Clinical Assessment of NanoFat as Treatment for Partial Rotator Cuff Tear
Article Information
Ronald B Barreto1*, Ricardo E R Silva Jr2, Juliana M C Lira2, Joao M M de A Santos3, Bernard P R Barbosa3 and Jose F S D Lana4, David Sadigursky1
1Department of Orthopedics, Instituto Repara, Aracaju, Sergipe, Brazil.
2Federal University of Sergipe, Aracaju, Sergipe, Brazil.
3Tiradentes University, Aracaju, Sergipe, Brazil.
4Department of Orthopedics, Brazilian Institute of Regenerative Medicine, Idaiatuba, Sao Paulo, Brazil.
*Corresponding Author: Ronald B Barreto, Department of Orthopedics, Instituto Repara, Aracaju, Sergipe, 49025740, Brazil.
Received: 26 June 2023; Accepted: 10 July 2023; Published: 17 July 2023
Citation:
Ronald B Barreto, Ricardo E R Silva Jr, Juliana M C Lira, Joao M M de A Santos, Bernard P R Barbosa, Jose F S D Lana, David Sadigursky. Clinical assessment of NanoFat as treatment for Partial Rotator Cuff Tear. Journal of Orthopedics and Sports Medicine. 5 (2023): 316 - 324.
View / Download Pdf Share at FacebookAbstract
Despite the various available options for managing Rotator Cuff Tear, achieving definitive treatment remains a major challenge. Considering a conservative treatment failure rate of 41% and a retear rate of 21%, new therapeutic approaches are necessary. The utilization of mesenchymal stem cells has garnered increasing attention due to their capacity for tissue regeneration and reduction of local joint inflammation. The goal of this study is to evaluate the impact of NanoFat intratendineous injection on patients diagnosed with Rotator Cuff Tear. This study involved a total of 14 shoulders (7 right and 7 left) from 12 patients diagnosed with Rotator Cuff Tear, who underwent one-year follow-up after ultrasound-guided intratendineous NanoFat injection. The collection of material for injection into the affected tendon was obtained through abdominal liposuction. All patients underwent evaluations at 1 week, 1-, 3-, 6-, and 12-months post-procedure. During all follow-up visits, the UCLA, SPADI, and CMS questionnaires were administered as primary outcome measures to assess the clinical impact of the proposed intervention. Following one year of the procedure, significant improvements of 79.88% and 76% were observed in the UCLA and SPADI scores, respectively (p < 0.001). The CMS questionnaire did not exhibit a statistically significant difference in scores at the conclusion of the evaluation. Regarding sociodemographic data, the mean age observed was 50 years, the mean BMI was 28.1kg/m2, the mean weight was 75.1kg, and mean height was 1.63m. This study has provided evidence of functional improvement and pain reduction in patients with Rotator Cuff Tear undergoing NanoFat treatment.
Keywords
Rotator cuff injuries; Adipocytes; Mesenchymal stem cells; Shoulder pain; Signal transduction; Case report
Orthopedics articles Orthopedics Research articles Orthopedics review articles Orthopedics PubMed articles Orthopedics PubMed Central articles Orthopedics 2023 articles Orthopedics 2024 articles Orthopedics Scopus articles Orthopedics impact factor journals Orthopedics Scopus journals Orthopedics PubMed journals Orthopedics medical journals Orthopedics free journals Orthopedics best journals Orthopedics top journals Orthopedics free medical journals Orthopedics famous journals Orthopedics Google Scholar indexed journals Non-steroidal anti-inflammatory drugs articles Non-steroidal anti-inflammatory drugs Research articles Non-steroidal anti-inflammatory drugs review articles Non-steroidal anti-inflammatory drugs PubMed articles Non-steroidal anti-inflammatory drugs PubMed Central articles Non-steroidal anti-inflammatory drugs 2023 articles Non-steroidal anti-inflammatory drugs 2024 articles Non-steroidal anti-inflammatory drugs Scopus articles Non-steroidal anti-inflammatory drugs impact factor journals Non-steroidal anti-inflammatory drugs Scopus journals Non-steroidal anti-inflammatory drugs PubMed journals Non-steroidal anti-inflammatory drugs medical journals Non-steroidal anti-inflammatory drugs free journals Non-steroidal anti-inflammatory drugs best journals Non-steroidal anti-inflammatory drugs top journals Non-steroidal anti-inflammatory drugs free medical journals Non-steroidal anti-inflammatory drugs famous journals Non-steroidal anti-inflammatory drugs Google Scholar indexed journals Rotator Cuff Tendinopathy articles Rotator Cuff Tendinopathy Research articles Rotator Cuff Tendinopathy review articles Rotator Cuff Tendinopathy PubMed articles Rotator Cuff Tendinopathy PubMed Central articles Rotator Cuff Tendinopathy 2023 articles Rotator Cuff Tendinopathy 2024 articles Rotator Cuff Tendinopathy Scopus articles Rotator Cuff Tendinopathy impact factor journals Rotator Cuff Tendinopathy Scopus journals Rotator Cuff Tendinopathy PubMed journals Rotator Cuff Tendinopathy medical journals Rotator Cuff Tendinopathy free journals Rotator Cuff Tendinopathy best journals Rotator Cuff Tendinopathy top journals Rotator Cuff Tendinopathy free medical journals Rotator Cuff Tendinopathy famous journals Rotator Cuff Tendinopathy Google Scholar indexed journals Joint movement articles Joint movement Research articles Joint movement review articles Joint movement PubMed articles Joint movement PubMed Central articles Joint movement 2023 articles Joint movement 2024 articles Joint movement Scopus articles Joint movement impact factor journals Joint movement Scopus journals Joint movement PubMed journals Joint movement medical journals Joint movement free journals Joint movement best journals