Challenges in Hematologic Care: A Case Report of Sickle Cell Disease and Multiple Myeloma Concurrence
Article Information
Supriya Peshin*, 1, FNU Warsha1, Tamanna Taznin1, Shagun Singh2, FNU Vineesha3, Marissa G Vito Cruz1
1Norton community Hospital, Norton, Virginia
2University of Arizona, Tucson, Arizona
3Liquat medical college, Karachi, Pakistan
*Corresponding author: Supriya Peshin, Norton community Hospital, Norton, Virginia, USA.
ORCID: https://orcid.org/0000-0002-3895-9474
Received: 11 October 2024; Accepted: 18 October 2024; Published: 25 October 2024
Citation: Supriya Peshin, FNU Warsha, Tamanna Taznin, Shagun Singh, FNU Vineesha, Marissa G Vito Cruz. Integrating Prediabetes Management into Workplace Health Programs: Outcomes and Challenges. Fortune Journal of Health Sciences.7 (2024): 590-593.
View / Download Pdf Share at FacebookAbstract
This case report details the complex medical journey of a 69-year-old African American female with a multifaceted history, including sickle cell anemia with hemoglobin C disease, hypothyroidism, hypercholesteremia, severe obesity, autoimmune hemolytic anemia, and multiple myeloma. Initially referred for evaluation of asymptomatic pancytopenia, the patient was later diagnosed with IgG kappa monoclonal paraproteinemia, consistent with multiple myeloma, ISS stage II. Throughout her treatment, she underwent several cycles of chemotherapy, facing multiple complications, such as recurrent sickle cell crises, significant anemia, and elevated liver enzymes. These complications necessitated careful management and adjustments to her therapeutic regimen, reflecting the intricate interplay between her coexisting conditions. Despite these challenges, the patient exhibited partial improvement, underscoring the need for a tailored and multidisciplinary approach to managing such complex cases. This report highlights the diagnostic and therapeutic difficulties in treating patients with concurrent sickle cell disease and multiple myeloma, emphasizing the importance of personalized care to optimize outcomes.
Keywords
FLT3-ITD, FLT3-TKD, Next-generation sequencing (NGS), MDS/AML, acute myeloid leukemia (AML)
FLT3-ITD articles, FLT3-TKD articles, Next-generation sequencing (NGS) articles, MDS/AML articles, acute myeloid leukemia (AML) articles
Article Details
Introduction
Sickle cell disease (SCD) is a hereditary hemoglobinopathy characterized by chronic hemolysis, vaso-occlusive crises, and end-organ damage [1]. The disease is most commonly seen in individuals of African descent and represents a significant global health burden [2]. Patients with SCD, particularly those with concurrent hemoglobin C disease, are at increased risk for severe complications, including stroke, acute chest syndrome, and a heightened susceptibility to infections [1]. These complications arise due to the sickling of red blood cells, which leads to vascular occlusion, ischemia, and subsequent organ damage [3]. The coexistence of SCD with multiple myeloma, a malignant plasma cell disorder characterized by clonal proliferation of plasma cells and the presence of monoclonal immunoglobulins, is uncommon [4]. Multiple myeloma typically presents with hypercalcemia, renal impairment, anemia, and bone lesions, but the overlap with SCD complicates both diagnosis and management [5]. The pathophysiological interplay between these two conditions can exacerbate each disease’s manifestations, making treatment decisions more complex and outcomes less predictable [4]. Managing a patient with concurrent SCD and multiple myeloma presents unique challenges. For instance, therapeutic interventions such as chemotherapy may trigger vaso-occlusive crises in SCD patients, while the underlying hemoglobinopathy can complicate the treatment response and overall prognosis of multiple myeloma [6]. This case report illustrates the diagnostic and therapeutic complexities of treating a patient with SCD and multiple myeloma, highlighting the need for a tailored, multidisciplinary approach to optimize outcomes [7].
Case Presentation
A 69-year-old African American female with a known history of sickle cell anemia with hemoglobin C disease, hypothyroidism, hypercholesteremia, severe obesity, autoimmune hemolytic anemia, osteoporosis, and thrombocytopenia presented to Dr. Cook's office at SW Cancer Center for an evaluation. She was initially referred for the assessment of asymptomatic pancytopenia and abnormal marrow signals observed on an MRI of the lumbar spine.
The patient had been diagnosed with sickle cell hemoglobin C disease, with her last significant crisis occurring over a decade ago, although she had experienced multiple crises during her youth. Her medical history was further complicated by the fact that her son also had a similar diagnosis. Initial laboratory investigations conducted during this period revealed normal iron studies and vitamin B12 levels, which ruled out other common causes of anemia and pancytopenia.
