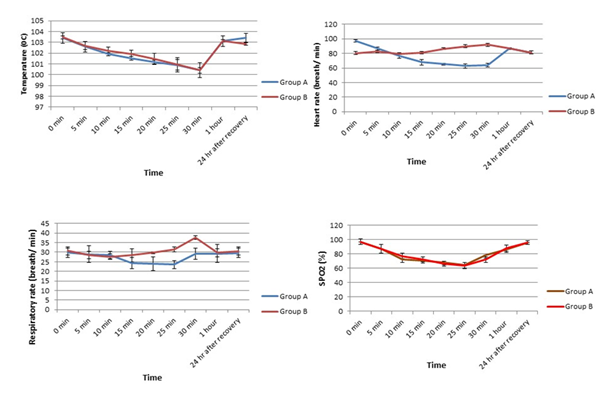
Heart rate, respiratory rate, rectal temperature, and SpO2 were at recorded before and every 5 minutes after administration of the anaesthetics in both the groups up to 24h after recovery. From Figure-2, it was revealed that both in group A and group B, the rectal temperature decreased slowly up to 30 minutes and then started elevating gradually towards the initial level from 1h onward.
Distinct different pattern in heart rate was observed in group A and group B. Heart rate remarkably decreased after induction of anaesthesia in group A up to 25mins and then started to increase until 1h, again declined at 24h post-recovery (Figure 2). On the other hand, the administration of ketamine alone in group B led to the elevation of heart rate at 10 min post-induction and followed the pattern until 30mins post-induction. The heart rate then declined at 1h post-induction and the trend followed even after 24h of recovery (Figure 2).
The respiratory rate in group A was declined significantly from 5mins to 25mins following combination anaesthesia and then returned to its initial control value. In group B, a fluctuated pattern of respiration was observed after the injection of ketamine alone (Figure 2). In case of SpO2, there was a similar changing pattern in groups A and B. In both groups, SPO2 decreased until 25mins post-induction and then gradually returned to its basal value (Figure 2).
3.2 Blood pictures of anaesthetized sheep following Diazepam- Ketamine and Ketamine Injection
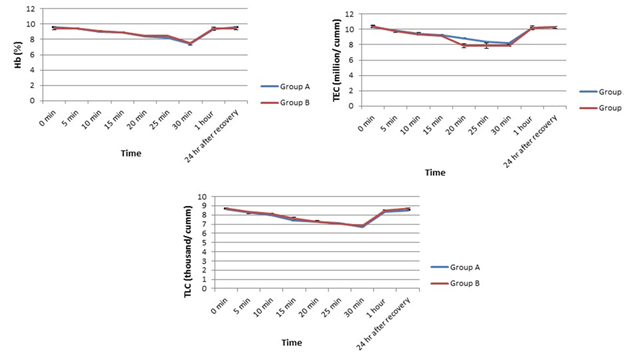
In this experiment, we found a gradual fall down in hemoglobin throughout the experiment in both groups until 30mins post-induction from their pre-anaesthetic control values and then the values started elevating toward the basal level in both the groups (Figure 3). On the other hand, a remarkable change in TLC was recorded in both Group A and B compared to their basal values (Figure 3).
In both groups, the values were gradually decreased until 30mins post-induction and then elevated toward the initial control value which followed even after 24h of recovery. In terms of TEC, it decreased in both the groups until 30mins but soon after that, TEC increased in both to restore their basal level in a similar pattern in group A and B.