Joint movement top journals Joint movement free medical journals Joint movement famous journals Joint movement Google Scholar indexed journals Tendinopathy articles Tendinopathy Research articles Tendinopathy review articles Tendinopathy PubMed articles Tendinopathy PubMed Central articles Tendinopathy 2023 articles Tendinopathy 2024 articles Tendinopathy Scopus articles Tendinopathy impact factor journals Tendinopathy Scopus journals Tendinopathy PubMed journals Tendinopathy medical journals Tendinopathy free journals Tendinopathy best journals Tendinopathy top journals Tendinopathy free medical journals Tendinopathy famous journals Tendinopathy Google Scholar indexed journals Non-surgical treatment articles Non-surgical treatment Research articles Non-surgical treatment review articles Non-surgical treatment PubMed articles Non-surgical treatment PubMed Central articles Non-surgical treatment 2023 articles Non-surgical treatment 2024 articles Non-surgical treatment Scopus articles Non-surgical treatment impact factor journals Non-surgical treatment Scopus journals Non-surgical treatment PubMed journals Non-surgical treatment medical journals Non-surgical treatment free journals Non-surgical treatment best journals Non-surgical treatment top journals Non-surgical treatment free medical journals Non-surgical treatment famous journals Non-surgical treatment Google Scholar indexed journals Orthopedic specialists articles Orthopedic specialists Research articles Orthopedic specialists review articles Orthopedic specialists PubMed articles Orthopedic specialists PubMed Central articles Orthopedic specialists 2023 articles Orthopedic specialists 2024 articles Orthopedic specialists Scopus articles Orthopedic specialists impact factor journals Orthopedic specialists Scopus journals Orthopedic specialists PubMed journals Orthopedic specialists medical journals Orthopedic specialists free journals Orthopedic specialists best journals Orthopedic specialists top journals Orthopedic specialists free medical journals Orthopedic specialists famous journals Orthopedic specialists Google Scholar indexed journals Connective tissue articles Connective tissue Research articles Connective tissue review articles Connective tissue PubMed articles Connective tissue PubMed Central articles Connective tissue 2023 articles Connective tissue 2024 articles Connective tissue Scopus articles Connective tissue impact factor journals Connective tissue Scopus journals Connective tissue PubMed journals Connective tissue medical journals Connective tissue free journals Connective tissue best journals Connective tissue top journals Connective tissue free medical journals Connective tissue famous journals Connective tissue Google Scholar indexed journals Physical therapy articles Physical therapy Research articles Physical therapy review articles Physical therapy PubMed articles Physical therapy PubMed Central articles Physical therapy 2023 articles Physical therapy 2024 articles Physical therapy Scopus articles Physical therapy impact factor journals Physical therapy Scopus journals Physical therapy PubMed journals Physical therapy medical journals Physical therapy free journals Physical therapy best journals Physical therapy top journals Physical therapy free medical journals Physical therapy famous journals Physical therapy Google Scholar indexed journals Magnetic resonance imaging articles Magnetic resonance imaging Research articles Magnetic resonance imaging review articles Magnetic resonance imaging PubMed articles Magnetic resonance imaging PubMed Central articles Magnetic resonance imaging 2023 articles Magnetic resonance imaging 2024 articles Magnetic resonance imaging Scopus articles Magnetic resonance imaging impact factor journals Magnetic resonance imaging Scopus journals Magnetic resonance imaging PubMed journals Magnetic resonance imaging medical journals Magnetic resonance imaging free journals Magnetic resonance imaging best journals Magnetic resonance imaging top journals Magnetic resonance imaging free medical journals Magnetic resonance imaging famous journals Magnetic resonance imaging Google Scholar indexed journals Rheumatologic articles Rheumatologic Research articles Rheumatologic review articles Rheumatologic PubMed articles Rheumatologic PubMed Central articles Rheumatologic 2023 articles Rheumatologic 2024 articles Rheumatologic Scopus articles Rheumatologic impact factor journals Rheumatologic Scopus journals Rheumatologic PubMed journals Rheumatologic medical journals Rheumatologic free journals Rheumatologic best journals Rheumatologic top journals Rheumatologic free medical journals Rheumatologic famous journals Rheumatologic Google Scholar indexed journals Coagulopathies articles Coagulopathies Research articles Coagulopathies review articles Coagulopathies PubMed articles Coagulopathies PubMed Central articles Coagulopathies 2023 articles Coagulopathies 2024 articles Coagulopathies Scopus articles Coagulopathies impact factor journals Coagulopathies Scopus journals Coagulopathies PubMed journals Coagulopathies medical journals Coagulopathies free journals Coagulopathies best journals Coagulopathies top journals Coagulopathies free medical journals Coagulopathies famous journals Coagulopathies Google Scholar indexed journals Body mass index articles Body mass index Research articles Body mass index review articles Body mass index PubMed articles Body mass index PubMed Central articles Body mass index 2023 articles Body mass index 2024 articles Body mass index Scopus articles Body mass index