As her condition was monitored, the patient was referred for a workup of worsening anemia. The evaluation revealed an elevated protein level characterized by a monoclonal M spike of 4.1 g/dL, without the presence of light chains in the urine. This finding prompted further investigation, and additional laboratory tests identified the presence of IgG kappa monoclonal paraproteinemia, suggestive of multiple myeloma, ISS stage II. A subsequent bone marrow biopsy confirmed the diagnosis of IgG kappa monoclonal antibody multiple myeloma, with a significant infiltration of plasma cells (50-60%) and the presence of 11q deletion, a genetic abnormality associated with a poorer prognosis. Consequently, the patient was started on induction therapy with a regimen that included lenalidomide, bortezomib, dexamethasone (RVD), and daratumumab (DARA), with the intention of considering autologous stem cell transplantation (ASCT) once remission was achieved.
During the initial cycles of treatment, the patient's hemoglobin levels dropped significantly, reaching a nadir of 7.3 g/dL. Despite this, transfusion was deferred as the patient remained asymptomatic. As treatment progressed, the patient tolerated Velcade and Darzalex but required two units of blood transfusion due to palpitations, which improved following the transfusion.The patient's treatment course was complicated by elevated liver function tests (LFTs), which prompted adjustments in her therapy. She received Darzalex alone during periods of elevated LFTs and was treated with topical nystatin for a concurrent yeast infection. As her LFTs returned to baseline, her therapy was resumed with Darzalex, Velcade, and dexamethasone. However, her liver enzymes became elevated again, necessitating a reduction in the dose of lenalidomide and subsequent discontinuation of this drug due to worsening LFTs. The treatment plan was adjusted to continue Velcade, dexamethasone, and Darzalex, with careful monitoring.The patient reported mild fatigue during periods when lenalidomide was held. Her condition further deteriorated when she presented to the emergency room with complaints of left arm and hip pain, coinciding with a significant drop in hemoglobin to 5.4 g/dL. This episode was likely due to an acute hemolytic and sickle cell pain crisis, prompting her admission to the hospital for pain management, hydration, and transfusion of two units of packed red blood cells (PRBCs).
As her chemotherapy resumed, the patient’s laboratory reports showed some improvement. However, due to the complexity of her sickle cell disease and the risks associated with ASCT, she was deemed ineligible for the procedure. The treatment focused on optimizing her response with daratumumab, Velcade, and pomalidomide. Later, she experienced another sickle cell crisis, likely precipitated by bortezomib, which was suspected to have contributed to her cardiomyopathy. Consequently, her treatment regimen was adjusted to continue with Darzalex alone. Further complications arose when her hemoglobin levels remained suboptimal, and she was not considered a good candidate for ASCT due to the rarity of concurrent multiple myeloma and hemoglobin SC disease. Discussions about initiating pomalidomide were held, leading to the adjustment of her treatment regimen. The patient was later admitted to the emergency department with another sickle cell crisis, along with multiple infections. Her treatment regimen was again modified, discontinuing bortezomib and lenalidomide due to intolerance, and pomalidomide was started at a reduced dose.
Over time, the patient reported adherence to the pomalidomide regimen, although she experienced neuropathy in her hands, legs, and feet, which worsened at night. Despite these challenges, she continued with her treatment, experiencing another sickle cell pain crisis with symptoms including right hip and mid-sternal pain. Treatment with antibiotics was initiated for suspected pneumonia, and her liver enzymes normalized after discontinuing pomalidomide. With the stabilization of her multiple myeloma under the continued administration of daratumumab and dexamethasone, plans were made to add carfilzomib if further progression occurred. This detailed account of the patient's course of treatment underscores the complexity and challenge of managing multiple concurrent conditions that independently carry high risks and require meticulous, individualized treatment strategies.
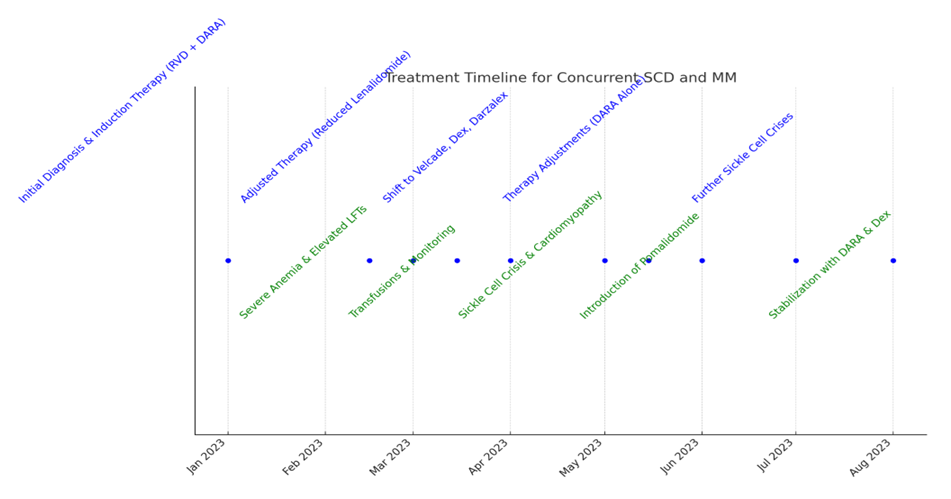
Figure 1: Here is the updated treatment timeline for the concurrent Sickle Cell Disease (SCD) and Multiple Myeloma (MM) patient.
