Circulating 25-hydroxyvitamin D levels and Risk of Lung Cancer: A Meta-epidedmiological Meta-analysis
Article Information
Jong-Myon Bae*
Department of Preventive Medicine, Jeju National University College of Medicine, Jeju Province, Korea
*Corresponding Author: Dr. Jong-Myon Bae, 102 Jaejudaehak-ro, Jeju-si, Jeju Special Self-Governing Province, 63243, Republic of Korea
Received: 24 July 2019; Accepted: 05 August 2019; Published: 14 August 2019
Citation: Jong-Myon Bae. Circulating 25-hydroxyvitamin D levels and Risk of Lung Cancer: A Meta-epidedmiological Meta-analysis. Journal of Cancer Science and Clinical Therapeutics 3 (2019): 105-113.
View / Download Pdf Share at FacebookAbstract
Background: Five previous systematic reviews (SR) evaluating the hypothesis that serum vitamin D deficiency was associated with risk of lung cancer showed unstable and uncertain results. Especially antipathic relative risks (RR) of selected articles as well as missed articles were found among them.
Objective: The aim of this SR was to conduct a meta-epidemiolgical meta-analysis (MEMA) based on them.
Methods: Using citation discovery tools, additional articles were selected from cited lists based on 16 selected articles. Fixed effect model was applied if I2 value was less than 50%. A publication bias was evaluated using Egger’s test.
Results: Of 13 articles, the summary RRs (and their 95% confidence intervals) (I2 value) were 1.17 (1.09, 1.26) (18.3%). The sRR from 10 articles adjusted for sex and 12 articles adjusted for smoking habits showed stable RRs with keeping the statistical significance. Egger’s test showed no publication bias.
Conclusion: This MEMA supported the lower level of serum vitamin D was associated with an increased risk of lung cancer.
Impact: This might be an evidence for conducting some public health programs against vitamin D deficiency status in order to prevent lung cancer.
Keywords
<p>Vitamin D; Lung neoplasms; Risk factors; Systematic Review; Meta-analysis</p>
Vitamin D articles, Lung neoplasms articles, Risk factors articles, Systematic Review articles, Meta-analysis articles
Article Details
1. Introduction
In the last several decades, vitamin D has been shown to be involved in processes such as cell differentiation, proliferation, and apoptosis [1]. And low vitamin D levels have been reported to be associated with risk of breast cancer [2], colorectal cancer [3], and prostate cancer [4]. Meanwhile, lung cancer is known to be the most common cancer worldwide, while also being fatal [5]. Accordingly, in addition to early diagnosis, prevention of lung cancer is of the utmost importance. Among previous 5 meta-analyses on the lung cancer risk associated with reduced plasma 25-hydroxyvitamin D [25(OH)D] [6-10], in the most recent study, by Wei et al. [10], the summary relative risk (sRR) reversed direction to 0.95 and lost its statistical significance. In other words, the meta-analysis results reported to date are unstable and uncertain (Table 1).
|
FA (RF) |
Zhang (6) |
Chen (7) |
Feng (8) |
Liu (9) |
Wei (10) |
||
|
PY |
2015 |
2015 |
2017 |
2017 |
2018 |
||
|
Search upto |
Oct 2014 |
May 2015 |
Aug 2017 |
NA |
Dec 2017 |
||
|
sRR |
1.19 |
1.05 |
1.19 |
1.39 |
0.95 |
||
|
(95% CI) |
1.11-1.28 |
1.01-1.10 |
1.05-1.35 |
1.18-1.64 |
086-1.06 |
||
|
I-squared (%) |
0 |
51.3 |
50.3 |
61.3 |
37.9 |
||
|
Lists of PAS |
RF |
PY |
|||||
|
(18) |
2006 |
√ |
√ |
√ |
|||
|
(11) |
2008 |
√ |
√ |
√ |
√ |
√ |
|
|
(16) |
2010 |
√ |
|||||
|
(12) |
2011 |
√ |
√ |
√ |
√ |
√ |
|
|
(17) |
2012 |
√ |
√ |
√ |
|||
|
(13) |
2013 |
√ |
√ |
√ |
√ |
√ |
|
|
(19) |
2013 |
√ |
|||||
|
(14) |
2014 |
√ |
√ |
√ |
√ |
√ |
|
|
(15) |
2014 |
√ |
√ |
√ |
√ |
√ |
|
|
(20) |
2014 |
√ |
√ |
√ |
|||
|
(21) |
2015 |
√ |
√ |
||||
|
(22) |
2015 |
√ |
√ |
||||
|
(23) |
2016 |
√ |
√ |
||||
|
(24) |
2017 |
√ |
|||||
|
(25) |
2017 |
√ |
|||||
|
(26) |
2017 |
√ |
*CI: confidence intervals; FA: First author; NA: not available; PY: publication year; RF: reference number; sRR: summary relative risks
Table 1: Summary table of prospective studies selected (PAS) from five systematic reviews.