impact factor journals Body mass index Scopus journals Body mass index PubMed journals Body mass index medical journals Body mass index free journals Body mass index best journals Body mass index top journals Body mass index free medical journals Body mass index famous journals Body mass index Google Scholar indexed journals Adipose tissue articles Adipose tissue Research articles Adipose tissue review articles Adipose tissue PubMed articles Adipose tissue PubMed Central articles Adipose tissue 2023 articles Adipose tissue 2024 articles Adipose tissue Scopus articles Adipose tissue impact factor journals Adipose tissue Scopus journals Adipose tissue PubMed journals Adipose tissue medical journals Adipose tissue free journals Adipose tissue best journals Adipose tissue top journals Adipose tissue free medical journals Adipose tissue famous journals Adipose tissue Google Scholar indexed journals Liposuction articles Liposuction Research articles Liposuction review articles Liposuction PubMed articles Liposuction PubMed Central articles Liposuction 2023 articles Liposuction 2024 articles Liposuction Scopus articles Liposuction impact factor journals Liposuction Scopus journals Liposuction PubMed journals Liposuction medical journals Liposuction free journals Liposuction best journals Liposuction top journals Liposuction free medical journals Liposuction famous journals Liposuction Google Scholar indexed journals
Article Details
Abbreviations:
NSAIDs: Non-steroidal anti-inflammatory drugs; ADSCs: Adipose-derived mesenchymal cells; UH – FUS: University Hospital of Federal University of Sergipe; MRI: Magnetic resonance imaging; BMI: The mean body mass index; Kg: Kilograms; m: Meters; CMS: Constant-Murley Shoulder Score; SPADI: Shoulder Pain and Disability Index; UCLA: University of California Shoulder Rating Scale; ASES: American Shoulder and Elbow Surgery Society
1. Introduction
Rotator Cuff Tendinopathy is an inflammatory condition characterized by pain during joint movement, local edema, reduced performance, and alteration of adjacent tendon structures [1]. This condition has a multifactorial etiology and is associated with various intrinsic factors, such as age, obesity, and anatomical variations. Regarding extrinsic risk factors, trauma, occupation, and overuse can be mentioned [2]. Given the potential progression of tendinopathy to partial or complete ruptures, this condition leads to work absences and has a significant socioeconomic impact, ranking as the fourth leading cause of such absences [3-6]. Tendinopathies are estimated to account for approximately 30 million repair procedures in the United States and European countries, with a cost exceeding 150 billion euros [7].
Initially, upon diagnosing the disease through anamnesis, physical examinations, and imaging, non-surgical treatment is usually prioritized. Conservative treatment aims to alleviate and improve joint function and includes resting, non-steroidal anti-inflammatory drugs (NSAIDs), physical therapy sessions, and intra-articular corticosteroid injections. In cases of persistent symptoms or more severe tears, surgical intervention becomes the preferred method for joint repair [8]. Despite the various available options for managing Rotator Cuff Tendinopathy, achieving definitive treatment remains a major challenge for orthopedic specialists. The resulting scar tissue in the tendon is more fragile than the native tissue due to higher levels of type III collagen, at the expense of type I collagen [3,9]. Considering a conservative treatment failure rate of approximately 41% after one year and a retear rate of 21% after three months of surgical procedure, new therapeutic approaches are necessary [3,10].
In this context, the utilization of mesenchymal stem cells has garnered increasing attention due to their capacity for tissue regeneration and reduction of local joint inflammatory response [7,11]. Although the exact mechanism remains unknown, it is hypothesized that elevated levels of inflammatory and catabolic markers such as PGE2, IL-1, IL-6, TNF-α, and mast cells are implicated in the pathogenesis of the disease [4,12,13]. Animal and human studies have demonstrated that mesenchymal stem cell therapy can decrease these apoptotic and antiangiogenic markers, while also promoting tissue regeneration through the secretion of growth factors such as connective tissue growth factor, TGF-β, PDGF, FGF, HGF, VEGF, and angiopoietin, ultimately resulting in symptom improvement and enhanced joint function [14-18].
Among the various sources of mesenchymal stem cells, adipose-derived mesenchymal cells (ADSCs) have emerged as the most promising therapeutic option. Not only are they effective, but they can also be easily obtained through subcutaneous tissue liposuction. ADSCs exhibit high cellularity with multipotent potential and demonstrate a low incidence of adverse effects [7,11,19]. Studies have indicated that NanoFat, the liquid suspension form of emulsified and filtered liposuction-derived adipose tissue, containing a high number of stem cells (1.9 – 3.0 × 106) from the vascular fraction, has shown promising results in various medical fields due to its residual regeneration capacity and straightforward application [17,20-23].
Therefore, the present study aims to evaluate the impact of abdominal liposuction-derived NanoFat intratendineous injection on the symptomatology and joint functionality of patients diagnosed with Rotator Cuff Tendinopathy, presenting with partial tendon tears and a lack of response to initial conservative treatment.