3.3 Anaesthetic effects on serum biochemistry
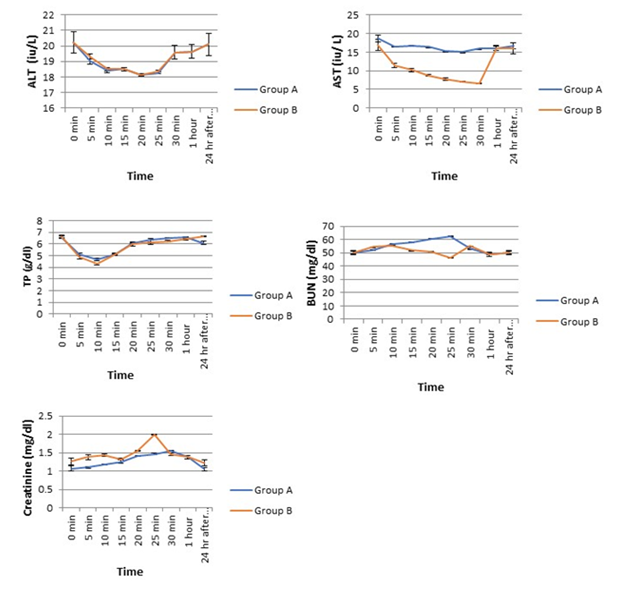
Changes in ATL was similar in both groups. ALT rapidly decreased and after 10-15mins it started elevating and became stable at 24h after recovery (Figure 4). On the other hand, there was marked dissimilarity in the changes of AST in between the groups. Combination anaesthesia did not lead to any major alternation of AST whereas ketamine alone drastically reduced AST from pre-experiment control value. After 30min of ketamine injection, it increased and become stable until complete recovery (Figure 4). The TP decreased from initial control value and after 10 mins TP level was increased and become stable from 30min post-induction onward (Figure 4).
We found a gradual increase of BUN until 25mins of induction in combination anaesthesia group and then decreased toward the basal value at 30mins post-induction. In Group B, a highly fluctuating trend in BUN value was registered until the end of the experiment. Level of serum creatinine exhibited a steady increase followed by a decrease toward the basal value after 30mins of injection in group A, whereas, in group B, a highly fluctuating and inconsistent changing pattern was registered.
Table 2: Induction and duration of anaesthetic combinations used in sheep.
|
Anaesthetic combination |
Induction time (min) |
Duration of anaesthesia (min) |
|
Group A (atropine-diazepam- ketamine) |
11.33±0.08 |
41.67±0.10 |
|
Group B (atropine- ketamine) |
18.00±0.07 |
23.67±0.05 |
3.4 Anaesthetic efficacy of tested combination
The effects of the different anaesthetic combination on the onset of induction and duration of anaesthesia in sheep is presented in Table 2. Results proved that the duration of general anaesthesia was much higher (41.67±0.10 mins) when ketamine was used with a pre-anaesthetic and muscle relaxant, diazepam than ketamine alone (23.67±0.05 mins).
4. Discussion
Ideal balanced anaesthesia produces sleep, amnesia, analgesia and muscle relaxation with no or minimal adverse effects. Before administration of the anaesthetic compounds, sedative and analgesic drugs should be given to induce anaesthesia in sheep [9]. The use of such pre-anaesthetics has many advantages in terms of decreasing anxiety, reducing the dosage of anaesthetic medications, supplying analgesia during subsequent surgery or treatment and contributing to smooth induction and recovery from anaesthesia [9].
In our study, we found prominent variation in certain clinical and biochemical traits in between the anaesthetic groups. After a significant decrease in rectal temperature in both groups at 5 minutes after administration of Diazepam-Ketamine and Ketamine alone, temperature gradually increased after 25 min and returned to the level of initial control value. This was per the findings of Gebremedhin et al. [10]. The decrease in rectal temperature might be due to the blocking of the hypothalamic thermo-regulatory center by the anaesthetics [11, 12].
Following induction, a decrease in heart rate may be attributed to the bradycardia effect which was somewhat similar to that noted by Gebremedhin et al. [10]. The decrease in heart rate could be due to inhibition of the release of the neurotransmitter noradrenaline or depression of the sympathetic activity [13]. The changes in heart rate in group A were smooth and steady than that of group B where a fluctuated pattern was recorded and that may be because of light anaesthesia or as a result of the cardiac stimulatory effect of ketamine.