Table 1 highlights several issues with the processes of these 5 systematic reviews. First, there is no consistency in the studies selected for meta-analysis. If the selection criteria were the same, more recent searches should obviously include studies selected by the authors of previous meta-analyses. However, there were only 5 studies that were included in all 5 meta-analyses [11-15]. Second, the selection criteria differed between meta-analyses studies. If the endpoint in the hypothesis is lung cancer risk, the two studies dealing with lung cancer mortality [16, 17] should be excluded. Third, the results extracted from the selected studies show differences between the meta-analyses. These differences in the extracted values can be found in the forest plots presented as the results of each meta-analysis. Fourth, there was a lack of subgroup analysis or sensitivity analysis for lung cancer-related variables. It is important to examine the effects of plasma vitamin D depending on gender, smoking history, and histological type of lung cancer.
Accordingly, the list of studies selected in the previous 5 meta-analyses [6-10] needs to be reorganized, and the information extracted from those studies needs to be re-examined. In this study, we aimed to investigate hypothesis that decreased plasma 25(OH)D levels increase the risk of lung cancer. To this end, we performed a meta-epidemiological meta-analysis (MEMA) aiming to utilize and update the 5 previous meta-analyses.
2. Materials and Methods
In accordance with the aims of this study to update the previous 5 meta-analyses [6-10], it was necessary to add relevant studies that were published after the meta-analyses were performed. Utilizing the list of 16 studies [11-26] selected by the authors of the previous meta-analyses (Table 1), we made a search list using the ‘cited by’ option as citation discovery tools (CDT) provided by PubMed [27]. We set the end of the search period as the end of March 2019. To select relevant studies from the search list, we applied the same selection criteria as the systematic reviews in Table 1. Specifically, we selected analytic epidemiological studies of lung cancer risk that obtained plasma 25(OH)D levels at constructing a cohort and used a prospective observational design.
From the studies selected according to the criteria, we extracted the RR and 95% confidence intervals (CI) using the ‘highest versus lowest’ method (HLM), only extracting data for the group with the lowest plasma 25(OH)D compared to the highest plasma 25(OH)D. In cases using the lowest 25(OH)D group as the reference, we obtained the inverse values, such that the highest 25(OH)D group would become the reference. This was to reflect the aim of our study, examining the risk associated with low plasma 25(OH)D levels. Since Giovannucci et al. [18] presented their results in the form of a graph, we used the values suggested by Zhang et al [6]. Wu et al. [23] presented results separately for the smoking group and the non-smoking group; therefore, we took values derived from a meta-analysis of the results from these two groups and used these as the results for that study. From the RR and 95%CIs of each study, we calculated the logarithm RR (logRR) and standard error of logRR (SElogRR).
Study heterogeneity was assessed using the I-squared value (%); a random effect model was applied for I2 ≥50%, and a fixed effect model was applied for I2 <50% [28]. Subgroup analysis was performed by histological type of lung cancer (total vs. non-small cell lung cancer (NSCLC)), sex (men vs. women), and smoking status, as well as by study design (cohort (CO) vs. case-control (CC)). We constructed a funnel plot and performed Egger’s test to examine publication bias. If a publication bias was detected, we limited SElogRR and performed a sensitivity analysis. The statistical significance level was set to 0.05.