2. Materials and Methods
The current study comprises a case series involving patients diagnosed with rotator cuff tendinopathy, without a control group, who were followed up for a period of one year after intratendineous NanoFat injection guided by ultrasound. The study was conducted at the University Hospital of Federal University of Sergipe (UH – FUS) between February 2020 and April 2022. The study protocol obtained approval from the Research Committee of UH – FUS (26262319.7.0000.5546), and all participants provided signed informed consent. The study recruited participants aged between 40 and 75 years with symptoms of shoulder pain or limited range of motion, despite having undergone at least three months of conservative treatment (NSAIDs, physical therapy), and the presence of partial rotator cuff tear confirmed by magnetic resonance imaging (MRI). Exclusion criteria for this study encompassed prior intra-articular corticosteroid injections, history of previous rotator cuff surgery, diagnoses of rheumatologic or systemic comorbidities that could potentially confound the cause of pain, physical examination findings indicating an etiology other than rotator cuff tendinopathy for the reported joint pain, diagnoses of coagulopathies, and occurrence of trauma subsequent to the study intervention.
This study comprised a total of 14 shoulders (7 right and 7 left) from 12 patients diagnosed with Rotator Cuff Tear. The mean age observed was 50 ± 8.8 years, with the majority of patients being female, representing 58.3% of the sample. The mean body mass index (BMI) among patients was 28.1 ± 5.2 kg/m2. The mean weight was 75.1 ± 16.3 kilograms (Kg), and the mean height was 1.63 ± 0.1 meters (m), as depicted in Table 1.
|
n |
% |
Mean (SD) |
Median (IQR) |
|
|
Sex |
||||
|
Female |
7 |
58,3 |
||
|
Male |
5 |
41,7 |
||
|
Age |
50 (8,8) |
47 (45-53) |
||
|
Weight |
75,1 (16,3) |
69 (65,4-90,8) |
||
|
Height |
163,2 (10,2) |
160,5 (157,5-167,8) |
||
|
BMI |
28,1 (5,2) |
27,5 (24,6-31,4) |
||
|
Side of the shoulder affected |
||||
|
Right |
7 |
50 |
||
|
Left |
7 |
50 |
||
|
Subtitle: n – absolute frequency. % – relative percentage frequency. SD – Standard Deviation. IQR – Interquartile Range. |
||||
Table 1: Sociodemographic and clinical characterization of patients affected by Rotator Cuff Tear.
2.1 Study design
The recruitment of participants was conducted through media outlets and communications, and their screening was performed at the orthopedic outpatient clinic of UH – FUS. A total of 16 participants were deemed eligible for the research. Prior to the proposed intervention, functional assessment, and shoulder pain questionnaires, namely the Constant-Murley Shoulder Score (CMS), the Shoulder Pain and Disability Index (SPADI), and the University of California Shoulder Rating Scale (UCLA), were utilized [24-26]. Four participants withdrew their consent, resulting in a final sample of 12 patients, including two bilateral involvements, leading to a total of 14 interventions. No participants withdrew or were excluded from the study after the proposed intervention.
2.2 Collection and processing of mesenchymal cells
Under the guidance of a licensed plastic surgeon, the collection of material for injection into the affected tendon was performed at the surgical center of UH – FUS through subcutaneous tissue liposuction at four points in the periumbilical region. The procedure was carried out without the administration of sedatives, following the technique described by Tonnard et al. [22]. Aseptic technique was applied in the region, and local anesthesia was administered using Klein`s tumescent solution. The solution consisted of 50 mL of 1% lidocaine and 1 mL of 1mg/mL epinephrine in 1000 mL of saline solution. The safety of this solution has been demonstrated in previous studies [22,23,27]. Adipose tissue was then collected using negative pressure. The collection process utilized two 2.1 mm × 200 mm infiltration cannulas, each accompanied by a three-port spiral cannula of 2.1 mm × 150 mm attached to a 20 mL Luer Lock syringe. Approximately 80 mL of tissue was obtained through liposuction in each patient.
To obtain NanoFat, the aspirated adipose tissue was processed using the NanoTransfer device from Tulip (Tulip Medical, San Diego, CA) according to the manufacturer`s technique [20-22]. The purpose of this device is to emulsify and mechanically filter the adipose tissue until it acquires a liquid suspension form, which can be easily injected with needles [22]. The aspirated tissue was initially decanted for 3 minutes. Subsequently, pre-emulsification was performed using a 2.4 mm collector, with the content transferred 30 times between syringes. Emulsification was then carried out using the 1.2 mm collector, repeating the transfer of content between syringes 30 times. For filtration, the NanoTransfer kit consists of an upper and lower component, each with connectors of 2.4 and 1.2 mm, a single-use cartridge, and two filters for each component. The upper component filter has a pore size of 629 micrometers, while the lower component filter has a pore size of 394 micrometers.
2.3 Intratendinous ultrasound-guided injection
All procedures were performed by the same orthopedic surgeon. Patients were positioned in a supine posture with the arm in a neutral position. Following an inspection of the joint cavity and identification of the tendon rupture as shown in the preoperative imaging examination, the puncture site was aseptically prepared, and local anesthesia with 2% lidocaine was administered. The interventions were guided by ultrasound (GE Venou 50, linear transducer, 4-15 MHz). A volume of 3 to 5 mL of ADSCs was injected into the posterior region of the shoulder using an 18 G disposable needle. The entire intervention, from collection to injection of the mesenchymal cells, lasted approximately one and a half hours. After the procedure, patients remained immobilized for 15 minutes and were discharged on the same day, with a prescription for acetaminophen in case of pain, instructions for absolute rest in the first 24 hours following the procedure, and a referral for physical therapy sessions.