Discussion
The case of a 69-year-old African American female with concurrent sickle cell anemia with hemoglobin C disease and multiple myeloma exemplifies the complex interplay between two serious and chronic conditions that independently carry significant morbidity and mortality. The coexistence of these diseases presents a formidable challenge in clinical management, as each condition can exacerbate the manifestations and complications of the other [1,4]. For instance, the chronic hemolysis and anemia inherent to sickle cell disease (SCD) can obscure the typical presentation of multiple myeloma, making diagnosis more difficult and potentially delaying appropriate treatment [5]. Moreover, the pathophysiological mechanisms underlying SCD, such as the sickling of red blood cells, lead to recurrent vaso-occlusive crises and multi-organ damage, which can be further aggravated by the bone marrow infiltration and immunosuppression caused by multiple myeloma [3,6]. In treating this patient, the use of standard multiple myeloma therapies such as lenalidomide and bortezomib posed additional risks [7]. Lenalidomide, while effective in targeting myeloma cells, is associated with an increased risk of thrombosis, which can be particularly dangerous in SCD patients already prone to vaso-occlusion [7]. Bortezomib, another cornerstone of myeloma therapy, is known to cause peripheral neuropathy, adding to the already significant burden of neuropathic pain and dysfunction in SCD patients [4]. In this case, the patient’s treatment was complicated by recurrent sickle cell crises, severe anemia requiring multiple transfusions, and elevated liver enzymes, necessitating the discontinuation of these therapies and the initiation of pomalidomide at a reduced dose [8]. This approach underscores the necessity of balancing the efficacy of myeloma treatment with the need to minimize the exacerbation of SCD-related complications [5].
Autologous stem cell transplantation (ASCT) is typically considered a potentially curative option for eligible multiple myeloma patients, offering the possibility of prolonged remission [4]. However, in patients with SCD, ASCT introduces significant risks, including the potential for severe vaso-occlusive crises and acute chest syndrome triggered by the high-dose chemotherapy required for conditioning [9]. In this case, the patient was ultimately deemed ineligible for ASCT due to the high complexity of her dual diagnosis, highlighting the limitations of standard myeloma treatment strategies in the context of SCD [9]. Instead, the decision to continue treatment with daratumumab, Velcade, and pomalidomide reflects a more cautious approach, aiming to control the myeloma while mitigating the risk of severe SCD complications [6].
This case emphasizes the critical need for an interdisciplinary approach in managing patients with concurrent SCD and multiple myeloma [10]. Such an approach involves close collaboration between hematologists, oncologists, and other specialists to ensure comprehensive monitoring and management of both conditions [10]. Tailoring treatment plans to the individual patient is essential, as standard protocols may need to be adjusted to reduce the risk of exacerbating SCD-related complications [7]. Involving the patient in decision-making is also crucial, given the complexity of managing dual diagnoses and the potential impact on the patient’s quality of life [6]. Additionally, there is a pressing need for further research into the optimal management of patients with these concurrent conditions [10]. Prospective studies and clinical trials focused on this unique patient population could lead to the development of more effective and safer treatment protocols, ultimately improving outcomes for individuals with both SCD and multiple myeloma [9].
Conclusion
This case underscores the importance of a multidisciplinary approach in managing patients with multiple comorbidities, particularly those with rare and complex conditions such as concurrent sickle cell disease and multiple myeloma. Tailoring treatment plans to account for the unique challenges posed by each condition is crucial for optimizing patient outcomes. Further research is needed to develop treatment strategies that minimize the risk of exacerbating sickle cell crises while effectively managing multiple myeloma in this patient population.
References
- Piel FB, Steinberg MH, Rees DC. Sickle Cell Disease. N Engl J Med 376 (2017): 1561-1573.
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15 (2014).
- Gaziev J, Lucarelli G. Stem cell transplantation for hemoglobinopathies. Curr Opin Pediatr 15 (2003): 24-31.
- Lonial S, Durie BGM, Palumbo A, et al. Monoclonal antibodies in the treatment of multiple myeloma: current status and future perspectives. Leukemia 30 (2016): 526-535.
- Korde N, Roschewski M, Zingone A, et al. Treatment of newly diagnosed multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines 2013. Mayo Clin Proc 89 (2014): 192-211.
- Landgren O, Rajkumar SV. New Developments in Diagnosis, Prognosis, and Assessment of Response in Multiple Myeloma. Clin Cancer Res (2016): 5428-5433.
- Baz R, Lin HM, Hui AM, et al. Predictors of outcome in patients with multiple myeloma treated with lenalidomide as initial therapy. Am J Hematol 85 (2010): 836-840.
- Mateos MV, Cavo M, Blade J, et al. Emergence of secondary primary malignancies in multiple myeloma: therapeutic implications. Blood 127 (2016): 2293-2303.
- Hari P, Pasquini M, Bonini C, et al. Current state of haploidentical stem cell transplantation. Br J Haematol 165 (2014): 27-38.
- Kazandjian D, Landgren O. A New Era for Multiple Myeloma: The Promise of Immunotherapy. Cancer J 22 (2016): 49-54.