3. Results
Using PubMed’s CDT, we retrieved a total of 389 studies citing the 16 studies in Table 1. Of these we selected 3 studies that satisfied the selection criteria [29-31]. All studies had been published after January 2018. Notably, Muller et al. [31] derived their results from a CC study using cases obtained from internationally famous cohorts. In order to exclude duplicate data in the meta-analysis, this study was only included in the meta-analysis of CC studies.
Of the 16 studies in Table 1, we excluded 2 studies that used lung cancer deaths as outcomes [16, 17]. Ananthakrishnan et al. [20] was also excluded because the subject were patients with inflammatory bowel diseases. And, of the 3 studies using results from the ESATER cohort [19, 21, 26], we selected Ordóñez-Mena et al. [21] as the representative study, since they presented their results in the most detail. Therefore, we selected 14 studies finally to include in the meta-analysis [11-15, 18, 21-25, 29-31]. Table 2 shows the logRR and SElogRR calculated by applying the HLM to these studies.
|
RN |
First Author |
Year |
Design |
Sex |
logRR |
SElogRR |
Study or Nation |
|
18 |
Giovannucci |
2006 |
CO |
M |
0.19 |
0.18 |
HPF |
|
11 |
Kilkkinen |
2008 |
CO |
B |
0.33 |
0.26 |
Mini-Finland Health Survey |
|
12 |
Weinstein |
2011 |
CC |
M |
0.19 |
0.23 |
ATBC |
|
13 |
Afzal |
2013 |
CO |
B |
0.17 |
0.05 |
Copenhagen City Heart |
|
14 |
Wong |
2014 |
CO |
M |
0.32 |
0.27 |
HIMS |
|
15 |
Skaaby |
2014 |
CC |
B |
0.09 |
0.29 |
Monica10, Inter99, Health2006 |
|
22 |
Wang |
2015 |
CC |
B |
0.89 |
0.4 |
China |
|
21 |
Ordóñez-Mena |
2016 |
CO |
B |
0.33 |
0.29 |
CHANCES |
|
23 |
Wu |
2016 |
CC |
B |
0.12 |
0.14 |
HongKong |
|
24 |
Gromowski |
2017 |
CC |
B |
0.48 |
0.25 |
Poland |
|
25 |
Cheng |
2017 |
CC |
W |
-0.06 |
0.14 |
WHI CTs & OS |
|
29 |
Budhathoki |
2018 |
CO |
B |
0.33 |
0.17 |
HPHC |
|
30 |
Sun |
2018 |
CO |
B |
-0.15 |
0.14 |
HUNT |
|
31 |
Muller |
2018 |
CC |
B |
0.01 |
0.07 |
LC3 |
* CC: case-control study; CI: confidence interval; CO: cohort study logRR: logarithm relative risk; RN: reference number; Sex: B(men and women) M(men) W(women); SElogRR: standard error of logarithm relative risk
Table 2: Summary of the extracted information of 14 selected articles.
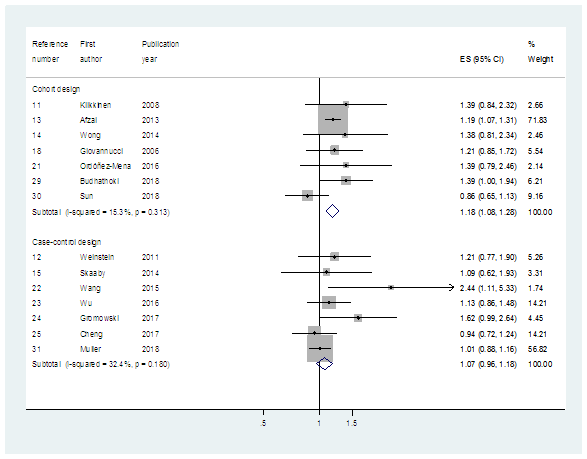
When we performed meta-analysis of the 13 selected studies excluding Muller et al. [31], the sRR (95% CI) (I2 value, %) was 1.17 (1.09-1.26) (18.3%) (Table 3). When we analyzed the 7 CO studies and 7 CC studies separately, the sRRs were 1.18 (1.08-1.28) (15.3%) and 1.07 (0.96-1.18) (32.4%), respectively (Table 3, Figure 1).