2.4 Postoperative follow-up
All patients were evaluated at 1 week, 1, 3, 6, and 12 months after the procedure. During all follow-up consultations, the UCLA, SPADI, and CMS questionnaires were administered to assess the clinical impact of the proposed intervention as the primary outcome. Additionally, the presence of intervention-related adverse effects and the impact on patients` sleep were also observed. For strength evaluation in the CMS questionnaire, the Lafayette Manual Muscle Test System 01165 digital dynamometer was utilized, enabling objective grading of strength in kilograms.
2.6 Statistical analysis
Categorical variables were described using absolute and relative frequencies. Continuous variables were described using mean, standard deviation, median, and interquartile range. The hypothesis of equality of means was tested using analysis of variance (ANOVA). Multiple comparisons were tested using the Tukey test. The hypothesis of no correlation and the strength of the relationship between continuous variables were evaluated using Spearman`s correlation. The adopted significance level was 5%, and the software used for analysis was R Core Team 2021.
3. Results
3.1 Application of pre-procedure questionnaires
Initially, the results of the questionnaires administered prior to the proposed intervention were observed. The mean score obtained on the UCLA questionnaire was 16.9 ± 5.6, while on the SPADI questionnaire, the mean score obtained was 58 ± 26.7, and on the CMS questionnaire, it was 60.1 ± 19.4. The average strength was measured using a digital dynamometer and yielded a value of 8.36 ± 6.26 kg.
The correlation between the test results at the pre-procedure consultation was evaluated. Negative correlations were observed between SPADI and UCLA (-0.573), as well as between SPADI and CMS (-0.687). A positive correlation of 0.845 was observed between CMS and UCLA.
When comparing the data between genders, no statistical difference was found in the questionnaire responses. However, regarding strength, women exhibited an average strength of 4.38 ± 2.67 kg, while men displayed an average strength of 13.67 ± 5.72 kg (p = 0.002). No difference was observed in the results obtained between the shoulders regarding laterality.
3.2 Comparison of questionnaires in follow-up
In the comparative analysis of UCLA questionnaire results between the pre-procedure phase and one month after the intervention, a 66% increase (11.2 points) was observed. At 3 months, the average increase compared to the initial evaluation was 62% (10.6 points). This increasing trend persisted in the assessments conducted at 6 months and 1 year after the procedure, with an increase of 70.4% (11.9 points) and 79.88% (13.5 points), respectively (p < 0.001).
Analyzing the average scores of the SPADI questionnaire as a percentage, an improvement of 45.6% (26.5 points) was observed between the initial evaluation and 1 month after the intervention. There was also an improvement of 61.37% (35.6 points) when comparing the pre-intervention period with the evaluation performed 3 months later and 50.5% (29.3 points) when compared to the 6-month evaluation. Regarding the results obtained 1 year after the procedure, an improvement of 76% (44.1 points) was observed. The p-value obtained was less than 0.001.
In the CMS questionnaire, a statistically significant improvement of 26.5% (12 points) was observed when comparing the assessment conducted 7 days after the procedure with the one conducted at 1 month (p = 0.023). However, there was no difference between the score obtained before the procedure and the subsequent evaluations. The average scores obtained in each follow-up questionnaire are expressed in Table 2 and showed in Figures 1, 2 and 3.
|
Visit |
UCLA |
SPADI |
CMS |
|
Mean (SD) |
Mean (SD) |
Mean (SD) |
|
|
Preoperative |
16.9 (5.6) |
58.0 (26.7) |
60.1 (19.4) |
|
7 days |
22.0 (7.4) |
56.2 (24.8) |
57.0 (14.8) |
|
1 month |
28.1 (4.8) |
31.5 (23.5) |
72.1 (7.4) |
|
3 months |
27.5 (7.1) |
22.4 (21.6) |
67.6 (13.7) |
|
6 months |
28.8 (6.4) |
28.7 (28.0) |
70.9 (12.6) |
|
1 year |
30.4 (5.4) |
13.9 (17.1) |
71.8 (9.7) |
|
p-value |
<0.001 |
<0.001 |
0.023 |
|
Subtitle: SD – Standard Deviation. ANOVA. |
|||
Table 2: Mean scores obtained from questionnaires in follow-up visits of patients with Rotator Cuff Tear.
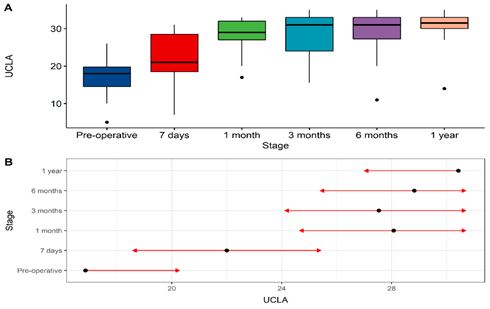
Figure 1: Comparison of results obtained with the application of UCLA questionnaire before the procedure and during follow-up. 1 month vs. pre-operative, p < 0.001; 3 months vs. pre-operative, p < 0.001; 6 months vs. pre-operative, p < 0.001; 1 year vs. pre-operative, p < 0.001.