There was a significant decrease in respiratory rate (RR) in group A at 30 mins and soon after that it gradually increased towards the basal value which became stable at the end of the experiment. A decrease in respiratory rate after induction is also documented by [14]. The profound decrease in respiratory rate after induction may be attributed to the respiratory depression of ketamine in the initial stage [15]. In group B (Ketamine alone), we found fluctuated and inconsistent patterns of respiration at different time points throughout the experiment. Our results on the changes in SpO2 following induction is similar to the report of Abdel-Hady et al. [16]. The initial decrease in SpO2 might be due to the depression of the ventilatory function of the lungs caused by the anaesthetic agents. Low pulse oximeter readings are indicative of reduced arterial oxygenation and diminished tissue perfusion during the period of anaesthesia [13, 17].
In both groups, we found a decrease in Hb concentration, TLC, and TEC from pre-anaesthetic control value until a certain level and then gradually increased and became stable after complete recovery. This finding agreed with the findings of previous researchers [18-20]. The pooling of circulating blood cells in the spleen and other reservoirs secondary to decreased sympathetic activity could be the reason for a decrease in hemoglobin concentration, TEC, and TLC after administration of ketamine [21, 22].
We observed a decrease in ALT value from its pre-anaesthetic control value up to 10mins in group A and up to 15mins in group B, then again it increased. This finding agrees with the report of Çamkerten et al. [23]. The decrease in ALT activity in our study might be due to less alteration in cell membrane permeability in response to hemodynamic changes by the anaesthetic agents [4]. In this study, the value of TP fluctuated significantly in both group A and group B until a certain duration of anaesthesia and then returned to its baseline values. Kamal et al. [18] showed consistent, significant changes in TP in dogs after ketamine administration. Ismail et al. [17] emphasized that there were no significant changes in TP during the ketamine and diazepam anaesthesia in sheep and goats, which contrast to our findings. The increase in TP activities might be due to the suppression of liver function during ketamine anaesthesia [24].
BUN and creatinine were inversely affected by anaesthetics at different time intervals from pre anesthetized control value in both group A and group B. Chauhan and Pandey [25] showed a significant increase in BUN and creatinine after fentanyl-ketamine anaesthesia in dogs. The increase in serum BUN levels has been reported by Kamal et al. [18] after ketamine and xylazine administration in dogs. Ketamine might reduce renal cortical blood flow by constricting the blood vessels, hence decreases glomerular filtration rate and increases serum BUN and creatinine levels [4]. Long surgical anaesthesia time was achieved in groups A. This could be attributed to synergistic actions of the drugs used. Diazepam acts in synergism with ketamine to produce a better analgesic effect [19].
5. Conclusions
Based on the results from this study, intramuscular administration of ketamine as bolus doses produced safe and excitement free induction of anaesthesia when the patient is pre-treated with diazepam. Considering the parameters measured, diazepam-ketamine combination is graded superior to ketamine alone because of smoother induction, a long length of anaesthesia with minimal or no adverse dynamics on clinical and biochemical parameters.
Conflict of Interest
The authors do not have any conflict of interest.
References
- Bonhomme V, Staquet C, Montupil J, et al. General Anaesthesia: A Probe to Explore Consciousness. Frontiers in Systems Neuroscience 13 (2019): 36.
- Mohiuddin M, Hasan MM, Shohag M, et al. Surgical management of limb fractures in calves and goats. Bangladesh Veterinary Journal 52 (2018): 46-56.
- Alam MM, Juyena NS, Alam MM, et al. Use of wire suture for the management of fractures in calves. IOSR Journal of Agriculture and Veterinary Science 7 (2014): 90-96.
- Hossen MJ, Alam MM, Hashim MA, et al. Certain haemato-biochemical values in anesthetized dogs. Bangladesh Veterinary Journal 38 (2004): 97-104.
- Ashraf MB, Akter MA, Saha M, et al. Clinicopathological Evaluation on Capture Myopathy Due To Chemical Immobilization in Spotted Deer. Turkish Journal of Veterinary Research 3 (2019): 73-79.
- Marland S, Ellerton J. Ketamine: use in anaesthesia. Review. CNS Neuroscience and Therapeutics 19 (2013): 381-389.
- Craven R, Alkhafaji R. Ketamine in Anaesthetic Practice, ATOTW 27. World Anaesthesia Tutorial of the week (2006).