|
Subgroup |
All |
Cohort Design |
Case-control design |
|
Histological type |
|||
|
All histological |
1.17 (1.09-1.26) (18.3) {13} |
1.18 (1.08-1.28) (15.3) {7} |
1.07 (0.96-1.18) (32.4) {7} |
|
Non-small cell |
1.17 (0.92-1.49) (27.7) {3} |
- |
1.17 (0.92-1.49) (27.7) {3} |
|
Sex |
|||
|
Adjusted |
1.14 (1.06-1.22) (42.4) {10} |
1.17 (1.07-1.28) (40.4) {5} |
1.08 (0.96-1.21) (48.3) {5} |
|
Men |
1.21 (0.96-1.51) (0.0) {4} |
1.21 (0.93-1.57) (0.0) (3) |
1.21 (0.76-1.89) (-) {1} |
|
Women |
0.44 (0.08-2.46) (84.2) {2} |
0.16 (0.14-0.59) (-) {1} |
0.94 (0.54-0.94) (-) {1} |
|
Smoking habit |
|||
|
Adjusted |
1.15 (1.07-1.23) (32.4) {12} |
1.18 (1.08-1.28) (15.3) {7} |
1.08 (0.96-1.22) (49.3) {5} |
|
Non-smokers |
0.97 (0.77-1.23) (0.0) {2} |
- |
0.97 (0.77-1.23) (0.0) {2} |
*Summary relative risks (95% confidence intervals) (I-squared value, %) of {number} selected cohorts
Table 3: Subgroup analyses by study design.
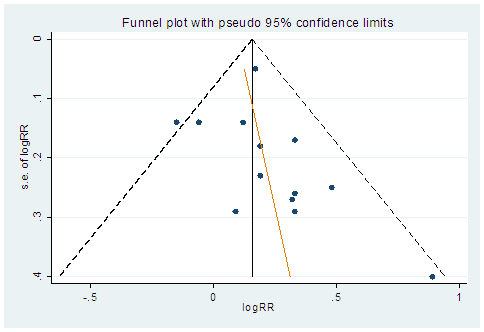
The 10 studies adjusting for sex and the 12 studies adjusting for smoking history each showed similar, significant risk. However, in studies restricted to NSCLC, men, women, or non-smokers, we no longer observed statistical significance. Egger’s test on the results of the 14 studies showed that there was publication bias (P=0.332) (Figure 2).
4. DiscussionThe above results show that low plasma 25(OH)D was associated with a significant, 1.17-fold (95% CI: 1.09-1.26) increase in lung cancer risk. In subgroup analysis, statistical significance was maintained in studies adjusting for sex and smoking history. In subgroup analyses, significances were also maintained in cohort design, sex-adjusted, or smoking habit-adjusted group. When the sRR from this MEMA was compared with the 5 previous meta-analyses in Table 1, the results were similar to 3 of the previous meta-analyses [6-8]. Meanwhile, the direction and significance of the sRR differed from the 2 more recently published meta-analyses [9, 10]. These facts demonstrates the importance of strict selection criteria, accurate data extraction, and selecting appropriate studies in order to obtain a valid sRR.
In order to fit the study objectives, this MEMA excluded 3 studies that had been selected in related meta-analyses [16, 17, 20], and selected only the study by Ordóñez-Mena et al. [21] to represent 3 studies presenting results from the same cohort [19, 21, 26]. In addition, we used PubMed’s CDT to search studies based on the list of studies selected in the previous 5 meta-analyses (Table 1), and we added 3 studies that had been published between January 2018 and March 2019 [29-31]. When we performed subgroup analyses for studies adjusting for sex and smoking history, we obtained more stable sRR values, and statistical significance was maintained.