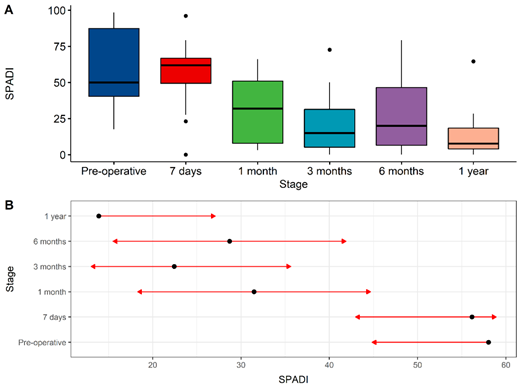
Figure 2: Comparison of results obtained with the application of SPADI questionnaire before the procedure and during follow-up. 1 month vs. pre-operative, p < 0.001; 3 months vs. pre-operative, p < 0.001; 6 months vs. pre-operative, p < 0.001; 1 year vs. pre-operative, p < 0.001.
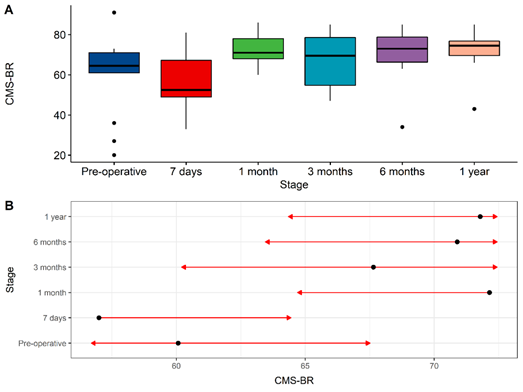
Figure 3: Comparison of results obtained with the application of CMS questionnaire before the procedure and during follow-up. 7 days vs. 1 month, p = 0.023.
4. Discussion
The present study demonstrated a significant improvement in pain and functionality in the affected shoulders during the final evaluation of the participants, as assessed by two out of three questionnaires administered after a 12-month period following the procedure. The UCLA questionnaire showed an increase from 16.9 to 30.4 points (p<0.001), while the SPADI questionnaire showed a decrease from 59.0 to 13.9 points (p<0.001). Adverse effects observed in the study included mild hematoma in the periumbilical region, which persisted for up to three days after the intervention in three out of 12 participants. Additionally, one participant reported joint stiffness within the first 24 hours post-procedure (Table 3 and Figure 4). No serious adverse effects or permanent damage were reported, and no participant was excluded from the final 12-month evaluation. Throughout the evaluation period, no patient experienced complete rupture of the tendon or presented functional or painful deterioration. Despite the absence of well-defined protocols and guidelines for the application of mesenchymal cells, several studies have demonstrated their safety and efficacy [7,11,28,29].
For the liposuction procedure, the participants received local anesthesia with tumescent solution, the efficacy and safety of which have been documented in the literature by numerous studies [14,22,23,30,31]. In 2009, Habbema [27] described a series of 3240 consecutive cases in which tumescent solution was utilized for abdominal liposuction procedures. Data analysis revealed no cases of mortality, hospitalization, severe complications, or legal complaints following the procedures. Minor adverse effects reported included two cases of panniculitis and two cases of skin necrosis smaller than 5 cm resulting from blister formation.
|
Your shoulder disrupts sleep, n (%) |
Preoperative |
7 days |
p-value |
|
Always |
6 (42,9) |
5 (35,7) |
0,044 |
|
Sometimes |
8 (57,1) |
4 (28,6) |
|
|
No |
0 (0) |
5 (35,7) |
|
|
Subtitle: n – absolute frequency. % – relative percentage frequency. Pearson's chi-square test. |
|||
Table 3: Impact of Rotator Cuff Tear on sleep before and after the procedure.
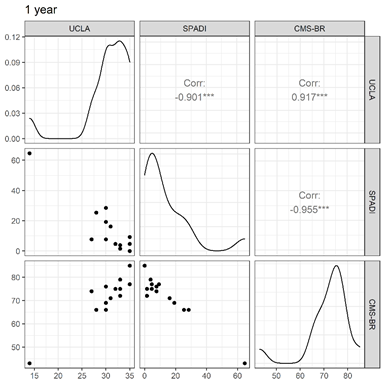
Figure 4: Correlation between questionnaires after 1 year of the procedure.
To evaluate the impact of ADSCs on rotator cuff tears, Jo et al. [3] divided the patients into three groups based on the number of stem cells injected: 1.0 × 107 (low-dose), 5.0 × 107 (mid-dose) and 1.0 × 108 (high dose). ADSCs were obtained through abdominal liposuction, and enzymatic digestion was performed for cell preparation. The effectiveness of the treatment was assessed using the SPADI and CMS questionnaires, while the regenerative potential was evaluated through magnetic resonance imaging. After 6 months, a significant improvement was observed in the SPADI questionnaire, with an 80% decrease (p = 0.20) and a 77% decrease (p<0.001) in scores for the high-dose and mid-dose groups, respectively, while no significant improvement was observed in the low-dose group. The CMS questionnaire demonstrated a significant increase in scores of 27% (p = 0.014) and 20% (p = 0.005) for the high-dose and mid-dose groups, respectively. Radiological findings revealed a 90% reduction in the volume of defects on the bursal side among patients in the high-dose group. Therefore, it was concluded that the use of ADSCs for the treatment of rotator cuff tears is both safe and effective in regenerating tendon defects, resulting in functional improvement and pain relief.