- Yohannes G, Negash G, Fantay Comparison the effects of ketamine alone and ketamine-diazepam combination in dogs of local breed in Mekelle, Ethiopia. MOJ Surgery 6 (2018): 119-124.
- Malik V. An update on general anaesthesia in ruminants. Indian Journal of Veterinary Surgery 35 (2014): 1-11.
- Gebremedhin Y, Negash G, Fantay H. Clinical Evaluation of Anaesthetic Combinations of Xylazine-Ketamine, Diazepam- Ketamine and Acepromazine-Ketamine in Dogs of Local Breed in Mekelle, Ethiopia. SOJ Veterinary Science 4 (2018): 1-9.
- Afshar S, Baniadam A, Marashipour P. Effect of xylazine ketamine on arterial blood pressure, arterial blood pH, blood gases, rectal temperature, heart rate and respiratory rate in goat. Bulletin of the Veterinary Institute in Pulawy 49 (2005): 481-484.
- Jaman MM, Mishra P, Rahman M, et al. Clinical and laboratory investigation on the recurrence of the umbilical hernia after herniorrhaphy in bovine calves. Journal of Bangladesh Agricultural University 16 (2018): 464-470.
- Munif MR, Alam MM, Alam MR. Pulse oximetry and clinical changes during electrosurgery in dogs anaesthetized with xylazine-thiopentone and xylazine-ketamine combinations. Research of Agriculture, Livestock and Fisheries 7 (2020): 97-105.
- Ragab GH, Seif MM, Fatma M, et al. Comparison of quality of anaesthetic effect between intramuscularly administered ketamine, intravenously administered ketamine and intravenously administered propofol in diazepam premedicated goats. Journal of Veterinary Medicine Research 24 (2017): 247-256.
- Hodgkinson O, Dawson L. Practial anaesthesia and analgesia in sheep, goats and calves. In Practice 29 (2007): 596-603.
- Abdel-Hady AAA, Abdelbasset KM, Soliman AS. Comparative experimental study on two designed intravenous anaesthetic combinations in dogs. EXCLI Journal 16 (2017): 770-779.
- Ismail ZB, Jawasreh K, Al-Majali A. Effects of xylazine–ketamine–diazepam anaesthesia on Blood cell counts and plasma biochemical values in sheep and goats. Comparative Clinical Pathology 19 (2010): 571-574.
- Kamal MM, Hasan, M, Akter MA, et al. Effects of two anaesthetic combinations (xylazine-ketamine and xylazine-propofol) on clinical and hematobiochemical profile in dogs. Bangladesh Veterinary Journal 53 ( 2019): 9-17.
- Mahmud A, Shaba P, Yisa H, et al. Comparative efficacy of Diazepam, Ketamine and Diazepam-Ketamine combination for sedation or anaesthesia. Journal of Advanced Veterinary Animal Research 1 (2014): 107-113.
- Sankar P, Jastin WB, Rao GD, et al. Cardiopulmonary and haematobiochemical alterations during ketamine or propofol anaesthesia in acepromazine-xylazine premedicated horses. Indian Journal of Veterinary Surgery 32 (2011): 23-26.
- Al-Redah SAA. A comparative study between using of Midazolam-Ketamine and Diazepam-Ketamine combinations as anaesthetic program in sheep. Al;Qadasiya. Journal of Veterinary Medical Science 10 (2011):15-18.
- Hossen MJ, Juyena NS, Hashim MA, et al. Evaluation of certain anaesthetic drugs in Dogs. Bangladesh Veterinary Journal 38 (2004): 87-96.
- Çamkerten I, Sindak N, Özkurt G, et al. Effect of Ketamine-Xylazine Anaesthesia on Some Hematological and Serum Biochemical Values of Bozova Greyhounds. Harran Üniversitesi Veteriner Fakültesi Dergisi 2 (2013): 27-31.
- Bateson AN. Basic pharmacologic mechanism involved in benzodiazepine tolerance and withdrawal. Current Pharmaceutical Design 8 (2002): 5-21.
- Chauhan A, Pandey SK. Haemato-Biochemical effects of epidural fentanyl-ketamine combinations in dogs. The Journal of Bombay Veterinary College 14 (2006): 96-99.