On the other hand, as shown in Table 3, when the number of studies analyzed was 5 or lower, or when analyzing only CC studies, statistical significance was lost and sRR was unstable. This can be interpreted as showing that the meta-analysis results are affected by the number of subjects. Given that a CC analysis by Muller et al. [31] found plasma 25(OH)D to be unrelated to lung cancer incidence, it will be necessary to perform a cohort analysis using individual patient data. It will also be necessary to perform a MEMA that adds further relevant studies by extending the range of publication dates in the selection criteria [32].
Meanwhile, author did not evaluate the quality of selected articles using the NewcastleOttawa Scale (NOS) or Grading of recommendation, assessment, development and evaluation. Instead, author did conduct subgroup analyses by study design for observational studies in nutritional epidemiology. The reason was based on the suggestion by Bae JM [33], which concluded that 'it is more reasonable to control for quality level by performing subgroup analysis according to study design rather than by using high quality based on the NOS quality assessment tool.
Until new MEMA results are published, this MEMA is evidence that increasing circulating vitamin D levels could be a measure to help prevent lung cancer. Amidst recent environmental changes such as the spread of electronic cigarettes and increased levels of particulate matter, these results can be utilized in planning and promoting public health projects for lung cancer prevention.
Conflicts of Interest
The author declares no potential conflicts of interest.
References
- Holick MF. Vitamin D and sunlight: strategies for cancer prevention and other health benefits. Clin J Am Soc Nephrol 3 (2008):1548-1554.
- Estébanez N, Gómez-Acebo I, Palazuelos C, et al. Vitamin D exposure and Risk of Breast Cancer: a meta-analysis. Sci Rep 8 (2018): 9039.
- Maalmi H, Walter V, Jansen L, et al. Association between Blood 25-Hydroxyvitamin D Levels and Survival in Colorectal Cancer Patients: An Updated Systematic Review and Meta-Analysis. Nutrients 10 (2018). pii: E896.
- Song ZY, Yao Q, Zhuo Z, et al. Circulating vitamin D level and mortality in prostate cancer patients: a dose-response meta-analysis. Endocr Connect 7 (2018): R294-R303.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 69 (2019): 7-34.
- Zhang L, Wang S, Che X, et al. Vitamin D and lung cancer risk: a comprehensive review and meta-analysis. Cell Physiol Biochem 36 (2015): 299-305.
- Chen GC, Zhang ZL, Wan Z, et al. Circulating 25-hydroxyvitamin D and risk of lung cancer: a dose-response meta-analysis. Cancer Causes Control 26 (2015): 1719-1728.
- Feng Q, Zhang H, Dong Z, et al. Circulating 25-hydroxyvitamin D and lung cancer risk and survival: A dose-response meta-analysis of prospective cohort studies. Medicine (Baltimore) 45 (2017): e8613.
- Liu J, Dong Y, Lu C, et al. Meta-analysis of the correlation between vitamin D and lung cancer risk and outcomes. Oncotarget 8 (2017): 81040-81051.
- Wei H, Jing H, Wei Q, et al. Associations of the risk of lung cancer with serum 25-hydroxyvitamin D level and dietary vitamin D intake: A dose-response PRISMA meta-analysis. Medicine (Baltimore) 97 (2018): e12282.
- Kilkkinen A, Knekt P, Heliövaara M, et al. Vitamin D status and the risk of lung cancer: a cohort study in Finland. Cancer Epidemiol Biomarkers Prev 17 (2008): 3274-3278.
- Weinstein SJ, Yu K, Horst RL, et al. Serum 25-hydroxyvitamin D and risk of lung cancer in male smokers: a nested case-control study. PLoS One 6 (2011): e20796.
- Afzal S, Bojesen SE, Nordestgaard BG. Low plasma 25-hydroxyvitamin D and risk of tobacco-related cancer. Clin Chem 59 (2013): 771-780.
- Wong YY, Hyde Z, McCaul KA, et al. In older men, lower plasma 25-hydroxyvitamin D is associated with reduced incidence of prostate, but not colorectal or lung cancer. PLoS One 9 (2014): e99954.