In accordance with this, Kim et al. [32] demonstrated the efficacy of ADSCs usage by conducting a comparative study involving 35 patients with full-thickness rotator cuff tears who underwent exclusive arthroscopic repair and 35 patients who received ADSCs injections as an adjunct to surgical treatment. The functional improvement and pain reduction of the participants were evaluated using the UCLA and CMS questionnaires. Although patients who received adjunctive treatment exhibited a significant improvement in CMS questionnaire scores (65.2 vs. 78.3; p < 0.001) and UCL scores (26.5 vs. 20.8; p = 0.037) 28 months post-intervention, there was no statistically significant difference compared to participants treated solely with surgical repair. However, patients who received ADSCs demonstrated a lower incidence of new tears (14.3% vs. 28.5%, p < 0.001). Consequently, it can be concluded that while not superior in terms of functional improvement and pain reduction, the utilization of ADSCs in conjunction with surgical treatment reduces the risk of retear.
In a prospective randomized study conducted by Hurd et al. [14], the aim was to compare patients with partial rotator cuff tears who received intra-articular injections of 80 mg of methylprednisolone with patients who received ADSCs injections. The ASES (American Shoulder and Elbow Surgery Society) questionnaire and RAND Short Form-36 Total Score were employed to evaluate efficacy. The study revealed a significantly greater improvement in patients who received mesenchymal cells at 24- and 52-weeks post-procedure, and no severe adverse effects were reported.
Regarding the specific use of intra-articular NanoFat, Mahmmood et al. [23] described its efficacy as a therapeutic option for temporomandibular disorder. After evaluating 19 joints, they concluded that the utilizations of NanoFat had a significant impact on pain level, joint clicking, joint deviation, and maximum mouth opening after a 2-week period.
However, this study has several limitations. Firstly, the small number of participants and the short follow-up period should be noted. Furthermore, the absence of a control group prevented the comparison of the efficacy of the performed treatment with the conventional approach. The inability to obtain magnetic resonance imaging for all participants post-procedure hindered the observation of possible tissue regeneration. Despite all patients being referred for physical therapy sessions, as our research was conducted in the Brazilian public healthcare system, the inability to standardize the responsible professional and the follow-up period limited the sample uniformity. Finally, the lack of histological analysis and evaluation of cell viability of ADSCs through fluoroscopic microscopy can also be considered a limiting factor. Nevertheless, the use of ADSCs as a therapeutic treatment for patients with rotator cuff tears shows promise [33]. Therefore, further randomized clinical studies are necessary to develop standardized protocols and guidelines.
5. Conclusion
The current study has provided evidence of functional improvement and pain reduction in patients undergoing NanoFat treatment, as evaluated through the UCLA and SPADI questionnaires. Furthermore, the absence of severe adverse effects aligns with the findings from other studies in the scientific literature, indicating procedural safety. Finally, a noteworthy enhancement of sleep quality was observed among the participants.
References
- Scott A, Squier K, Alfredson H, et al. International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br J Sports Med 54 (2020): 260-262.
- Wang HN, Rong X, Yang LM, et al. Advances in Stem Cell Therapies for Rotator Cuff Injuries. Front Bioeng Biotechnol 10 (2022): 866195.
- Jo CH, Chai JW, Jeong EC, et al. Intratendinous Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Rotator Cuff Disease: A First-In-Human Trial. Stem Cells 36 (2018): 1441-1450.
- Arvind V, Huang AH. Reparative and Maladaptive Inflammation in Tendon Healing. Front Bioeng Biotechnol 9 (2021): 719047.
- Yelin E, Weinstein S, King T. The burden of musculoskeletal diseases in the United States. Seminars in Arthritis and Rheumatism 46 (2016): 259-260.
- Tashjian RZ. Epidemiology, Natural History, and Indications for Treatment of Rotator Cuff Tears. Clinics in Sports Medicine 31 (2012): 589-604. [PMID: 23040548 DOI: 10.1016/j.csm.2012.07.001]
- Senesi L, De Francesco F, Marchesini A, et al. Efficacy of Adipose-Derived Mesenchymal Stem Cells and Stromal Vascular Fraction Alone and Combined to Biomaterials in Tendinopathy or Tendon Injury: Systematic Review of Current Concepts. Medicina 59 (2023): 273.
- Longo UG, Risi Ambrogioni L, Candela V, et al. Conservative versus surgical management for patients with rotator cuff tears: a systematic review and META-analysis. BMC Musculoskelet Disord 22 (2021): 50.
- Ko JY, Huang CC, Chen WJ, et al. Pathogenesis of partial tear of the rotator cuff: A clinical and pathologic study. Journal of Shoulder and Elbow Surgery 15 (2006): 271-278.
- Longo UG, Carnevale A, Piergentili I, et al. Retear rates after rotator cuff surgery: a systematic review and meta-analysis. BMC Musculoskelet Disord 22 (2021): 749.
- Itro A, Trotta MC, Miranda R, et al. Why Use Adipose-Derived Mesenchymal Stem Cells in Tendinopathic Patients: A Systematic Review. Pharmaceutics 14 (2022): 1151.