- Skaaby T, Husemoen LL, Thuesen BH, et al. Prospective population-based study of the association between serum 25-hydroxyvitamin-D levels and the incidence of specific types of cancer. Cancer Epidemiol Biomarkers Prev 23 (2014):1220-1229.
- Freedman DM, Looker AC, Abnet CC, et al. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988-2006). Cancer Res 70 (2010): 8587-8597.
- Cheng TY, Neuhouser ML. Serum 25-hydroxyvitamin D, vitamin A, and lung cancer mortality in the US population: a potential nutrient-nutrient interaction. Cancer Causes Control 23 (2012): 1557-1565.
- Giovannucci E, Liu Y, Rimm EB, et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 98 (2006): 451-459.
- Ordóñez-Mena JM, Schöttker B, Haug U, et al. Serum 25-hydroxyvitamin d and cancer risk in older adults: results from a large German prospective cohort study. Cancer Epidemiol Biomarkers Prev 22 (2013): 905-916.
- Ananthakrishnan AN, Cheng SC, Cai T, et al. Association between reduced plasma 25-hydroxy vitamin D and increased risk of cancer in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 12 (2014): 821-827.
- Ordóñez-Mena JM, Schöttker B, Fedirko V, et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: an analysis of cohorts participating in the CHANCES consortium. Eur J Epidemiol 31 (2016): 311-323.
- Wang X, Cui J, Gu J, et al. Plasma 25-hydroxyvitamin D deficiency is associated with the risk of non-small cell lung cancer in a Chinese population. Cancer Biomark 15 (2015): 663-668.
- Wu X, Cheng J, Yang K. Vitamin D-Related Gene Polymorphisms, Plasma 25-Hydroxy-Vitamin D, Cigarette Smoke and Non-Small Cell Lung Cancer (NSCLC) Risk. Int J Mol Sci 17 (2016). pii: E1597.
- Gromowski T, Gapska P, Scott RJ, et al. Serum 25(OH)D concentration, common variants of the VDR gene and lung cancer occurrence. Int J Cancer 141 (2017): 336-341.
- Cheng TD, Song X, Beresford SAA, et al. Serum 25-hydroxyvitamin D concentrations and lung cancer risk in never-smoking postmenopausal women. Cancer Causes Control 28 (2017):1053-1063.
- Ordóñez-Mena JM, Schöttker B, Saum KU, et al. No Association of Vitamin D Pathway Genetic Variants with Cancer Risks in a Population-Based Cohort of German Older Adults. Cancer Epidemiol Biomarkers Prev 26 (2017): 1459-1461.
- Bae JM, Kim EH. Citation Discovery Tools for Conducting Adaptive Meta-analyses to Update Systematic Reviews. J Prev Med Public Health 49 (2016): 129-133.
- Harris RJ, Bradburn MJ, Deeks JJ, et al. Fixed- and random-effects meta-analysis. Stata J 8 (2008): 3-28.
- Budhathoki S, Hidaka A, Yamaji T, et al. Plasma 25-hydroxyvitamin D concentration and subsequent risk of total and site-specific cancers in Japanese population: large case-cohort study within Japan Public Health Center-based Prospective Study cohort. BMJ 360 (2018): k671.
- Sun YQ, Langhammer A, Wu C, et al. Associations of serum 25-hydroxyvitamin D level with incidence of lung cancer and histologic types in Norwegian adults: a case-cohort analysis of the HUNT study. Eur J Epidemiol 33 (2018): 67-77.
- Muller DC, Hodge AM, Fanidi A, et al. No association between circulating concentrations of vitamin D and risk of lung cancer: an analysis in 20 prospective studies in the Lung Cancer Cohort Consortium (LC3). Ann Oncol 29 (2018): 1468-1475.
- Bae JM. Meta-epidemiology. Epidemiol Health. 36 (2014): e2014019.
- Bae JM. A suggestion for quality assessment in systematic reviews of observational studies in nutritional epidemiology. Epidemiol Health 38 (2016): e2016014.