- Bergqvist F, Carr AJ, Wheway K, et al. Divergent roles of prostacyclin and PGE2 in human tendinopathy. Arthritis Res Ther 21 (2019): 74.
- Bedi A, Maak T, Walsh C, et al. Cytokines in rotator cuff degeneration and repair. Journal of Shoulder and Elbow Surgery 21 (2012): 218-227.
- Hurd JL, Facile TR, Weiss J, et al. Safety and efficacy of treating symptomatic, partial-thickness rotator cuff tears with fresh, uncultured, unmodified, autologous adipose-derived regenerative cells (UA-ADRCs) isolated at the point of care: a prospective, randomized, controlled first-in-human pilot study. J Orthop Surg Res 15 (2020): 122.
- Viganò M, Lugano G, Perucca Orfei C, et al. Autologous microfragmented adipose tissue reduces inflammatory and catabolic markers in supraspinatus tendon cells derived from patients affected by rotator cuff tears. International Orthopaedics (SICOT) 45 (2021): 419-426.
- De Francesco F, Ricci G, D’Andrea F, et al. Human Adipose Stem Cells: From Bench to Bedside. Tissue Engineering Part B: Reviews 21 (2015): 572-584.
- Kamat P, Frueh FS, McLuckie M, et al. Adipose tissue and the vascularization of biomaterials: Stem cells, microvascular fragments and nanofat—a review. Cytotherapy 22 (2020): 400-411.
- Chen L, Tredget EE, Wu PYG, et al. Paracrine Factors of Mesenchymal Stem Cells Recruit Macrophages and Endothelial Lineage Cells and Enhance Wound Healing. PLoS ONE 3 (2008): e1886.
- Torres-Torrillas M, Rubio M, Damia E, et al. Adipose-Derived Mesenchymal Stem Cells: A Promising Tool in the Treatment of Musculoskeletal Diseases. IJMS 20 (2019): 3105.
- Cohen SR, Tiryaki T, Womack HA, et al. Cellular Optimization of Nanofat: Comparison of Two Nanofat Processing Devices in Terms of Cell Count and Viability. Aesthetic Surgery Journal Open Forum 1 (2019): ojz028.
- Ding P, Lu E, Li G, et al. Research Progress on Preparation, Mechanism, and Clinical Application of Nanofat. Journal of Burn Care and Research 43 (2022): 1140-1144.
- Tonnard P, Verpaele A, Peeters G, et al. Nanofat Grafting: Basic Research and Clinical Applications. Plastic and Reconstructive Surgery 132 (2013): 1017-1026.
- Mahmmood VH, Shihab SM. Assessment of Therapeutic Effect of Intra-Articular Nanofat Injection for Temporomandibular Disorders: Journal of Craniofacial Surgery 30 (2019): 659-662.
- Martins J, Napoles BV, Hoffman CB, et al. Versão Brasileira do Shoulder Pain and Disability Index: tradução, adaptação cultural e confiabilidade. Rev bras fisioter 14 (2010): 527-536.
- Barreto RPG, Barbosa MLL, Balbinotti MAA, et al. The Brazilian version of the Constant–Murley Score (CMS-BR): convergent and construct validity, internal consistency, and unidimensionality. Revista Brasileira de Ortopedia (English Edition) 51 (2016): 515-520.
- Oku EC, Andrade AP, Stadiniky SP, et al. Tradução e adaptação cultural do Modified-University of California at Los Angeles Shoulder Rating Scale para a língua portuguesa. Rev Bras Reumatol 46 (2006): 246-252.
- Habbema L. Safety of Liposuction Using Exclusively Tumescent Local Anesthesia in 3,240 Consecutive Cases. Dermatologic Surgery 35 (2009): 1728-1735.
- Khoury MA, Chamari K, Tabben M, et al. Expanded adipose derived mesenchymal stromal cells are effective in treating chronic insertional patellar tendinopathy: clinical and MRI evaluations of a pilot study. J EXP ORTOP 8 (2021): 49.
- Khoury M, Tabben M, Rolón AU, et al. Promising improvement of chronic lateral elbow tendinopathy by using adipose derived mesenchymal stromal cells: a pilot study. J EXP ORTOP 8 (2021): 6.
- Striano RD. Refractory Shoulder Pain with Osteoarthritis, and Rotator Cuff Tear, Treated With Micro-Fragmented Adipose Tissue 2 (2018).
- Usuelli FG, Grassi M, Maccario C, et al. Intratendinous adipose-derived stromal vascular fraction (SVF) injection provides a safe, efficacious treatment for Achilles tendinopathy: Results of a randomized controlled clinical trial at a 6-month follow-up. Knee Surg Sports Traumatol Arthrosc 26 (2018): 2000-2010.
- Kim YS, Sung CH, Chung SH, et al. Does an Injection of Adipose-Derived Mesenchymal Stem Cells Loaded in Fibrin Glue Influence Rotator Cuff Repair Outcomes? A Clinical and Magnetic Resonance Imaging Study. Am J Sports Med 45 (2017): 2010-2018.
- Zhang X, Wang D, Wang Z, et al. Clinical perspectives for repairing rotator cuff injuries with multi-tissue regenerative approaches. Journal of Orthopaedic Translation 36 (2022): 91-108.
